Abstract
Aims
Well-differentiated leiomyosarcoma show morphologically recognizable smooth muscle differentiation, while poorly differentiated tumors may form a spectrum with a subset of undifferentiated pleomorphic sarcomas. Expression of certain muscle markers has been reported to have prognostic impact. We investigated the correlation between morphologic spectrum and muscle-marker expression profile of leiomyosarcoma and the impact of these factors on patient outcomes.
Methods and Results
Tissue microarrays including 203 non-uterine and 181 uterine leiomyosarcoma with a spectrum of tumor morphologies were evaluated for expression of immunohistochemical markers of muscle differentiation. Poorly differentiated tumors frequently lost one or more conventional smooth muscle markers (smooth muscle actin, desmin, h-caldesmon and smooth muscle myosin (p<0.0001)), as well as more recently described markers SLMAP, MYLK and ACTG2 (p<0.0001). In primary tumors, both desmin and CFL2 expression predicted improved overall survival in multivariate analyses (p=0.0111 and p=0.043, respectively). Muscle-marker enriched tumors (expressing all 4 conventional markers or any 3 of ACTG2, CFL2, CASQ2, MYLK, and SLMAP, had improved overall survival (p<0.05) in univariate analyses.
Conclusions
Morphologically and immunohistochemically, poorly differentiated leiomyosarcoma can masquerade as undifferentiated pleomorphic sarcoma with progressive loss of muscle markers. Expression of muscle markers has prognostic significance in primary leiomyosarcoma independent of tumor morphology.
Keywords: Leiomyosarcoma, differentiation, immunohistochemistry, prognosis, desmin
Introduction
Leiomyosarcomas account for up to 20% of all adult soft tissue sarcomas,1–7 and are characterized by an aggressive clinical course and relative insensitivity to cytotoxic chemotherapy.4, 5 Leiomyosarcomas demonstrate smooth muscle differentiation, and are typically subclassified for diagnostic and therapeutic purposes as uterine and non-uterine (somatic) origin. Uterine leiomyosarcoma is distinguished from the more common myometrial leiomyomas, by a combination of mitotic activity at least 5/10 high power fields in the presence of necrosis and cellular atypia, or mitotic activity >10/10 high power fields in the absence of necrosis or atypia.8–11 In somatic tissues outside of the dermis or gastrointestinal tract, leiomyomas are extremely rare. Thus, smooth muscle tumors with any mitotic activity are generally considered to be leiomyosarcoma, and commonly graded using the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) criteria.6
Well-differentiated leiomyosarcomas demonstrate a growth pattern of perpendicularly-intersecting cellular fascicles composed of elongated spindle-shaped tumor cells with abundant, brightly eosinophilic, fibrillary cytoplasm. Nuclei generally have an elongated, blunt-ended oval “cigar-like” shape. Variable numbers of markedly pleomorphic “monster” cells may be seen. In poorly differentiated tumors, classic features may be focal or difficult to appreciate as the proportion of pleomorphic cells increases. Additional morphologic variants include epithelioid and myxoid types, which are more commonly associated with uterine origin. While the majority of cases can be diagnosed on biopsy based on classic histologic features on H&E stained sections, morphologic variants, poorly differentiated tumors, and those with unusual features may be difficult to distinguish from other sarcomas and may require additional immunohistochemical evaluation. In anaplastic variants, or extremely poorly differentiated tumors, myogenic markers may be lost.12
Commonly used markers of muscle differentiation in clinical practice include desmin, seen in all types of muscle, smooth muscle actin (SMA), most commonly present in smooth muscle or myofibroblasts, h-caldesmon and smooth muscle myosin (SMMS), both of which are relatively specific for smooth muscle. More recently, expression profiling studies identified an additional set of muscle markers, including smooth muscle gamma actin (ACTG2, typically expressed in enteric smooth muscle), calsequestrin 2 (CASQ2, cardiac and skeletal muscle), human muscle cofilin 2 (CFL2, skeletal muscle), myosin light chain kinase (MYLK, smooth muscle) and sarcolemmal membrane associated protein (SLMAP, all muscle types) as being associated with improved outcomes when 3 or more were co-expressed – so called “muscle-enriched” leiomyosarcomas.13
Few studies have investigated the utility of muscle marker expression as a more objective measure of differentiation status compared to histologic appearance on H&E sections. We therefore queried how well muscle marker expression correlated with histologic assessment of tumor differentiation, and if muscle marker expression could independently predict tumor behavior and survival outcomes in primary leiomyosarcoma.
Materials and Methods
Patents and tumor tissues
Acquisition of tissue specimens and clinical information and subsequent analyses were approved by the Institutional Review Board (IRB) of The University of Texas M. D. Anderson Cancer Center (UTMDACC).
Tissue microarray construction
All available formalin-fixed, paraffin-embedded leiomyosarcoma specimens collected between 1993 and 2010 were retrieved from UTMDACC pathology archives. Hematoxylin and eosin (H&E)-stained sections were reviewed to confirm the diagnosis, define areas of viable tumor, and select one or more areas (if there was morphologic variability) for inclusion in tissue microarrays. Extremely poorly differentiated tumors or those that had heterologous elements were only included in the study if they had a prior documented history leiomyosarcoma with at least focal typical immunohistochemical and/or morphologic features.
An automated tissue microarray apparatus (ATA-27, Beecher Instruments, Sun Prairie, WI) was used to obtain and format paired 1.2 mm punch samples from each case into recipient blocks.14 H&E staining of 4 μm tissue microarray sections was used to verify all samples. Individual cores on the tissue microarray were screened by an experienced soft tissue pathologist and classified by differentiation score as 1) well, 2) moderately or 3) poorly differentiated (Figure 1).6 Tumors with myxoid or epithelioid morphology were considered to be moderately and poorly differentiated, respectively. Only the most poorly differentiated pair of cores from each case was considered in data analysis.
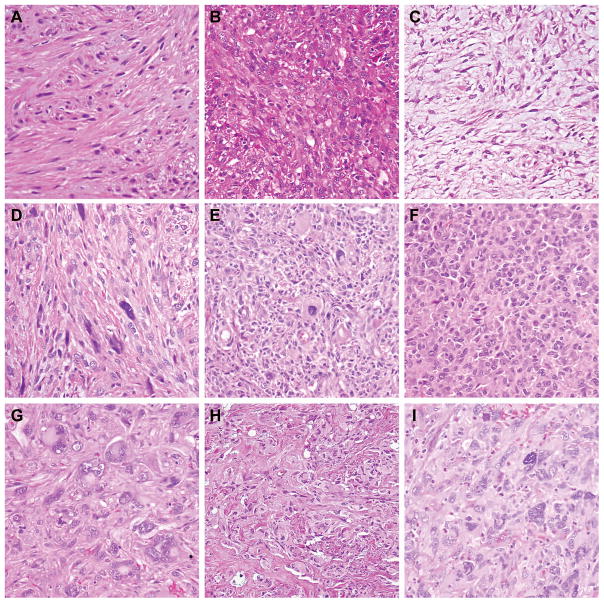
Figure 1. Morphologic Variation in Leiomyosarcoma.
A) Well differentiated tumor demonstrating intersecting fascicles of slightly atypical eosinophilic spindle cells. B) Moderately differentiated tumor with nuclear variability and increased disorganization of fascicles. C) Moderately differentiated myxoid tumor with bland spindle cells in a storiform to fascicular arrangement. D) Moderately differentiated tumor with scattered “monster cells.” E) Poorly differentiated tumor with loss of fascicular architecture, and increased rounded to epithelioid cells. F) Poorly differentiated tumor with epithelioid features. G) Poorly differentiated tumor showing therapy effect and marked nuclear pleomorphism. H) Poorly differentiated tumor with pleomorphism, loss of architecture, and numerous mitoses. I) Poorly differentiated “undifferentiated pleomorphic sarcoma-like” tumor showing storiform growth, marked pleomorphism and scattered inflammatory infiltrate. (All panels at 200X).
Clinicopathologic Data Collection
Patient and tumor variables, including age, gender, tumor site, size, and disease status were recorded. Sites of primary tumors were categorized thusly: uterine; vascular (apparently arising from or extensively invading a large caliber vein); retroperitoneal/pelvic (not clearly arising in association with a specific intra-abdominal organ or vascular structure); extremity (leg or arm, including dermal tumors); trunk (chest wall, superficial abdominal wall, back, paraspinal); uterine; or other miscellaneous sites (including viscera, bone and head and neck).
Outcome data including survival and disease recurrence were tabulated for primary tumors only. Complete FNCLCC grading criteria (mitotic figures / 10 high power fields, percent necrosis) were not tabulated because these were not equivalently applicable in primary uterine leiomyosarcoma.
Immunohistochemical analysis
Immunohistochemical staining was performed on 4-μm-thick tissue microarray sections using an automated stainer (Dako, Carpinteria, CA) following the manufacturer’s instructions, and commercially available antibodies (Table 1). Positive and negative controls were run in parallel. Sections were counterstained with hematoxylin. SMA, desmin, SMMS, and h-caldesmon were scored as absent (0), focal (<10%) or diffuse (≥10%). ACTG2, CASQ2, CFL2, MYLK and SLMAP were scored as completely absent (0), or by intensity if any staining present as weak (1) or strong (2). For the purposes of outcome analysis, only diffuse/strong staining was considered positive. Muscle enriched tumors were defined as those expressing 3 or more of: ACTG2, CASQ2, CFL2, MYLK and SLMAP.
Table 1.
Antibodies used for immunohistochemistry
| Marker | Catalog # | Company | dilution |
|---|---|---|---|
| Smooth muscle actin | A2547 | Sigma, St Louis, MO | 1:80,000 |
| Desmin | M0760 | Sigma | 1:200 |
| Caldesmon | M3557 | Dako, Carpenteria, CA | 1:50 |
| Smooth muscle myosin | IR066 | Dako | 1:200 |
| Smooth muscle gamma actin (AC TG2) | H00000072-A01 | Novus Biologicals, Littleton CO | 1:2,000 |
| Calsequestrin 2 (CASQ2) | GTX90833 | GeneTex Inc, Irvine, CA | 1:100 |
| Human muscle cofilin 2 (CFL2) | GTX92818 | GeneTex Inc | 1:100 |
| Myosin light chain kinase (MYLK) | GTX91044 | GeneTex Inc | 1:25 |
| Sarcolemmal associated protein (SLMAP) | GTX94163 | GeneTex Inc | 1:2,500 |
Statistical methods
Associations between histopathological features and immunophenotype were examined using Fisher’s exact test or χ2 test, with an alpha of p≤0.05 (adjusted to p≤0.025 for multiple comparisons) considered significant. For primary tumors only, the method of Kaplan and Meier was used to assess outcomes. Univariable and multivariable Cox proportional hazards regression models were used to estimate the association between histopathologic features or biomarker expression with overall survival (OS), disease-specific survival (DSS) and time to first metastasis, with alpha of 0.05 considered as significant.
Results
Leiomyosarcoma characteristics
Tumors included 384 leiomyosarcoma from 257 patients, including 198 women and 59 men. Primary sites of origin included the uterus (41%), retroperitoneum/pelvis (19%), large vessels (16%), extremities (11%), trunk (3%), and miscellaneous sites (9%). Vascular leiomyosarcoma predominately arose in the inferior vena cava and renal vessels, with a few cases originating from other deep veins. Tumors in the head and neck arose in the nasal cavity, sinuses and mandible, while organs giving rise to leiomyosarcoma included lung/bronchus, bowel, bladder, liver and penis. Extremity tumors included 4 leiomyosarcomas of dermal origin. There were 98 primary leiomyosarcomas, 93 local recurrences (including both those recurring within a prior surgical site and intra-abdominal spread) and 192 distant metastases. The median size of primary leiomyosarcomas was 8 cm (range 0.7 to 30 cm).
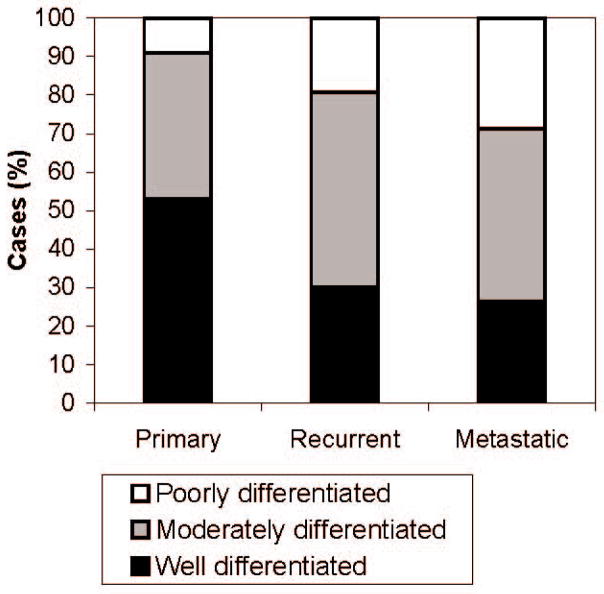
When tumors were classified by their microarray core with the least-differentiated histology, there were 130 (34%) well-differentiated leiomyosarcomas, 170 (45%) moderately differentiated and 82 (21%) poorly differentiated. Loss of differentiation correlated with disease progression (p<0.0001), with primary tumors being more frequently well differentiated (53%) than either recurrent (30%, p=0.0013) or metastatic tumors (26%, p<0.0001) (Figure 2). There was no significant further loss of differentiation between recurrent and metastatic tumors.
Figure 2. Histologic Features of Leiomyosarcoma by Site and Stage.
Tumor status correlates with differentiation. Primary tumors are most frequently well-differentiated (53%) compared to recurrence or metastases (30%, p=0.0013 and 26%, p<0.0001 respectively). Poorly differentiated tumors are most commonly metastases (29%) compared to 19% recurrences (p=0.087), and 9% of primaries (p=0.0001).
Muscle marker expression
Smooth muscle markers SMA and ACTG2 were the most frequently expressed (91% and 90% of cases, respectively), while striated muscle marker CASQ2 was least prevalent (18% of cases) (Table 2). In sum, 235/377 (62%) tumors expressed all 4 conventional muscle markers, and 292 (77%) expressed 3 or more, while 56 (15%) expressed only one or no markers. Similarly, muscle-enriched phenotype was seen in 298/357 cases (83%).
Table 2.
Muscle marker expression by differentiation status.
| Total (n=384) | Well differentiated (n=131) | Moderately differentiated (n=170) | Moderately vs. well- differentiated (p value) | Poorly differentiated (n=82) | Poorly vs. well- differentiated (p value) | Poorly vs. moderately differentiated (p value) | |
|---|---|---|---|---|---|---|---|
| SMA | 91% | 99% | 93% | 0.0082 | 73% | <0.0001 | <0.0001 |
| Desmin | 74% | 89% | 75% | 0.0045 | 50% | <0.0001 | <0.0001 |
| SMMS | 76% | 91% | 79% | 0.0065 | 47% | <0.0001 | <0.0001 |
| Caldesmon | 78% | 94% | 78% | 0.00013 | 51% | <0.0001 | <0.0001 |
| SLMAP | 83% | 93% | 84% | 0.028 | 63% | <0.0001 | 0.003 |
| CASQ2 | 18% | 23% | 17% | 0.24 | 12% | 0.044 | 0.34 |
| ACTG2 | 90% | 99% | 94% | 0.047 | 66% | <0.0001 | <0.0001 |
| CFL2 | 69% | 77% | 64% | 0.0196 | 64% | 0.0537 | 1 |
| MYLK | 82% | 95% | 77% | <0.0001 | 71% | <0.0001 | 0.42 |
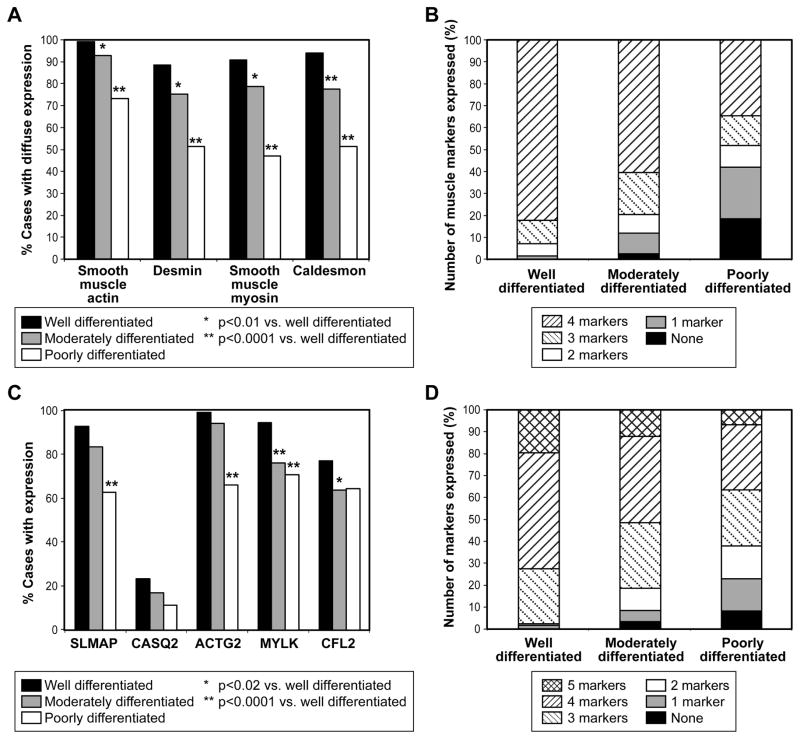
Expression of muscle markers correlated with morphologic differentiation (Figure 3). Expression of all four conventional muscle markers decreased from 82% (106/129) in well to 60% (100/167) in moderate to 36% (24/81) in poorly differentiated tumors (p<0.0001). The most dramatic loss of expression was seen in SMMS, which was expressed in 118/130 (91%) well differentiated tumors, 133/169 (79%) moderately differentiated tumors and only 38/81 (47%) poorly differentiated, while SMA was retained at the highest levels, being present in 129/130 (99%) of well-differentiated, 158/170 (93%) moderately differentiated and 60/82 (73%) poorly differentiated tumors. Statistically significant loss of expression of SLMAP, ACTG2, MYLK, and COFL2 was also seen as tumors became more poorly differentiated.
Figure 3. Expression of Markers of Smooth Muscle Differentiation in Leiomyosarcoma.
A) Diffuse expression of smooth muscle actin, desmin, smooth muscle myosin, and caldesmon are reduced in less-well-differentiated tumors. B) Total number of conventional muscle markers with diffuse expression is reduced in more poorly differentiated tumors, p<0.0001. C) Expression of SLMAP, CASQ2, ACTG2, MYLK and CFL2 is less frequent in poorly differentiated tumors. D) Muscle-enriched phenotype (3 or more of SLMAP, CASQ2, ACTG2, MYLK, CFL2) is less frequent in more poorly differentiated tumors (p=0.0089).
Prognostic utility of differentiation markers in primary leiomyosarcoma
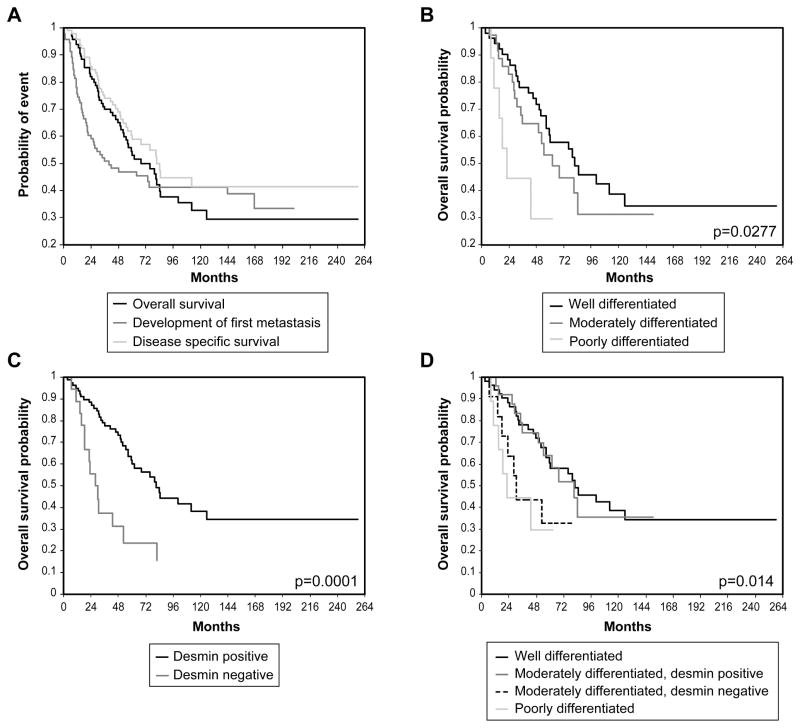
Clinical follow-up was available for 97 primary tumors, and ranged from 2.6-258 months (median 50). Five- and 10-year OS were 54% and 33%, respectively, while 5-and 10-year DSS were 60 % and 41% (Figure 4a). Four patients had distant metastases at the time of diagnosis, while 52/97 patients developed metastases during the course of follow-up. Of these 52 patients, median time to development of first metastasis after primary resection was 15 months (range 8–167 months).
Figure 4. Survival Outcomes in Primary Leiomyosarcoma.
A) Overall survival, disease-specific survival and time to development of first metastasis for entire cohort. B) Patients with well-differentiated leiomyosarcoma have improved overall survival than those with poorly differentiated tumors. C) Expression of desmin correlates with improved overall survival. D) Moderately differentiated tumors expressing desmin have identical survival to well differentiated tumors, while moderately differentiated tumors without desmin behave like poorly differentiated tumors.
There was no difference in outcome by sex or site (uterine vs. somatic). Primary tumor size >5 cm was associated with poor OS and DSS but not time to first metastasis (Tables 3–5). Histopathological differentiation status alone was a modest predictor of clinical outcomes (Figure 4b). Poor differentiation predicted a worse OS compared to well-differentiated tumors (HR, 3.281 [1.311–8.212], p=0.0112), and trended to shorter time to first metastasis (p=0.0587), but was not predictive of DSS (p=0.0897). There was no significant difference in outcome between well- and moderately-differentiated tumors.
Table 3.
Overall survival, univariate and multivariate
| Variable | Level | N | # all cause deaths | Hazard Ratio | 95% CI Lower | 95% CI upper | P- Value |
|---|---|---|---|---|---|---|---|
| Univariate analyses | |||||||
| Actin, smooth muscle | >10% pos | 96 | 51 | 1.2191 | 0.1673 | 8.8841 | 0.845 |
| Caldesmon | >10% pos | 97 | 52 | 0.4154 | 0.2011 | 0.8583 | 0.0177 |
| Desmin | >10% pos | 97 | 52 | 0.3058 | 0.1617 | 0.5783 | 0.0003 |
| Myosin, smooth muscle | >10% pos | 95 | 51 | 0.7951 | 0.3867 | 1.635 | 0.5331 |
| Standard muscle markers | all 4 markers expressed | 94 | 50 | 0.4721 | 0.2576 | 0.865 | 0.0151 |
| ACTG2 | strong, diffuse | 96 | 51 | 1.8255 | 0.2507 | 13.2914 | 0.5524 |
| CASQ2 | strong, diffuse | 96 | 51 | 0.8104 | 0.4433 | 1.4816 | 0.4946 |
| CFL2 | strong, diffuse | 91 | 48 | 0.4191 | 0.2288 | 0.7676 | 0.0049 |
| MYLK | strong, diffuse | 95 | 50 | 0.5227 | 0.2046 | 1.3356 | 0.1753 |
| SLMAP | strong, diffuse | 97 | 52 | 0.6123 | 0.2981 | 1.2576 | 0.1816 |
| Muscle enriched phenotype | any 3/5 | 94 | 49 | 0.4177 | 0.1763 | 0.9897 | 0.0473 |
| differentiation score | moderate poor |
97 | 52 | 1.4259 3.2807 |
0.7843 1.3107 |
2.5923 8.2119 |
0.2447 0.0112 |
| gender (M/F) | M | 97 | 52 | 1.068 | 0.6086 | 1.8742 | 0.8186 |
| site (U/S) | Uterine vs all other | 97 | 52 | 1.2194 | 0.5489 | 2.709 | 0.6262 |
| Size | >5 cm | 97 | 52 | 2.7263 | 1.1624 | 6.3944 | 0.0211 |
| Multivariate analyses | |||||||
| Differentiation | Moderate poor | 91 | 48 | 0.9084 3.5215 |
0.4373 1.2365 |
1.8869 10.0286 |
0.7967 0.0184 |
| Desmin | >10% pos | 91 | 48 | 0.3077 | 0.1216 | 0.7788 | 0.0129 |
| CFL2 | strong, diffuse | 91 | 48 | 0.4943 | 0.2615 | 0.9343 | 0.0301 |
| Size | >5 cm | 91 | 48 | 4.5457 | 1.7251 | 11.9782 | 0.0022 |
Table 5.
Time to first metastasis, univariate analyses
| Variable | Level | N | # with metastases | Hazard Ratio | 95% CI Lower | 95% CI upper | P- Value |
|---|---|---|---|---|---|---|---|
| Univariate analyses | |||||||
| Actin, smooth muscle | >10% pos | 92 | 51 | 0.9286 | 0.1276 | 6.7582 | 0.9417 |
| Caldesmon | >10% pos | 93 | 52 | 0.6418 | 0.288 | 1.4304 | 0.2781 |
| Desmin | >10% pos | 93 | 52 | 0.4643 | 0.2407 | 0.8957 | 0.0221 |
| Myosin, smooth muscle | >10% pos | 91 | 50 | 0.8576 | 0.4156 | 1.7696 | 0.6777 |
| Standard muscle markers | all 4 markers expressed | 90 | 49 | 0.5986 | 0.3255 | 1.101 | 0.0988 |
| ACTG2 | strong, diffuse | 92 | 51 | 1.789 | 0.2462 | 13.0021 | 0.5654 |
| CASQ2 | strong, diffuse | 92 | 51 | 0.6913 | 0.367 | 1.302 | 0.253 |
| CFL2 | strong, diffuse | 87 | 46 | 0.559 | 0.2876 | 1.0868 | 0.0864 |
| MYLK | strong, diffuse | 91 | 50 | 0.9674 | 0.3463 | 2.7026 | 0.9496 |
| SLMAP | strong, diffuse | 93 | 52 | 0.8091 | 0.3794 | 1.7254 | 0.5835 |
| Type-1 leiomyosarcoma markers | muscle enriched (any 3/5) | 90 | 49 | 0.8023 | 0.2872 | 2.2412 | 0.6743 |
| differentiation score | moderate poor |
93 | 52 | 1.104 2.4072 |
0.6043 0.9684 |
2.0171 5.9836 |
0.7476 0.0587 |
| gender (M/F) | M | 93 | 52 | 1.324 | 0.766 | 2.2886 | 0.3148 |
| site (U/S) Size |
Uterine vs all other >5 cm |
93 93 |
52 52 |
0.5359 1.7609 |
0.1927 0.881 |
1.4903 3.5196 |
0.2319 0.1093 |
Muscle enriched primary leiomyosarcoma predicted improved OS in univariate analysis (HR 0.4177 [0.1763–0.9897], p=0.0473), as did expression of all 4 conventional smooth muscle (HR 0.4887 [0.2711–0.8809], p=0.0172). As individual markers, CFL2, desmin, and h-caldesmon all associated with improved survival (p=0.0049, p=0.0003, and p-0.0177, respectively) (Table 3). The small sample size limited multivariate analyses of OS, and only the 2 known risk factors (tumor size, differentiation score) and 2 muscle markers (desmin and CFL2) were included in the model. All 4 retained independent prognostic significance.
Significant prognostic biomarkers for DSS were similar to those of overall survival (Table 4). Loss of any one of the conventional smooth muscle markers increased risk (HR 2.5031 [1.3078–4.7916]; p=0.0056), while muscle-enriched tumors trended to improved DSS (p=0.0535). Both CFL2 and desmin were associated with decreased risk (p=0.0076 and p<0.0001), while expression of h-caldesmon trended to be protective (p=0.061) in univariate analyses. In multivariate analysis (including size, expression of all 4 conventional muscle markers, CFL2 and desmin expression in the model), only size >5 cm retained prognostic significance.
Table 4.
Disease specific survival, univariate and multivariate
| Variable | Level | N | # all cause deaths | Hazard Ratio | 95% CI Lower | 95% CI upper | P-Value |
|---|---|---|---|---|---|---|---|
| Univariate analyses | |||||||
| Actin, smooth muscle | >10% pos | 96 | 40 | 0.9083 | 0.1239 | 6.658 | 0.9246 |
| Caldesmon | >10% pos | 97 | 41 | 0.4042 | 0.1567 | 1.0426 | 0.061 |
| Desmin | >10% pos | 97 | 41 | 0.2398 | 0.121 | 0.4753 | <0.0001 |
| Myosin, smooth muscle | >10% pos | 95 | 40 | 0.8018 | 0.3544 | 1.8145 | 0.5961 |
| Standard muscle markers | all 4 markers expressed | 94 | 39 | 0.3995 | 0.2087 | 0.7646 | 0.0056 |
| ACTG2 | strong, diffuse | 96 | 40 | 1.457 | 0.199 | 10.6664 | 0.711 |
| CASQ2 | strong, diffuse | 96 | 40 | 0.6548 | 0.3087 | 1.3887 | 0.2696 |
| CFL2 | strong, diffuse | 91 | 37 | 0.3978 | 0.2022 | 0.7829 | 0.0076 |
| MYLK | strong, diffuse | 95 | 39 | 0.503 | 0.1761 | 1.4368 | 0.1994 |
| SLMAP | strong, diffuse | 97 | 41 | 0.5184 | 0.2391 | 1.1239 | 0.0961 |
| Type-1 leiomyosarcoma markers | muscle enriched (any 3/5) | 94 | 38 | 0.3928 | 0.1521 | 1.0141 | 0.0535 |
| differentiation score | moderate poor |
97 | 41 | 1.3427 2.5765 |
0.6902 0.8634 |
2.6119 7.6884 |
0.3854 0.0897 |
| gender (M/F) | M | 97 | 41 | 1.2493 | 0.6692 | 2.3325 | 0.4847 |
| site (U/S) | Uterine vs all other | 97 | 41 | 0.8502 | 0.3025 | 2.3895 | 0.7583 |
| Size | >5 cm | 97 | 41 | 2.561 | 1.0028 | 6.5405 | 0.0493 |
| Multivariate analyses | |||||||
| Standard muscle markers | all 4 markers expressed | 91 | 37 | 0.6245 | 0.139 | 2.8055 | 0.5391 |
| Size | >5cm | 91 | 37 | 4.2788 | 1.4224 | 12.8719 | 0.0097 |
| Desmin | >10% pos | 91 | 37 | 0.2378 | 0.0474 | 1.1923 | 0.0707 |
| CFL2 | strong, diffuse | 91 | 37 | 0.5996 | 0.2769 | 1.2986 | 0.1946 |
Desmin was the only significant predictor of time to first metastasis in univariate analysis (HR 0.46343 [0.2407–0.8957]; p=0.0221).
Discussion
Although immunohistochemistry is frequently used to support a diagnosis of leiomyosarcoma, few studies have specifically addressed the correlation between morphologic evidence of smooth muscle differentiation and immunohistochemical markers. We examined muscle marker expression in a diverse array of leiomyosarcomas to better understand the patterns of marker loss over the course of disease progression (from well- to poorly differentiated tumors, and from primary to metastatic disease). Of particular interest was whether immunohistochemical markers of muscle differentiation could provide a more objective measure of tumor differentiation than morphology in predicting patient outcomes.
When we reviewed the leiomyosarcoma tissue cored on our tissue microarray, we identified a number of poorly differentiated cores which showed few, if any, classic features of leiomyosarcoma and which could easily have been mistaken for undifferentiated pleomorphic sarcoma if examined out of context. These cores demonstrated a significant loss of markers of smooth muscle differentiation compared to well and moderately differentiated cores. While Carvalho et al., reported no correlation of muscle marker expression (SMA, desmin, caldesmon, calponin, and myosin) with histologic differentiation in a series of 78 cases,15 other studies report similar finding as ours, including retention of diffuse SMA in poorly differentiated tumors, with loss of both desmin and caldesmon (59% vs. 83% and 19% vs. 68%, respectively), compared with well-to-moderately differentiated tumors,16–18 and loss of smooth muscle marker expression in the less-differentiated areas of pleomorphic and “dedifferentiated” variants of leiomyosarcoma.12, 19, 20
Not only were individual muscle markers frequently lost in poorly differentiated tumors in our series, but the overall total number of muscle markers was reduced, with less than half of poorly differentiated tumors expressing 3 or more of SMA, desmin, h-caldesmon and SMMS, compared to over 90% of well differentiated tumors. This is consistent with reports that myosin and caldesmon expression are frequently co-expressed.15 Similarly to Mills et al.,21 we found that poorly differentiated “undifferentiated pleomorphic sarcoma-like” leiomyosarcoma less frequently expressed a muscle-enriched phenotype—that is, positivity for 3 or more of CASQ2, SLMAP, CFL2, MYLK, and ACTG2, with only 62% of poorly differentiated tumors falling into this category, compared to 98% of well-differentiated.
A significant proportion of so-called undifferentiated pleomorphic sarcomas (UPS, formerly termed malignant fibrous histiocytoma) are likely related to leiomyosarcoma and indeed may represent anaplastic (“dedifferentiated”) leiomyosarcoma. In multiple studies using protein expression analyses,22–26 gene expression analysis27–30 and/or comparative genomic hybridization,31 a subset of UPS consistently cluster with leiomyosarcoma, while up to 5% of UPS demonstrate a muscle enriched phenotype.21 These studies suggest that a subset of UPS represent a form of tumor progression from leiomyosarcoma.26, 27, 31, 32 Effectively differentiating between the two classes may have implications for prognostication and therapeutic selection as we more fully understand disease biology.
In our series, desmin and CFL2 were associated with improved OS independent of histologic differentiation or tumor size. Further analysis of desmin expression demonstrated that the impact of this marker in predicting outcome appeared to rest mainly in its ability to segregate moderately differentiated tumors into 2 groups- those that behaved like well-differentiated tumors and those which behaved like poorly differentiated tumors (Figure 4c and d). We also confirmed, in univariate analyses, the reported association of muscle-enriched phenotype with improved outcome. Of note, our study was underpowered for robust analysis of DSS, due to limited available clinical follow-up. However, in general, prognosticators of OS trended to predict DSS, implying that tumor-related deaths may have been more frequent in our cohort than we were able to confirm.
While we found that increased muscle marker expression predicted improved outcomes in leiomyosarcomas, other studies have found that UPS expressing muscle markers behave more aggressively than those without.22, 33–35 Thus, we are left with the seeming paradox that evidence of myoid differentiation is a positive predictor in leiomyosarcomas, and negative one in true UPS. This contradiction merits further study and reinforces the need to carefully evaluate biomarkers only in context with morphology.
In summary, we assessed the diagnostic and prognostic utility of an array of muscle markers in one of the largest cohorts of uterine and non-uterine leiomyosarcoma to date. We demonstrated that morphologically less-well-differentiated leiomyosarcoma are associated with loss of markers of muscle differentiation, thereby reducing the diagnostic value of immunohistochemistry in biopsies of such cases. Moreover, our findings are congruent with the theory that a subset of UPS represent pleomorphic or very poorly differentiated variants of leiomyosarcoma. Lastly, we identified desmin as an independent predictor of survival, which seems to be of most value in moderately differentiated tumors. Taken together our findings help to more clearly delineate patterns of muscle expression in leiomyosarcoma, which may help to improve diagnostic algorithms, and provide evidence for overlooked prognostic significance of everyday immunohistochemical assays.
Acknowledgments
The authors would like to thank Kim Vu for her invaluable assistance in preparation of the figures. Funding for this research was provided in part by the MD Anderson Physician Scientist Program (A.J. Lazar), a NIH/NCI K08CA160443 (K. Torres), NIH CA 112270 (M. van de Rijn), The Sally M. Kingsbury Sarcoma Research Foundation (K. Torres), NIH/NCI 5T32CA009599-21 (K. Lusby) and NIH/NCI S T32 CA009599-22 training grant (G. Boland). EGD, KET, DL, WLW, and AJL designed the study. GMB, KBS, KL, KLW and MB assembled the clinical database. EDY, DI, XG JLH, MvdR performed the immunohistochemistry. EGD analyzed the data and wrote the paper. WLW, AJL, JLH and MvdR provided manuscript critiques. All authors approved the final version.
Footnotes
Disclosure/ Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray-Coquard I, Thiesse P, Ranchere-Vince D, et al. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann Oncol. 2004;15:307–315. doi: 10.1093/annonc/mdh058. [DOI] [PubMed] [Google Scholar]
- 3.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 4.Borden EC, Baker LH, Bell RS, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- 5.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 6.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. New York: Springer; 2009. [Google Scholar]
- 8.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 9.Giuntoli RL, 2nd, Bristow RE. Uterine leiomyosarcoma: present management. Curr Opin Oncol. 2004;16:324–327. doi: 10.1097/01.cco.0000127721.55676.f6. [DOI] [PubMed] [Google Scholar]
- 10.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820–830. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, O'Connell F, Fletcher CD. Dedifferentiated leiomyosarcoma: clinicopathological analysis of 18 cases. Histopathology. 2011;59:1135–1143. doi: 10.1111/j.1365-2559.2011.04070.x. [DOI] [PubMed] [Google Scholar]
- 13.Beck AH, Lee CH, Witten DM, et al. Discovery of molecular subtypes in leiomyosarcoma through integrative molecular profiling. Oncogene. 2010;29:845–854. doi: 10.1038/onc.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lusby K, Savannah KB, Demicco EG, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol. 2013;20:2364–2372. doi: 10.1245/s10434-012-2834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho JC, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical markers in leiomyosarcoma delineates specific anatomic and gender subgroups. Cancer. 2009;115:4186–4195. doi: 10.1002/cncr.24486. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer. 1986;57:2077–2088. doi: 10.1002/1097-0142(19860515)57:10<2077::aid-cncr2820571033>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Hisaoka M, Wei-Qi S, Jian W, Morio T, Hashimoto H. Specific but variable expression of h-caldesmon in leiomyosarcomas: an immunohistochemical reassessment of a novel myogenic marker. Appl Immunohistochem Mol Morphol. 2001;9:302–308. doi: 10.1097/00129039-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama A, Hisaoka M, Hashimoto H. Vascular leiomyosarcoma: Clinicopathology and immunohistochemistry with special reference to a unique smooth muscle phenotype. Pathol Int. 2010;60:212–216. doi: 10.1111/j.1440-1827.2009.02508.x. [DOI] [PubMed] [Google Scholar]
- 19.Nicolas MM, Tamboli P, Gomez JA, Czerniak BA. Pleomorphic and dedifferentiated leiomyosarcoma: clinicopathologic and immunohistochemical study of 41 cases. Hum Pathol. 2010;41:663–671. doi: 10.1016/j.humpath.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030–1038. doi: 10.1097/00000478-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mills AM, Beck AH, Montgomery KD, et al. Expression of subtype-specific group 1 leiomyosarcoma markers in a wide variety of sarcomas by gene expression analysis and immunohistochemistry. Am J Surg Pathol. 2011;35:583–589. doi: 10.1097/PAS.0b013e318211abd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher CD, Gustafson P, Rydholm A, Willen H, Akerman M. Clinicopathologic reevaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol. 2001;19:3045–3050. doi: 10.1200/JCO.2001.19.12.3045. [DOI] [PubMed] [Google Scholar]
- 23.Franchi A, Massi D, Santucci M. The comparative role of immunohistochemistry and electron microscopy in the identification of myogenic differentiation in soft tissue pleomorphic sarcomas. Ultrastruct Pathol. 2005;29:295–304. doi: 10.1080/01913120590951293. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa T, Hasegawa F, Hirose T, Sano T, Matsuno Y. Expression of smooth muscle markers in so called malignant fibrous histiocytomas. J Clin Pathol. 2003;56:666–671. doi: 10.1136/jcp.56.9.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai A, Kondo T, Suehara Y, Kikuta K, Hirohashi S. Global protein-expression analysis of bone and soft tissue sarcomas. Clin Orthop Relat Res. 2008;466:2099–2106. doi: 10.1007/s11999-008-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal NH, Pavlidis P, Antonescu CR, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larramendy ML, Gentile M, Soloneski S, Knuutila S, Bohling T. Does comparative genomic hybridization reveal distinct differences in DNA copy number sequence patterns between leiomyosarcoma and malignant fibrous histiocytoma? Cancer Genet Cytogenet. 2008;187:1–11. doi: 10.1016/j.cancergencyto.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Carneiro A, Francis P, Bendahl PO, et al. Indistinguishable genomic profiles and shared prognostic markers in undifferentiated pleomorphic sarcoma and leiomyosarcoma: different sides of a single coin? Lab Invest. 2009;89:668–675. doi: 10.1038/labinvest.2009.18. [DOI] [PubMed] [Google Scholar]
- 29.Coindre JM, Hostein I, Maire G, et al. Inflammatory malignant fibrous histiocytomas and dedifferentiated liposarcomas: histological review, genomic profile, and MDM2 and CDK4 status favour a single entity. J Pathol. 2004;203:822–830. doi: 10.1002/path.1579. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama R, Nemoto T, Takahashi H, et al. Gene expression analysis of soft tissue sarcomas: characterization and reclassification of malignant fibrous histiocytoma. Mod Pathol. 2007;20:749–759. doi: 10.1038/modpathol.3800794. [DOI] [PubMed] [Google Scholar]
- 31.Derre J, Lagace R, Nicolas A, et al. Leiomyosarcomas and most malignant fibrous histiocytomas share very similar comparative genomic hybridization imbalances: an analysis of a series of 27 leiomyosarcomas. Lab Invest. 2001;81:211–215. doi: 10.1038/labinvest.3780229. [DOI] [PubMed] [Google Scholar]
- 32.Lee YF, John M, Edwards S, et al. Molecular classification of synovial sarcomas, leiomyosarcomas and malignant fibrous histiocytomas by gene expression profiling. Br J Cancer. 2003;88:510–515. doi: 10.1038/sj.bjc.6600766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deyrup AT, Haydon RC, Huo D, et al. Myoid differentiation and prognosis in adult pleomorphic sarcomas of the extremity: an analysis of 92 cases. Cancer. 2003;98:805–813. doi: 10.1002/cncr.11617. [DOI] [PubMed] [Google Scholar]
- 34.Massi D, Beltrami G, Capanna R, Franchi A. Histopathological re-classification of extremity pleomorphic soft tissue sarcoma has clinical relevance. Eur J Surg Oncol. 2004;30:1131–1136. doi: 10.1016/j.ejso.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Perot G, Mendiboure J, Brouste V, et al. Smooth muscle differentiation identifies two classes of poorly differentiated pleomorphic sarcomas with distinct outcome. Mod Pathol. 2013 doi: 10.1038/modpathol.2013.205. [DOI] [PubMed] [Google Scholar]