Abstract
This study aimed to examine the positive effects of polydeoxyribonucleotide (PDRN) on the wound-healing process in pressure ulcers. In this randomized controlled trial, the effects of PDRN were compared over time between an experimental group (n=11) and a control group (n=12). The former was administered the same dose of PDRN intramuscularly (1 ampule, 3 mL, 5.625 mg, for 5 days) for 2 weeks and perilesionally (1 ampule, 3 mL, 5.625 mg, twice a week) for 4 weeks. The primary endpoint for determining efficacy was wound healing in the pressure ulcers, which was reflected by the wound surface area determined using VISITRAK Digital (Smith & Nephew, Largo, FL). The secondary endpoint was the pressure ulcer scale for healing score, determined using pressure ulcer scale for healing (PUSH Tool 3.0 developed by the National Pressure Ulcer Advisory Panel). After the 4-week treatment period, PDRN therapy was found to significantly reduce the wound size and PUSH score, without adverse effect during the treatment. The findings indicate that PDRN can positively modify the wound healing process in pressure ulcers, and its use could improve the clinical outcomes of patients and lower the need for additional therapies or hospital stay.
Graphical Abstract
Keywords: Pressure Ulcers, Polydeoxyribonucleotides, Re-epithelialization
INTRODUCTION
A pressure ulcer, which is a type of chronic wound, reflects damage occurring on skin and tissue through continuous pressure imposed on the tissue, and it is mostly found in patients with limited mobility and certain health issues (1, 2). In acute care settings in the United States, the prevalence of pressure ulcers has been reported to be 10%-18% (3). In the United Kingdom, Germany, and the Netherlands, it reportedly ranges from 10%-11%, while in Korea, the reported prevalence ranges from 5.9%-20.2% (4, 5). Pressure ulcers not only cause pain and make patients susceptible to morbidity and mortality but also result in increased medical spending and prolonged hospital stay, eventually leading to economic losses as well as deteriorated quality of life for patients (6). For these reasons, pressure ulcers are receiving increasing attention globally, and many associations and government institutions are exerting efforts to reduce the burden of treating chronic wounds including pressure ulcers (7, 8).
Polydeoxyribonucleotide (PDRN) helps healing in wounds involving skin damage, such as burn injuries and chronic wounds, by stimulating tissue reconstruction without any side effects. As a deoxyribonucleotide linear polymer, which is a combination of purine and phosphodiester bonds forming the monometric unit of pyrimidine nucleotides, PDRN is known to selectively act on the A2 purinergic receptor to help cell growth and neogenesis (9, 10). Previous research has shown that at skin graft donor sites, PDRN injections resulted in a very high rate of re-epithelialization, and no side effects were reported (11, 12). In another study, the diabetic patient group, which received PDRN injections in the DM foot, showed improved wound healing, relief of skin edema around the wound area, and reduced pain (13).
Based on the previous studies, we hypothesized that PDRN will also promote wound healing of pressure ulcers. Therefore, authors aim to verify the efficacy and safety of PDRN on wound healing in pressure ulcers. Further, this pilot study was conducted to assess the feasibility of a large clinical trial.
MATERIALS AND METHODS
Study design
This study is a randomized control trial. The participants were randomly allocated into two groups by having their representatives choose a red or white ball from a container. The trial was conducted at the Seoul National University Bundang Hospital, Korea.
Setting and participants
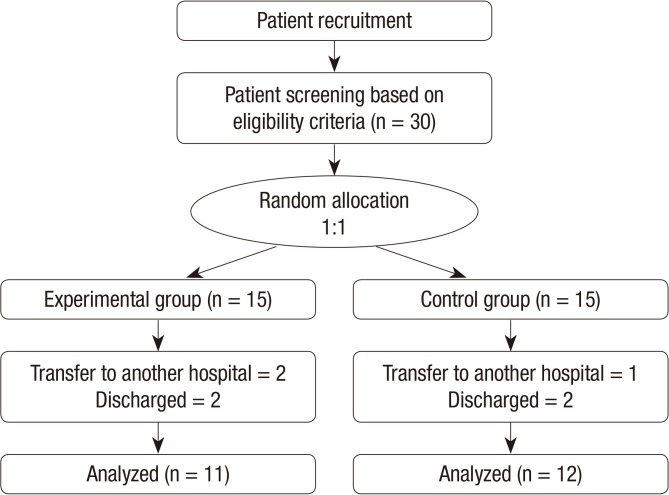
This pilot study lasted 4 weeks, and the study subjects were 30 patients (age range, 18-85 yr) who were hospitalized at Seoul National University Bundang Hospital between July 2010 and June 2012. The inclusion criteria were as follows: agreement to participate in the study; a clean wound bed; delay in treatment of pressure ulcers for over 2 weeks; ulcers higher than stage 2 in the National Pressure Ulcer Advisory Panel-European Pressure Ulcer Advisory Panel (NPUAP-EPUAP) pressure ulcer classification system; ulcers no longer than 20 cm2 horizontally without any secondary infection or local infection. This study excluded patients with allergies, drug shock history, or a history of alcoholism or drug addiction; those who had received or were receiving medication for wound healing other than dressing (e.g., cell therapy products, platelet-derived growth factor, epidermal growth factor, and collagen) 10 days prior to the clinical trial; those with subalimentation due to voluntary oral feeding disorder; those with albumin/total protein level <10% of the normal level; hypotensive patients with systolic pressure <100 and diastolic pressure <60; incontinent patients whose pressure ulcers were difficult to treat; patients with other serious systemic diseases (e.g., liver disease, uncontrolled diabetes, and platelet count ≤70,000 µL); patients who had taken other drugs in the past 3 months for different clinical trials; those sensitive to PDRN; and those deemed inappropriate by the clinical trial lead (Fig. 1). Thirty persons with pressure ulcer participated, and 7 persons withdrew after enrollment for total sample size of 23.
Fig. 1.
Flowchart of the study.
Procedure
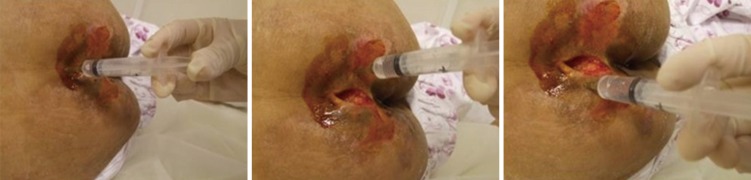
In addition to standard wound care, an intramuscular injection (1 ampule, 3 mL, 5.625 mg) of PDRN (Placentex® Integro; Mastelli Srl, Sanremo, Italy) was administered to all patients in the experimental group 5 times a week for 2 weeks. PDRN was also administered via perilesional infiltration twice a week (1 ampule, 3 mL, 5.625 mg) for 4 weeks (Table 1), as one-fourth of an ampule at four different points: superiorly, inferiorly, and left and right laterally (Fig. 2).
Table 1.
PDRN treatment protocol in patients with pressure ulcers
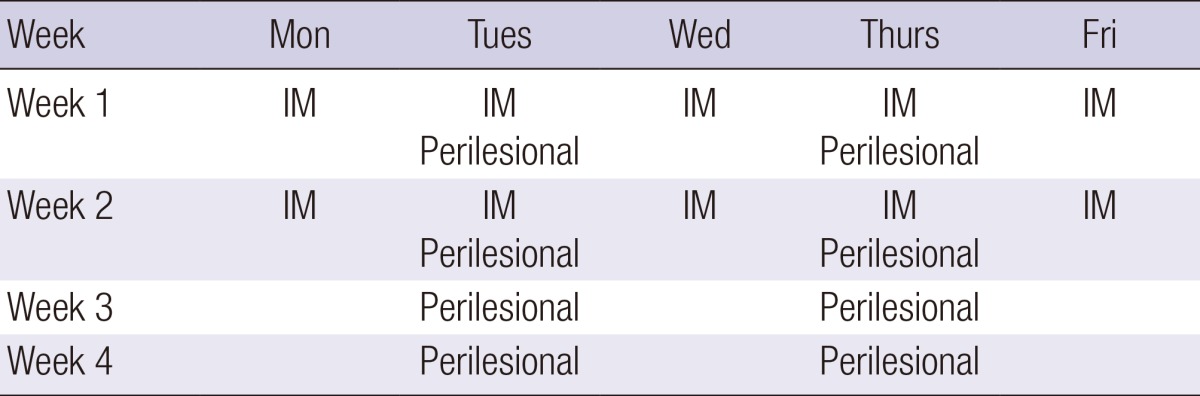
IM, intramuscular injection.
Fig. 2.
Perilesional infiltration.
The control group received standard wound care, which included position changing, use of air mattresses and cushions, a proper diet, and dressing. In both groups, pressure ulcers with dead space were irrigated with normal saline; Aquacel® Hydrofiber (ConvaTec, Princeton, NJ, USA) was used for filler dressing, while Baitain® Soft-Hold (Coloplast, A/S Denmark) was used for cover dressing. Depending on the amount of exudate, dressing was applied every day or every other day.
Measures
The primary outcome was the level of re-epithelialization in the pressure ulcers during the study. To determine this, the wound surface area was measured using VISITRAK® Digital (Smith & Nephew, Largo, USA).
The secondary outcome, that is, exacerbation of the condition, was determined by using the Pressure Ulcer Scale for Healing (PUSH Tool 3.0) developed by National pressure ulcer advisory panel (NPUAP). This tool comprises 3 items: length×width, amount of exudate, and tissue type. The total score equals the sum of the integer score of each item. Changes in the total score over time are used to quantify healing progress. The scale for each item begins with zero and a total score of PUSH ranges from 0 to 17 with a total score of zero representing a healed wound.
These primary and secondary outcomes were determined by a single researcher for all patients, at baseline and in weeks 1, 2, 3, and 4. At baseline, this was done to examine subject eligibility. Photos of the pressure ulcer area in eligible subjects were captured and analyzed using VISITRAK® Digital and PUSH Tool 3.0. Further, wound evaluation was conducted each week in accordance with the treatment protocol.
Adverse effects and safety
At baseline, the subjects were screened for medical history, drug history, vital signs, and hematological parameters (total protein; albumin; glutamic oxaloacetic transaminase; glutamate pyruvate transaminase; alkaline phosphatase; blood urea nitrogen; creatinine; glucose; total bilirubin; serum transferrin; counts of white blood cells, red blood cells, and platelets; and hematocrit). Adverse effects were evaluated every week during the next 4 weeks in which PDRN injections were administered as well as 1 week after the last visit; compliance and blood tests were also conducted as part of this drug safety study.
Statistical analysis
The data were analyzed using SPSS WIN (Version 18.0, SPSS Inc. Chicago, IL, USA). Fisher's exact test, independent t-tests, and the Mann-Whitney U-test were used to examine differences between groups. The latter test was used to identify differences in wound surface area and the PUSH score between the groups. The level of significance for all analyses was set below 0.013 (P value<0.013); the Bonferroni correction helped avoid the problem of multiple testing.
Ethics statements
This study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB Approval Number: B-1001-092-201). Patients were informed about the research protocol both verbally and in writing before they were asked to participate in this study and before they signed the informed consent form. At all times, patients were free to withdraw from the study without having to provide a reason for doing so.
RESULTS
Between-group differences in demographic and pressure ulcer related characteristics. Baseline data were analyzed for the entire patient population (n=23), and no significant differences were found in any characteristics between the groups (Table 2).
Table 2.
Homogeneity test of demographic and pressure ulcer-related characteristics comparing between the experimental and control groups (n = 23)
*Fisher's exact test; †Mann-Whitney U-test.
Evaluation of PDRN effects
Overall, in the experimental group, the area of the pressure ulcer decreased and the condition of pressure ulcer improved with PDRN therapy (Fig. 3, 4).
Fig. 3.
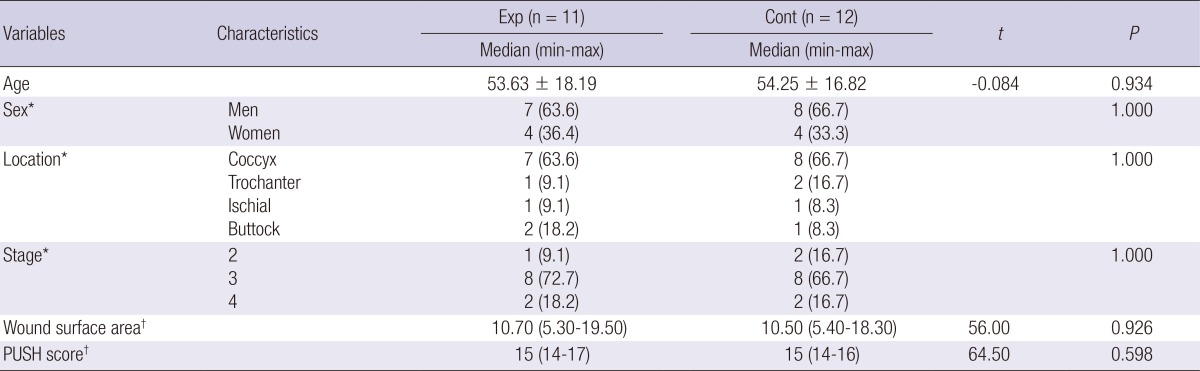
Change in wounds over time with PDRN administration (1). The patient was a 77-yr-old woman with a pressure ulcer over the coccyx. The wound size was 3.3 cm2 and PUSH score was 12. (A) At baseline and after (B) 1, (C) 3, and (D) 4 weeks of treatment.
Fig. 4.
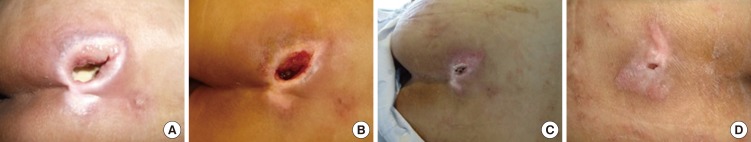
Changes in wounds over time with PDRN treatment (2). The patient was a 57-yr-old man with a pressure ulcer over the buttock area. The wound size was 6.7 cm2 and PUSH score was 13. (A) At baseline and after (B) 1, (C) 2, and (D) 4 weeks of treatment.
Wound surface area
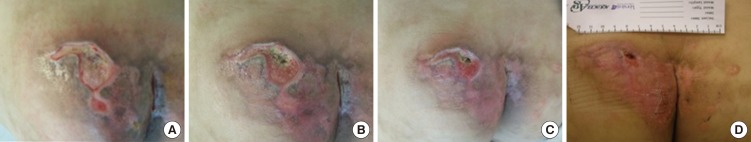
Significant differences were found between the two groups in terms of the wound surface area: compared to the control group, the experimental group showed an increasingly significant decrease in this parameter over the course of treatment (week 1, P=0.007; week 2, P=0.001; week 3, P=0.001; week 4, P<0.001) (Table 3).
Table 3.
Comparison of wound surface area between the experimental and control groups (n = 23)
Pressure ulcer scale for healing
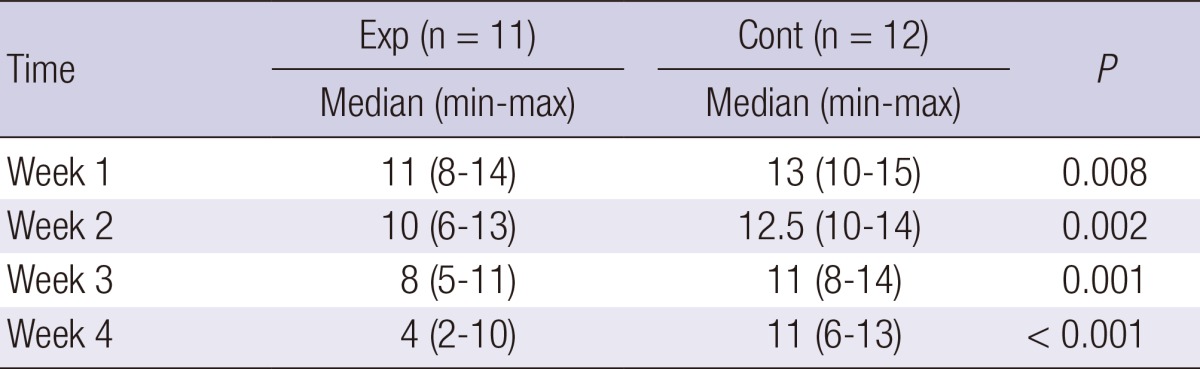
Similar to the primary outcome, that is, the wound surface area, the pressure ulcer scale for healing scale as the secondary outcome also showed significant differences between the groups: compared to the control group, the experimental group showed an increasingly significant decrease in the PUSH score with treatment (week 1, P=0.008; week 2, P=0.002; week 3, P=0.001; week 4, P<0.001) (Table 4).
Table 4.
Comparison of PUSH score between the experimental and control groups (n = 23)
Adverse effects and safety
In both groups, hematological examinations conducted in week 4 showed no abnormal results compared to the baseline values. Further, the patients' vital signs were within the normal ranges, and no adverse effects were reported.
DISCUSSION
Pressure ulcers are widespread chronic wound, which interests a large part of the population and whose incidence is likely to grow rapidly in the future due to the increase in life expectancy and aging. Because of their chronicity, pressure ulcers inflict extensive damage to the skin, and the recovery time often exceeds 3 months (14). The process of wound healing does not progress to the next level or may even stop altogether at the inflammation or proliferation stages (15). In addition, excessive production of matrix metalloproteinases causes uncontrollable tissue destruction. Further, although this finding is slightly controversial at present, the growth rate of fibroblasts in chronic wounds has been found to be slower than that of normal fibroblasts, and it has been reported that the lack of gene expression ability among fibroblasts hampers continued wound healing (16). Therefore, for chronic wounds like pressure ulcers, simple moist dressing is ineffective, necessitating active treatment efforts using various methods such as growth factors. Yet, no golden standard therapy for pressure ulcers has been established up to now. Since 2000, many alternative methods were developed and introduced. Various alternative methods for chronic wounds can be distributed into 2 groups. First, cell-based method was introduced. Shin et al. (17) had used platelet-rich plasma on chronic inflammatory wound, and Choi et al. (18) had demonstrated the efficacy and safety of PRP (Platelet-Rich Plasma) and ADSC (Adipose-Derived Stem Cells) in chronic wound. Also Yang et al. (19) reported the effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Cell based method wanted to demonstrate more basic roll of these cells as a key factor of the wound healing process. Second, artificial substitutes were developed. Kang et al. (20) compared wound healing effect of cellulose and gelatin. And Kim et al. (21) reported Amniotic Membrane-Collagen Dermal Substitute in the Management of Full-Thickness Skin Defects in a Pig. Also PDRN were introduced by Chung et al. (22), and PDRN can promote the survival of random pattern skin flap in rats. PDRN is a low-molecular weight DNA complex that is known to act on adenosine receptors and stimulate vascular endothelial growth factor, thereby accelerating DNA biosynthesis and boosting the healing process (13).
In this randomized clinical trial, our results showed that PDRN significantly reduced the wound surface area and improved the condition of the pressure ulcer with time in the experimental group. This finding is in agreement with those of previous research on skin graft donor sites, diabetic foot and burn wound. According to Dealoe et al.'s research (11), graft donor site was completely healed within 21 days after PDRN injections. Rubeqni et al.'s research (12) also revealed that donor site with PDRN had a more prompt re-epithelialization effect within 10 days after PDRN injections. In the other research, patients with Diabetic foot ulcer received PDRN 3 days a week for 8 weeks by intramuscular and perilesional route. As a result, PDRN facilitated the healing of wagner 1 or 2 diabetic foot ulcers (13). In vivo experiment, mice with deep dermal second degree burn was given PDRN (8 mg/kg/day intraperitoneally for 14 days). As a result, PDRN improved neoangiogenesis as suggested by the marked increase in microvessel density and enhanced vascular endothelial growth factor expression. These application cases suggested that PDRN was an effective therapeutic approach to improve clinical outcomes of wound (23). It is also in line with the results of pharmacology studies (24). Consequently, PDRN stimulates vascular endothelial growth factor production, increases the rate of granulation tissue in fibroblast differentiation and maturation, and thus accelerates the repair process. Acute and chronic toxicity studies regarding stability testing proved that PDRN caused neither mortality nor toxic effects on liver, lungs, brain, skeletal muscle, and heart evaluated macroscopically and by means of histologic analysis (16, 19). PDRN was approved in Italy for both parenteral and topical uses and in this study, no side effects were found when PDRN was applied to pressure ulcers. PDRN seems safe to use for this purpose.
This is the first study to identify the effectiveness of PDRN on pressure ulcers, and our results indicate that this agent is effective in treating chronic wounds. Additionally, most prior studies focused only on donor site and burn injuries while excluding other types of chronic wounds. From this aspect as well, the present study has significance, in that it was conducted on pressure ulcers.
The present study has some limitations. The effectiveness of PDRN was measured by setting the contour using a two-dimensional linear model to calculate the wound surface area, because of which it was impossible to measure the extent of tissue granulation and the depth of the pressure ulcer. While evaluating treatment efficacy, it is important to assess changes in size as well as reduction in ulcer depth. Thus, it was difficult to accurately evaluate the efficacy of PDRN in the case of subjects with deep yet small pressure ulcer areas. In the next phase of the study, we will evaluate the depth of the pressure ulcers in addition to their size. Further, in the present study, PDRN was administered in two different ways, that is, intramuscularly and perilesionally. Therefore, it was not possible to differentiate its systemic effects from its local effect like other research (11). In the next phase of the study, local infiltration and intramuscular infiltration of PDRN will be compared in order to examine the effects of these routes of administration. Our study was insufficient for accurately evaluating PU's completely healing because of short follow-up period (only 4 weeks). In the future studies, the time frame should be prolonged to observe and verify complete healing of pressure ulcer. Since PDRN requires invasive treatment, which may cause pain and discomfort to the patient, patient evaluation and adherence must also be included in the next phase of the study. Lastly, this study is pilot study that included a small number of subjects, and their outcomes were not generalized. Despite these limitations, this study is significant as it is the first study conducted specifically in pressure ulcer patients and because it was conducted as a blinded study in terms of subject selection, evaluation of PDRN efficacy against pressure ulcers, and the statistical analysis.
ACKNOWLEDGEMENTS
We thank the staff, management, and patients of the institutions for their willingness to participate in this research project. Special thanks are due to Hun Hee Lee, Min Hi Seo, and Pil Gyun Shin.
Footnotes
We do not have anything to disclose.
References
- 1.Defloor T. The risk of pressure sores: a conceptual scheme. J Clin Nurs. 1999;8:206–216. doi: 10.1046/j.1365-2702.1999.00254.x. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarten M, Margolis DJ, Localio AR, Kagan SH, Lowe RA, Kinosian B, Holmes JH, Abbuhl SB, Kavesh W, Ruffin A. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61:749–754. doi: 10.1093/gerona/61.7.749. [DOI] [PubMed] [Google Scholar]
- 3.Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14:208–215. doi: 10.1097/00129334-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Stausberg J, Kröger K, Maier I, Schneider H, Niebel W. Pressure ulcers in secondary care: incidence, prevalence, and relevance. Adv Skin Wound Care. 2005;18:140–145. doi: 10.1097/00129334-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lee MJ. Risk factors of pressure ulcers among elderly in the Geriatric Hospital. Seoul: Ewha Woman University; 2010. Dissertation. [Google Scholar]
- 6.Hopkins A, Dealey C, Bale S, Defloor T, Worboys F. Patient stories of living with a pressure ulcer. J Adv Nurs. 2006;56:345–353. doi: 10.1111/j.1365-2648.2006.04007.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergstorm N. Patients at risk for pressure ulcers and evidence-based care for pressure ulcer prevention. In: Bader D, Bouten C, Colin D, Oomens C, editors. Pressure ulcer research: current and future perspectives. Berlin: Springer; 2005. pp. 35–50. [Google Scholar]
- 8.Parish LC, Dryjski M, Cadden S. Prospective clinical study of a new adhesive gelling foam dressing in pressure ulcers. Int Wound J. 2008;5:60–67. doi: 10.1111/j.1742-481X.2007.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guizzardi S, Galli C, Govoni P, Boratto R, Cattarini G, Martini D, Belletti S, Scandroglio R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: a new proposal for bone tissue repair. Life Sci. 2003;73:1973–1983. doi: 10.1016/s0024-3205(03)00547-2. [DOI] [PubMed] [Google Scholar]
- 10.Lazzarotto M, Tomasello EM, Caporossi A. Clinical evaluation of corneal epithelialization after photorefractive keratectomy in patients treated with polydeoxyribonucleotide (PDRN) eye drops: a randomized, double-blind, placebo-controlled trial. Eur J Ophthalmol. 2004;14:284–289. [PubMed] [Google Scholar]
- 11.De Aloe G, Rubegni P, Biagioli M, Taddeucci P, Fimiani M. Skin graft donor site and use of polydeoxyribonucleotide as a treatment for skin regeneration: a randomized, controlled, double-blind, clinical trial. Wounds. 2004;16:258–263. [Google Scholar]
- 12.Rubegni P, De Aloe G, Mazzatenta C, Cattarini L, Fimiani M. Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants: a pilot study. Curr Med Res Opin. 2001;17:128–131. [PubMed] [Google Scholar]
- 13.Squadrito F, Bitto A, Altavilla D, Arcoraci V, De Caridi G, De Feo ME, Corrao S, Pallio G, Sterrantino C, Minutoli L, et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial. J Clin Endocrinol Metab. 2014;99:E746–E753. doi: 10.1210/jc.2013-3569. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira MC, Tuma P, Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics (Sao Paulo) 2006;61:571–578. doi: 10.1590/s1807-59322006000600014. [DOI] [PubMed] [Google Scholar]
- 15.Jaul E. Non-healing wounds: the geriatric approach. Arch Gerontol Geriatr. 2009;49:224–226. doi: 10.1016/j.archger.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Shin HS, Oh HY. The effect of platelet-rich plasma on wounds of OLETF rats using expression of matrix metalloproteinase-2 and -9 mRNA. Arch Plast Surg. 2012;39:106–112. doi: 10.5999/aps.2012.39.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012;39:585–592. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, Chung HY, Cho BC, Byun JS. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013;40:496–504. doi: 10.5999/aps.2013.40.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang BS, Na YC, Jin YW. Comparison of the wound healing effect of cellulose and gelatin: an in vivo study. Arch Plast Surg. 2012;39:317–321. doi: 10.5999/aps.2012.39.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H, Son D, Choi TH, Jung S, Kwon S, Kim J, Han K. Evaluation of an amniotic membrane-collagen dermal substitute in the management of full-thickness skin defects in a pig. Arch Plast Surg. 2013;40:11–18. doi: 10.5999/aps.2013.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung KI, Kim HK, Kim WS, Bae TH. The effects of polydeoxyribonucleotide on the survival of random pattern skin flaps in rats. Arch Plast Surg. 2013;40:181–186. doi: 10.5999/aps.2013.40.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitto A, Galeano M, Squadrito F, Minutoli L, Polito F, Dye JF, Clayton EA, Calò M, Venuti FS, Vaccaro M, et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med. 2008;36:1594–1602. doi: 10.1097/CCM.0b013e318170ab5c. [DOI] [PubMed] [Google Scholar]
- 24.Thellung S, Florio T, Maragliano A, Cattarini G, Schettini G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: involvement of A2 purinergic receptor subtypes. Life Sci. 1999;64:1661–1674. doi: 10.1016/s0024-3205(99)00104-6. [DOI] [PubMed] [Google Scholar]