Abstract
Soft tissue augmentation is a process of implanting tissues or materials to treat wrinkles or soft tissue defects in the body. Over the years, various materials have evolved to correct soft tissue defects, including a number of tissues and polymers. Autogenous dermis, autogenous fat, autogenous dermis-fat, allogenic dermis, synthetic implants, and fillers have been widely accepted for soft tissue augmentations. Tissue engineering technology has also been introduced and opened a new venue of opportunities in this field. In particular, a long-lasting filler consisting of hyaluronic acid filler and living human mesenchymal cells called "injectable tissue-engineered soft tissue" has been created and applied clinically, as this strategy has many advantages over conventional methods. Fibroblasts and adipose-derived stromal vascular fraction cells can be clinically used as injectable tissue-engineered soft tissue at present. In this review, information on the soft tissue augmentation method using the injectable tissue-engineered soft tissue is provided.
Graphical Abstract
Keywords: Cell Therapy, Soft Tissue, Tissue Engineering
INTRODUCTION
Tissue engineering is a novel technology that combines biodegradable polymer scaffolds with various cells to generate new tissue for graft, repair, or replacement (1, 2, 3). Autogenic cultured dermal fibroblasts have been successfully used as key cells for tissue-engineering of soft tissue to correct dermal and subcutaneous deficiencies since 1995 (4). The introduction of this strategy opened a new possibilities for physicians. In this review, our experiences with soft tissue augmentation using tissue-engineering technology based on hyaluronic acid (HA) fillers and mesenchymal cells are presented.
SOFT TISSUE AUGMENTATION
What is soft tissue augmentation?
Collagen and elastic fibers in the skin begin to breakdown as we age, and wrinkles emerge. In addition, some subcutaneous fat is lost on our bodies. In particular, the soft tissue atrophy on the face leads to a hollow or skeletal look. Some people want to modify their facial contours for aesthetic purposes. For example, the most common complaints among Asians include a flat or low nasal dorsum.
Soft tissue augmentation is a process of implanting tissues or materials where needed to restore a youthful or more aesthetic look to the face. The tissues or materials can be implanted in the face, including the nasal dorsum, lips, the nasolabial folds, the orbital regions, and the cheeks. This strategy is minimally invasive and may also treat problems such as depressed scars. Furthermore, soft tissue augmentation has been used widely for reconstruction purpose to treat various conditions or defects due to congenital anomalies or trauma (5).
Who is a candidate for soft tissue augmentation?
Soft tissue augmentation has been performed on nearly all body areas including the face. However, the most commonly used areas are the nasal dorsum, lips, the nasolabial folds, the orbital regions, the glabella, and the forehead. This method has also been used to restore the depressed scars on the face and to augment soft tissue on the buttocks and sternum.
The area being treated should have appropriate blood circulation so that the transplanted tissues are properly nourished and form their own blood supply. Patients to be treated must be emotionally stable and have realistic expectations of the results of the treatment.
What kinds of methods are used?
Various materials have evolved to correct soft tissue defects, including a number of tissues, polymers, and cell populations (3, 6, 7, 8, 9, 10).
Autogenous dermis, fat, or dermis-fat grafts have long been used for soft tissue augmentation to treat wrinkles and soft tissue defects in the body. Allogenic dermis has also been a widely accepted acellular human dermal matrix for skin and soft tissue applications. The cells and epidermis are removed from donated human skin since they lead to tissue rejection and graft failure. The implanted allogenic dermis is transformed into the host tissue, ultimately producing the effect of soft tissue augmentation by revascularization and cell migration from the host tissue. Therefore, allogenic dermis has been commonly used as a biologic implant to correct soft tissue defects or wrinkles.
However, as more patients look for aesthetic improvement through less invasive procedures than before, the demand for injectable soft tissue fillers to treat wrinkles in the body or to augment soft tissues has grown dramatically (11). Various commercially available biologic injectable fillers such as collagen and HA have been developed and are widely used (12). Human (both autogenic and homogenic), animal, and micribial sources are now clinically available. Each product has its own advantages and disadvantages. HA fillers are promising novel products that have exciting potential for soft tissue augmentation. Compared with collagen, HA is identical across all species and is synthesized from various cells. It can be stored at room temperature for more than 1 yr. Furthermore, since HA has the identical chemical structure in all species, it does not create immunologic rejection or foreign body reactions. Therefore, preliminary skin testing is not necessary. HA filler has been approved to be close to the ideal filler material for soft tissue augmentation. Currently, HA-based fillers have become the material of choice for use in soft tissue and dermal correction.
Although injectable filler substances are replacing conventional tissue transfer and the number of biocompatible fillers continues to increase, rapid absorption and overall disappointing long-term outcomes are common drawbacks for nearly all natural fillers, which are available at present, including HAs.
TISSUE-ENGINEERED SOFT TISSUE
What is injectable tissue-engineered soft tissue?
Ideal filler should have properties that are autogenic and applied without difficulty, can bring long-term effects, require less invasive procedure, and demonstrate minimal donor site morbidity. Although many HAs have been presented with better biocompatibility, convenience of administration, and chemical modifications for a long-lasting effect, they have not obtained a long-term corrective effect due to their rapid absorption. HA is absorbed in several weeks to months, depending on the injected area. This variable degree of resorption requires repeated percutaneous injections.
The authors have devised a long-term filler system consisting of a mixture of HA filler and living human mesenchymal cells to sustain the effect of soft tissue augmentation (13, 14). This strategy is called "injectable tissue-engineered soft tissue".
Fibroblasts and adipose-derived stromal vascular fraction (SVF) cells can be clinically used for injectable tissue-engineered soft tissue.
What is the advantage of injectable tissue-engineered soft tissue?
Injecting tissue-engineered soft tissue has many advantages. It can be carried out simple and easy because the procedure is performed at an out-patient clinic without entering the surgery room. It provides long-term effects, requires less invasive procedure, and demonstrates minimal donor site morbidity. In addition, it can minimize communication errors between the pysician and patient, since patients can immediately notice the outcomes. Furthermore, patients are psychologically comfortable because autogenic tissue is applied. Therefore, this strategy may meet the demand as ideal filler.
Basic mechanism of injectable tissue-engineered soft tissue
Injecting tissue-engineered soft tissue is based on the hypothesis that injecting cells which produce extracellular matrices leads to a long-term corrective effect than that obtained using a HA filler without cells. HA filler is expected to show an early effect and cells to give a long-term effect.
We reported in previous animal and clinical studies that human fibroblasts and SVF cells suspended in HA filler can produce extracellular matrices with long-lasting stability. The cell-HA filler mixtures remained in place for >1 yr; demonstrating the advantage of adding cells to HA fillers.
CLINICAL APPLICATION
Injectable soft tissue using fibroblasts
A skin biopsy (around 1 cm2) is taken from a patient's inguinal or gluteal area and sent to a Food and Drug Administration (FDA)-approved laboratory for fibroblast culture. Briefly, fibroblasts are isolated using collagenase and propagated for subsequent passaging. About 6 weeks are required to obtain twenty million cultured fibroblasts, which is a sufficient number for injection. Cell density is determined using a hematocytometer, and viability is assessed by trypan blue exclusion. Second or third passage cultures are used for injection.
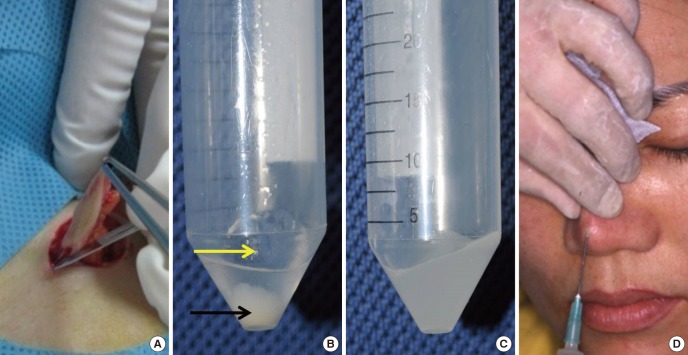
The twenty million cultured fibroblasts are suspended in 1.0-1.5 mL HA filler. After preparing the skin around the injection site with antiseptics, the fibroblast-filler mixture is injected into the intradermal, subdermal, and/or subcutaneous layers using a 23 and/or a 26-gauge needle (Fig. 1).
Fig. 1.
Application methods for injectable tissue-engineered soft tissue using cultured fibroblasts. (A) Skin harvesting. (B) Hyaluronic (HA) filler (yellow arrow) and a cultured fibroblast pellet (black arrow). (C) Cultured fibroblasts are mixed with the HA filler. (D) The fibroblasts suspended in the HA filler are injected into the nasal dorsum.
Finger pressure is applied for immediate molding to prevent possible irregularity of the injected implant. At a point that the patient is most satisfied with the augmentation effect, an 0.1-0.5 ml of the bioimplant is additionally injected to obtain approximately 30% overcorrection. The average total amount of injected bioimplant per patient is 0.6-1.5 mL.
This implant is not injected into an area with high densities of sebaceous glands or large skin pores, e.g. nasal tip.
Injectable soft tissue using adipose-derived SVF cells
Compared with the method using fibroblasts, SVF cells are easy to obtain in large quantities without culture. Therefore, they are readily available. Even though autogenic cells are used, patients do not need to wait because the cell culture step is omitted. In addition, SVF cell therapy does not need FDA approval in many countries including Korea. However, it is hypothesized that adipose-derived SVF cells mixed in HA filler may possibly generate variety of tissues after injection since they are a heterogenic cell population including fibroblasts, smooth muscle cells, preadipocytes, various progenitor cells, endothelial cells, and stem cells (15, 16, 17). We have performed a research to find out what kinds of tissues are created from injection of human SVF cells suspended in a HA filler. The conclusion was that transplanting human adipose-derived SVF cells mixed with HA filler produce fibrovascular tissue. No other tissue, including fat, cartilage, bone, or nerve is created (18). This finding suggests the possibility of using this material as a long-term filler for soft tissue augmentation.
Abdominal adipose tissues are taken from a patient by liposuction to obtain adipose-derived SVF cells. The samples are washed with Dulbecco's phosphate-buffered saline (DPBS), and then incubated in Dulbecco's Modified Eagle Medium/Ham's F-12 containing 0.075% collagenase type I for 2 hr at 37℃. The top layer of lipid is removed, and the remaining solution is centrifuged at 300×g for 10 min at 4℃. The resulting pellet is treated with 160 mM NH4Cl for 10 min to lyse the red blood cells. The remaining cells are then rinsed twice in 40 mL DPBS, resuspended in 5 ml DPBS, and filtered through a 100 µm nylon mesh. Cell density is determined with a hemocytometer, and cell viability is assessed by trypan blue exclusion. The number of isolated SVF cells has been assessed. Ashjian et al. (19) reported that processing 1 ml of suctioned adipose tissue routinely yields about 1.2×106 SVF cells. Suga et al. (20) demonstrated that 1.0×106 SVF cells could be obtained from 1 g of adipose tissue. However, based on our experience, 6.3×104 to 3.5×105 metabolically active SVF cells can be isolated per ml of aspirated adipose tissue (21).
The isolated SVF cells (as many as possible) are suspended in 1.0-1.5 mL HA filler. All conditions, including skin preparation and injection techniques, are identical to those used for dermal fibroblast injection.
Precautions
There is an important technical point that we would like to emphasize when applying this method for augmentation rhinoplasty. When injecting the cell-filler bioimplan in the nasal dorsum, the medical practitioner should be very careful to inject it at the exact midline. Although slight asymmetry may be corrected by using finger pressure, delayed deviation is possible. We have experienced this phenomenon in the first patient. Immediately after injecting the bioimplant, the augmented nasal dorsum was deviated slightly. This could be corrected with finger pressure immediately and the dorsum appeared to remain in the midline of the nose until 3 months after the injection. However, we noticed that the augmented nasal dorsum was deviated again after that period.
Clinical experience
We have applied the injectable tissue-engineered soft tissue with HA filler mixed with autologous cultured fibroblasts or SVF cells for augmentation rhinoplasty and wrinkle correction since 2002. Patients show mild redness immediately after the injection at the injected area, but this usually disappears completely within a couple of days. Patients feel that the soft tissue augmentation effect appears to reduce during the first 1-4 month postoperative period. However, the volume is well maintained after this early period. The level of volume decrease is 20%-40% in usual cases. The majority of patients are satisfied with the natural shape and feel comfortable with the augmentation (Fig. 2, 3, 4, 5).
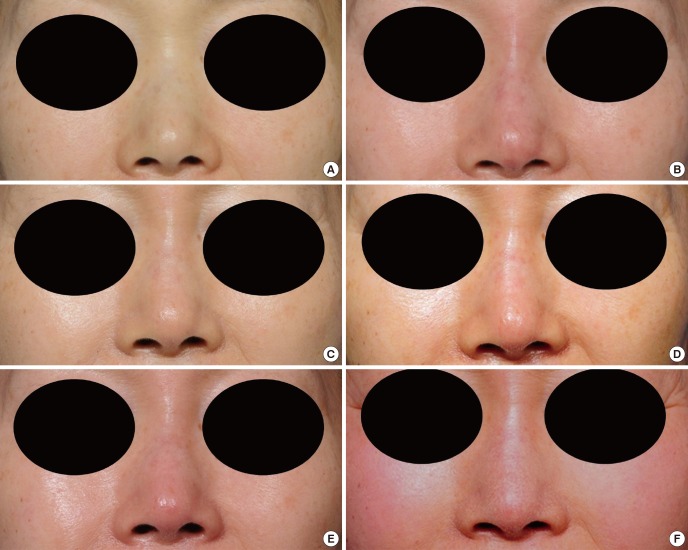
Fig. 2.
A 59-yr-old woman was treated with injectable tissue-engineered soft tissue using fibroblasts for augmentation rhinoplasty. (A) Preoperative view. (B) Immediate postoperative view. (C) Two weeks after the injection. (D-F) Six, 12, and 18 months after the injection.
Fig. 3.
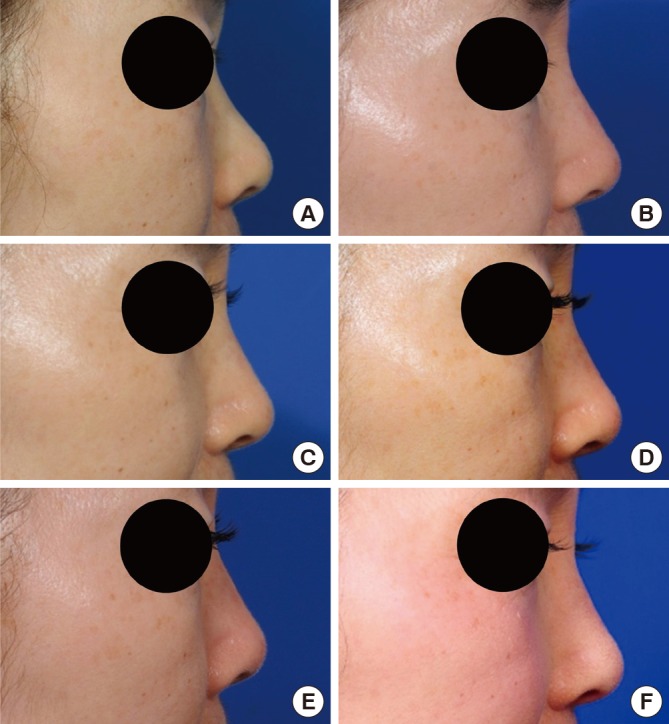
Profile views of the Fig. 2 case.
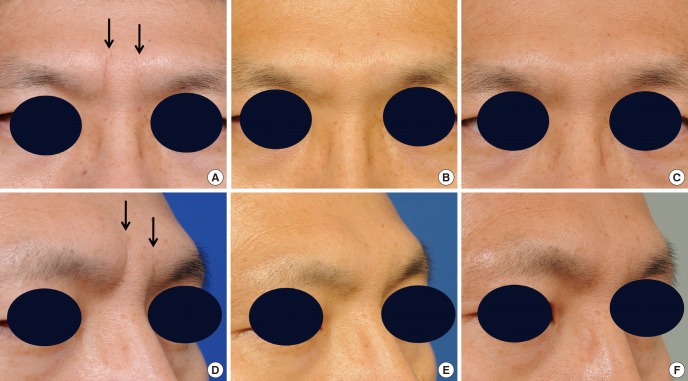
Fig. 4.
Deep glabellar wrinkles (arrows) of a 43-yr-old man were corrected by injecting stromal vascular fraction (SVF) cells suspended in a hyaluronic (HA) filler. (A) Preoperative view. (B) One year after the injection. (C) Three years after the injection. (D-F) Three-quarter views.
Fig. 5.
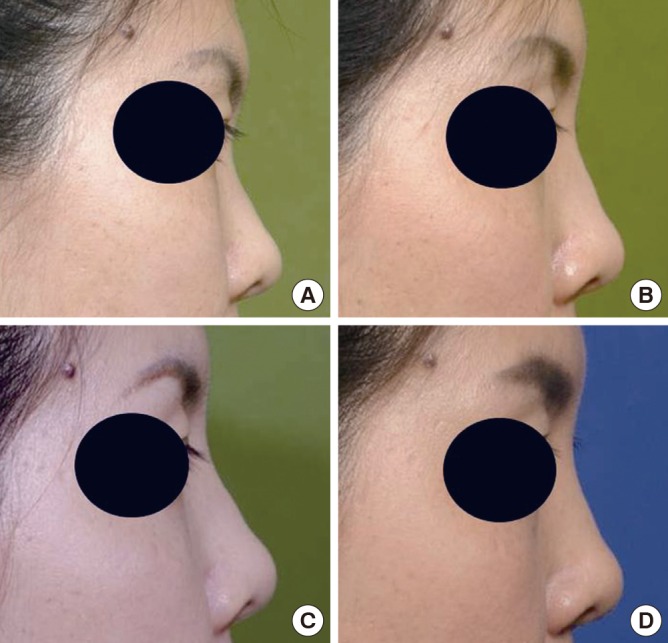
A 30-yr-old woman was treated with injectable tissue-engineered soft tissue using stromal vascular fraction (SVF) cells for augmentation rhinoplasty. (A) Preoperative view. (B) Six months after the injection. (C) One year after the injection. (D) Six years after the injection.
We have not experienced any abnormal laboratory findings during the follow-up period. No significant side effect such as overcorrection, granuloma or lump formation, keloid development, infection, or rejection have been observed.
The results of clinical use show that injection of the HA filler/cell mixture may have promising possibility as a long-term injectable soft tissue for soft tissue augmentation.
LIMITATION
What are the limitations of the present application?
Approval by the FDA and time-consuming culture steps are needed for human fibroblasts. Therefore, utilizing cultured cells for clinical purposes may be difficult realistically.
It has not been possible to obtain exact data of the absorption after injection in the body for the injected tissue-engineered soft tissue in each patient. Because soft tissue augmentation is mainly a cosmetic procedure, it is not only possible to harvest tissue samples from the injected sites to investigate amount of the newly created tissue, but most of patients objected to further quantitative evaluation such as radiological or imaging studies, based on the time and cost (22).
What kind of research is required?
It was not possible to obtain exact data on the absorption degree of the injected bioimplant in each patient. Additional studies will be needed to resolve this limitation. Moreover, since there were no reports of tissue biopsies for injection sites, we have no information if the tissue remains as cells themselves, cell excretes, or others. More studies on this issue may be required.
Research on the optimal conditions for the HA filler as a cell carrier should also be carried out. HA fillers have been basically developed to be used alone without cells. Studies of the optimal conditions of HA filler as cell delivery vehicles or as scaffolds, combined with cultured human dermal fibroblasts or SVF cells, to maximize the viability of injected cells is necessary.
Strategies to increase viability of suspended cells in HA fillers after injection should also be developed (23, 24, 25). Supplementing materials can possibly be added to the bioimplant during the preparation process to maximize viability of the injected cells in the cell-HA filler bioimplant. In particular, we have explored using prostaglandin E1 (PGE1) and vitamin C, which have been commonly used in clinical settings. PGE1 mediates vasodilation and improves microcirculation perfusion. PGE1 has been reported to stimulate fibroblast proliferation. Vitamin C promotes fibroblast production. Therefore, it can be hypothesized that a combination of PGE1 and vitamin C in the cell-HA filler might accelerate cell viability and/or proliferation after injection into the body.
CONCLUSION
Despite that more studies are required to definitely determine the value of this method, injection of human dermal fibroblasts or adipose-derived stromal vascular cells mixed in HA filler may be a promising treatment for soft tissue augmentation.
References
- 1.You HJ, Han SK, Lee JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes: a pilot study. Wound Repair Regen. 2012;20:491–499. doi: 10.1111/j.1524-475X.2012.00809.x. [DOI] [PubMed] [Google Scholar]
- 2.Han SK, You HJ. Wound coverage using advanced technology in Korea. J Korean Med Assoc. 2011;54:594–603. [Google Scholar]
- 3.Jeong SH, Han SK, Kim WK. Soft tissue augmentation using in vitro differentiated adipocytes: a clinical pilot study. Dermatol Surg. 2011;37:760–767. doi: 10.1111/j.1524-4725.2011.01950..x. [DOI] [PubMed] [Google Scholar]
- 4.Boss WK, Jr, Usal H, Chernoff G, Keller GS, Lask GP, Fodor PB. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin Plast Surg. 2000;27:613–626. [PubMed] [Google Scholar]
- 5.Lee TG, Chung S, Chung YK. A retrospective review of iatrogenic skin and soft tissue injuries. Arch Plast Surg. 2012;39:412–416. doi: 10.5999/aps.2012.39.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, Chacón-Martínez H, Mecott-Rivera G, Salazar-Lozano A, Valdes-Flores E. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg. 2012;39:659–662. doi: 10.5999/aps.2012.39.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SK, Kim DW, Dhong ES, Park SH, Yoon ES. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012;39:534–539. doi: 10.5999/aps.2012.39.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park DK, Song I, Lee JH, You YJ. Forehead augmentation with a methyl methacrylate onlay implant using an injection-molding technique. Arch Plast Surg. 2013;40:597–602. doi: 10.5999/aps.2013.40.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim EH, Sardinha JP, Myers S, Stevens M. Latent transforming growth factor-beta1 functionalised electrospun scaffolds promote human cartilage differentiation: towards an engineered cartilage construct. Arch Plast Surg. 2013;40:676–686. doi: 10.5999/aps.2013.40.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013;40:666–675. doi: 10.5999/aps.2013.40.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graf J. Appreciating the art and science of plastic and reconstructive surgery: a year in review. Arch Plast Surg. 2013;40:1–2. doi: 10.5999/aps.2013.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagien S, Klein AW. A brief overview and history of temporary fillers: evolution, advantages, and limitations. Plast Reconstr Surg. 2007;120:8S–16S. doi: 10.1097/01.prs.0000248788.97350.18. [DOI] [PubMed] [Google Scholar]
- 13.Yoon ES, Han SK, Kim WK. Advantages of the presence of living dermal fibroblasts within restylane for soft tissue augmentation. Ann Plast Surg. 2003;51:587–592. doi: 10.1097/01.sap.0000096424.23397.2a. [DOI] [PubMed] [Google Scholar]
- 14.Han SK, Shin SH, Kang HJ, Kim WK. Augmentation rhinoplasty using injectable tissue-engineered soft tissue: a pilot study. Ann Plast Surg. 2006;56:251–255. doi: 10.1097/01.sap.0000198549.64341.17. [DOI] [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183:133–140. doi: 10.1159/000095987. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Moon KC, Han SK, Jeong SH, Kim WK. What tissue is formed after graft of adipose-derived stromal vascular fraction cells? J Craniofac Surg. 2013;24:636–639. doi: 10.1097/SCS.0b013e318272dae9. [DOI] [PubMed] [Google Scholar]
- 19.Ashjian PH, De Ugarte DA, Katz AJ, Hedrick MH. Lipoplasty: from body contouring to tissue engineering. Aesthet Surg J. 2002;22:121–127. doi: 10.1067/maj.2002.122940. [DOI] [PubMed] [Google Scholar]
- 20.Suga H, Matsumoto D, Inoue K, Shigeura T, Eto H, Aoi N, Kato H, Abe H, Yoshimura K. Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg. 2008;122:103–114. doi: 10.1097/PRS.0b013e31817742ed. [DOI] [PubMed] [Google Scholar]
- 21.Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 2010;18:342–348. doi: 10.1111/j.1524-475X.2010.00593.x. [DOI] [PubMed] [Google Scholar]
- 22.Moon KM, Cho G, Sung HM, Jung MS, Tak KS, Jung SW, Lee HB, Suh IS. Nasal anthropometry on facial computed tomography scans for rhinoplasty in koreans. Arch Plast Surg. 2013;40:610–615. doi: 10.5999/aps.2013.40.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh Y, Heo CY. The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: an experimental study with a murine model. Arch Plast Surg. 2012;39:452–456. doi: 10.5999/aps.2012.39.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012;39:585–592. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Lee KH, Kim MH, Kim JP, Lee SJ, Yoon J. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocytes. Arch Plast Surg. 2012;39:593–599. doi: 10.5999/aps.2012.39.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]