Abstract
Due to the fast-acting nature of ricin, staphylococcal enterotoxin (SEB), and Clostridium perfringens epsilon toxin (ETX), it is necessary that therapeutic interventions following a bioterrorism incident by one of these toxins occur as soon as possible after intoxication. Moreover, because the clinical manifestations of intoxication by these toxins are likely to be indistinguishable from each other, especially following aerosol exposure, we have developed a cocktail of chimeric monoclonal antibodies that is capable of neutralizing all three toxins. The efficacy of this cocktail was demonstrated in mouse models of lethal dose toxin challenge.
Keywords: antibody, toxin, biodefense, therapeutic
The development of therapeutics directed against the Select Agents and Toxins poses significant and unique challenges. Foremost, the pathogens and toxins that are currently classified by the Centers for Disease Control and Prevention (CDC) as potential biothreat agents are genetically, evolutionarily, and structurally diverse, thereby necessitating therapeutics tailored against each agent (Mantis et al., 2011). Second, many of these agents, but in particular the toxins, can induce morbidity and even mortality within a matter of hours, which means that therapeutic interventions treatments will likely be initiated in the absence of definitive etiologic diagnosis (Wolfe et al., 2013). In addition, the earliest clinical manifestations of many select agents and toxins are expected to be indistinguishable from each other, which in a clinical setting may necessitate the administration of combinations of therapies (2007).
Ricin toxin, staphylococcal enterotoxin B (SEB), and Clostridium perfringens epsilon toxin (ETX) are fast acting, highly toxic and potentially lethal agents for which there are currently no available countermeasures (Mantis, 2005). The toxins are from unrelated sources and share no obvious functional domains or enzymatic activities (Table 1; Figure S1). Ricin toxin is a 65 kDa heterodimeric glycoprotein from the castor bean plant (Ricinus communis). The A subunit of ricin (RTA) is a ribosome-inactivating protein (RIP), while the B subunit (RTB) is a lectin that modulates toxin attachment and entry into mammalian cells. SEB is a 28 kDa superantigen produced by Staphylococcus aureus that, when ingested, causes symptoms that are classically associated with food poisoning, including cramps, vomiting and diarrhea (Krakauer and Stiles, 2013). While oral exposure to SEB is debilitating, it is rarely fatal. This is in contrast to SEB aerosol exposure, which in non-human primate models results in pulmonary endema and systemic complications (Lindsay and Griffiths, 2013; Mattix et al., 1995). Finally, ETX is a 33 kDa [.beta]-pore-forming toxin (PFT) secreted by Clostridium perfringens types B and D, which are economically important pathogens associated with enterotoxemia in several species of livestock (Stiles et al., 2013; Uzal et al., 2014). All three toxins cross epithelial barriers and can elicit mucosal and systemic damage following ingestion or inhalation (Mantis, 2005). Due to the capacity of these toxins to induce similar clinical signs, morbidity and mortality, and their recognized potential as biological warfare and bioterrorism agents, we reasoned that a tripartite antitoxin cocktail capable of neutralizing ricin, SEB, and ETX would be of significant medical benefit.
Table 1.
Characteristics of SEB, ETX and ricin toxin and their respective mAbs.
| Toxin | kDa | Toxin Class | mAb | Reference |
|---|---|---|---|---|
| SEB | 28 | super antigen | 19F | in preparation |
| ETX | 33 | β-pore forming | 4D7 | (Garcia et al., 2014) |
| Ricin | 65 | ribosome-inactivating | PB10 | (Sully et al., 2014) |
Neutralizing mAbs against ricin, SEB, and ETX have been previously described (Table 1); mAb PB10 is directed against ricin toxin (Sully et al., 2014), 19F1 against SEB (L.Zeitlin, manuscript in preparation), and 4D7 against ETX (Garcia et al., 2014; Hauer and Clough, 1999). The murine variable domains of each of the mAbs were synthesized (Life Technologies; San Diego, CA) and grafted onto human IgG1 frameworks, and transformed into Agrobacterium tumefaciens, which were then used for vacuum infiltration of Nicotiana benthamiana using the rapid antibody-manufacturing platform (RAMP) based on magnICON (Giritch et al., 2006; Hiatt and Pauly, 2006). RAMP makes use of transgenic N. benthamiana (Strasser et al., 2008) in which plant-specific glycosyl-transferases have been inhibited by RNAi, so the resulting mAbs contain mammalian N-glycans. The resulting chimeric (c-) derivatives of PB10, 19F1, and 4D7 have each been shown to retain potent toxin-neutralizing activity and to passively protect mice against a cognate toxin challenge (Garcia et al., 2014; Sully et al., 2014).
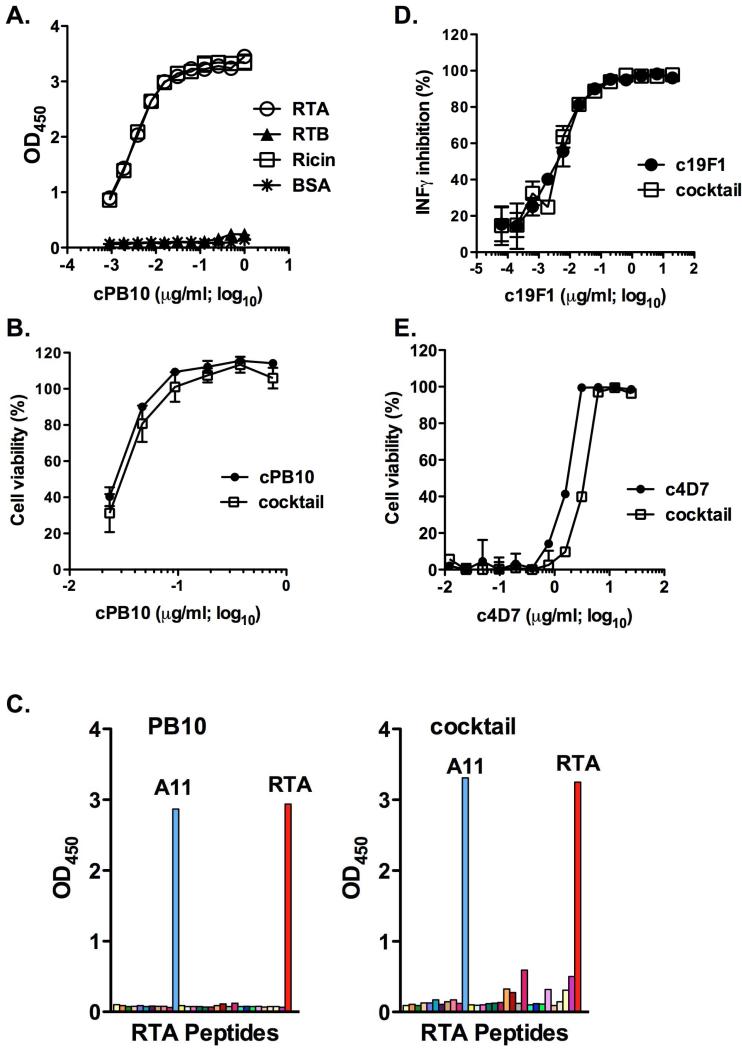
To examine the functional properties of a cocktail of plant-derived cPB10, c19F1, and c4D7, the three chimeric mAbs were combined at equimolar amounts and evaluated for toxin binding activity by ELISA and for toxin-neutralizing activities in cell-based cytotoxicity assays. We found that the binding profile of cPB10 as part of the tripartite cocktail was identical to cPB10 alone and its parenteral murine counterpart in terms of reactivity with RTA, ricin holotoxin, and its linear peptide epitope (Figure 1A,C). Moreover, the 50% inhibitory concentration (IC50) of cPB10 was the same whether cPB10 was tested by itself or combined with c19F1 and c4D7 (Figure 1B). The toxin-binding activities (data not shown) as well as toxin-neutralizing activities (Figure 1D,E) of c19F1 and c4D7 as a cocktail were also indistinguishable from the individual mAbs themselves. These data indicate that there is no evidence to suggest that the different chimeric mAbs interfere with each other's function activities.
Figure 1. Toxin binding and neutralizing activities associated with the chimeric mAbs in the context of the tripartite cocktail.
The tripartite cocktail was assessed for specificity for ricin (panels A-C), SEB (panel D) and ETX (panel E). (A) cPB10 (alone or in cocktail) reactivity with ricin holotoxin and subunits by ELISA. Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific) were coated by overnight incubation with 1 μg/ml ricin, RTA, RTB or BSA. Plates were developed using horseradish peroxidase (HRP)-labeled goat anti-human IgG (Invitrogen) and 3,3’,5,5’ tetramethylbenzidine (Kirkegaard & Perry Labs, Gaithersburg, MD), as described (Sully et al., 2014). (B) Toxin-neutralizing activity of cPB10. Serial dilutions (in triplicate) of cPB10, alone or the cocktail were mixed with ricin (10 ng/ml) and then applied to Vero cells, as described (Sully et al., 2014). Cell viability was assessed 48 h later; (C) cPB10 (alone or in cocktail) reactivity with RTA-peptide array. Overlapping 18-mer peptides spanning the length of RTA (O'Hara et al., 2013) were used to coat Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific) before being probed with cPB10. ELISA plates were developed as described in panel A. Peptide A11 (RTA residues Y91-F108) co corresponds to PB10's known epitope (Vance and Mantis, 2012). (D) The neutralizing activity of c19F1 was determined using peripheral blood mononuclear cells (PBMCs) and SEB toxin, as described (Karauzum et al., 2012). The resulting inhibition of INF-[.gamma] production by c19F1 or the antibody cocktail were indistinguishable (E) ETX neutralizing assays were performed by incubating ETX with indicated concentrations of c4D7, alone or in the context of the cocktail. Neutralizing assays were done using Madin-Darby Canine Kidney (MDCK II) cells, as described (Garcia et al., 2014; Robertson et al., 2011). ETX was obtained from BEI Resources (Manassas, VA).
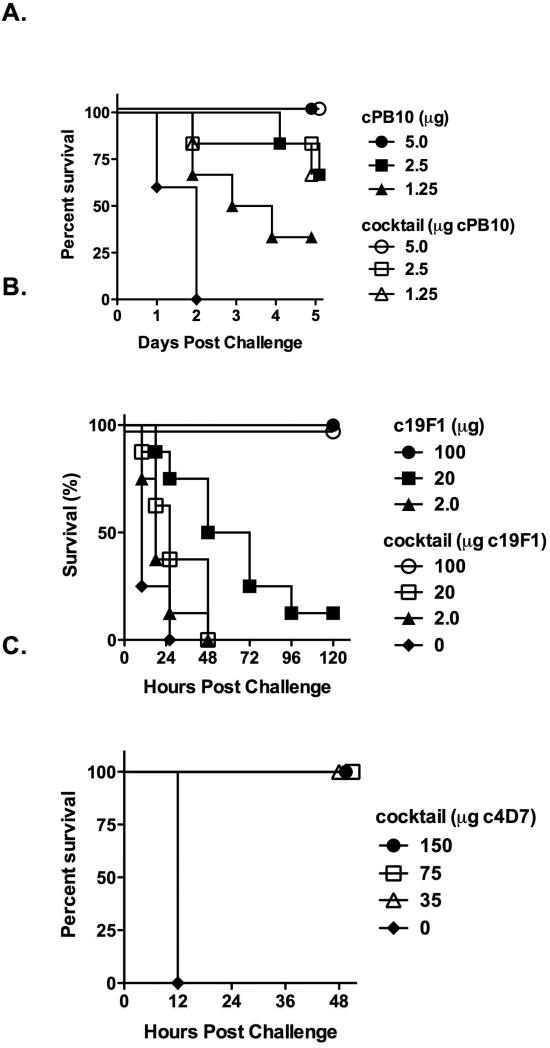
We next evaluated the tripartite cocktail for the ability to passively protect mice against ricin, SEB, and ETX in well-established mouse models of toxin challenge. For ricin toxin, mice received 5 μg, 2.5 μg or 1.5 μg of cPB10, by itself or as part of the tripartite cocktail, and were then challenged with 10 x LD50 ricin (Figure 2A). As expected, control mice succumbed to ricin intoxication within 48 h. Protection afforded by cPB10 was dose-dependent and was identical whether cPB10 was administered alone or in combination with c19F1 and c4D7, demonstrating that neither the anti-SEB or anti-ETX mAb interferes with cPB10. The reciprocal passive protection studies were done with the cocktail using mouse models of SEB and ETX intoxication. The SEB challenge model consisted of i.p. injection of 5 x LD50 SEB followed 4 h later by a potentiating dose of lipopolysaccharide (40 μg;List Biological Laboratories, Campbell, CA). Protection afforded by c19F1 was dose-dependent with complete survival observed in mice receiving 100 μg of c19F1, alone or in combination with c4D7 and cPB10 (Figure 2B). Finally, mAb c4D7 was able to fully protect mice when administered as part of the tripartite cocktail. The ETX challenge model involved i.p. administration of the cocktail to mice 24 h prior to intravenous injection of 3 x LD50 ETX, as described previously (Garcia et al., 2014). Control mice succumbed to toxin-induced death within 12 h, whereas cocktail-treated mice survived without showing any clinical abnormalities (Figure 2C). Additional control mice treated only with c4D7 also survived without showing any clinical abnormalities. These data demonstrate the potential of a mixture of cPB10, c19F1 and c4D7 to protect mice against lethal challenge doses of ricin, SEB, and ETX.
Figure 2. Protection afforded by the tripartite mAb cocktail in mice upon challenge with ricin, SEB and ETX.
The tripartite mAb cocktail was assessed for the ability to protect mice against ricin (panels A, D-F), SEB (panel B) and ETX (panel C). All studies involving mice were done in strict compliance the Institutional Animal Care and Use Committees (IACUC) at the Wadsworth Center, Iowa State University, and University of California, Davis. (A) BALB/c mice (female, 6-8 weeks of age; Taconic Labs, Hudson, NY) were housed under conventional, specific pathogen-free conditions. cPB10, alone or in the cocktail was administered to mice (n=10/group) by intraperitoneal (i.p.) injection 24 h prior to challenge with 10x LD50 ricin (~2 μg mouse; Vector Laboratories, Burlingame, CA), also by i.p. injection. Survival was monitored over a period of five days. (B) To evaluate c19F1, the chimeric mAb alone or in the context of the cocktail was mixed with SEB (1 μg) for 1 hr and then injected into BALB/c mice (Karauzum et al., 2012). Four hours later the animals received a potentiating dose of lipopolysaccharide (40 μg; List Biological Laboratories, Campbell, CA) and were monitored for survival for 5 days. (C) To evaluate c4D7, the chimeric mAb in the context of the cocktail was administered to female BALB/c mice by i.p. injection, as described previously (Garcia et al., 2014). Twenty-four hours later, the animals were challenged by intravenous injection 3xLD50 ETX.
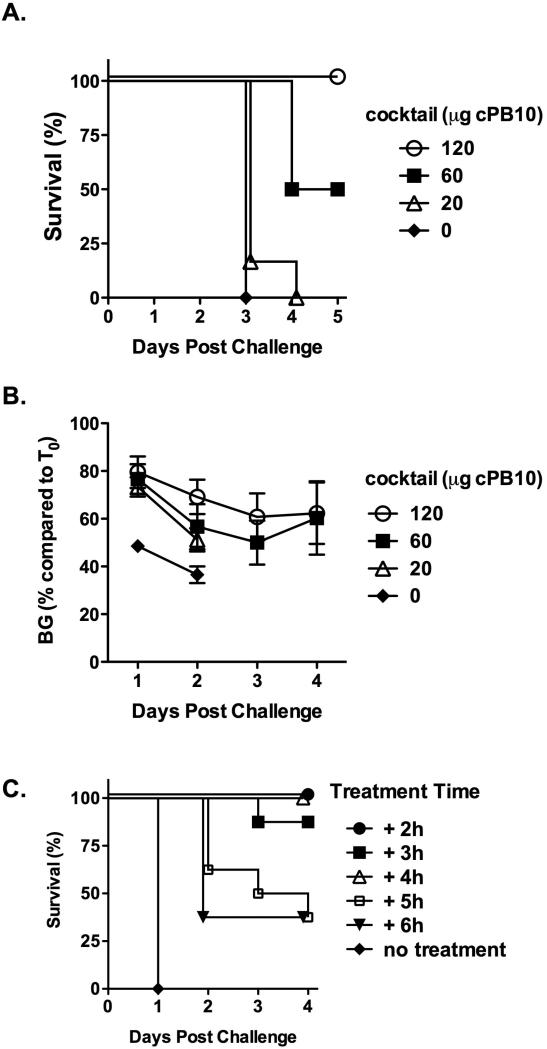
We next wished to further evaluate the tripartite antitoxin cocktail in a mucosal challenge model and as a possible therapeutic. We chose ricin toxin for these studies since cPB10 has been recently evaluated in respiratory tract challenge model and its therapeutic window has been established (Sully et al., 2014). Groups of mice received 120 μg, 60 μg or 20 μg of cPB10 in the context of the cocktail and then challenged with the same dose of ricin as above, but delivered via the intranasal (i.n.) route. Protection afforded by the tripartite cocktail was dose-dependent (Figure 3A); mice that received 120 μg cPB10 were protected from ricin challenge and experienced a transient reduction in blood glucose levels (Figure 3B); half the mice that received 60 μg cPB10 were protected against ricin, whereas the control mice (no cPB10) or mice that received 20 μg of cPB10 experienced a rapid onset of hypoglycemia and succumbed to toxin-induced death within 48 h. These data demonstrate cPB10 within the context of the cocktail is protective against respiratory tract challenge but that the amount of antibody required for protection was 10-20 times that required for systemic challenge. Because the exact LD50 for i.n. challenge is unknown, this requirement for increased dosing of mAb could be due to i.n. challenge being more lethal than systemic challenge or due to a need for higher mAb concentrations for protection on mucosal surfaces. In future studies, this observation will be validated in an aerosol challenge model as it may have important implications for ricin-based antibody prophylactics.
Figure 3. Mucosal protection and therapeutic potential of the tripartite cocktail against ricin toxin.
(A-B). Capacity of cPB10 to protect against intranasal ricin challenge. The tripartite cocktail was administered to mice (n=8 mice per group) by intraperitoneal (i.p.) injection 24 h prior to intranasal 10 x LD50 ricin challenge. Mice were monitored for survival (panel A) and morbidity (panel B), as determined by blood glucose levels (Sully et al., 2014); (C) Groups of BALB/c mice (n=8 per group) were challenged with 10 x LD50 ricin and then administered (by i.p. injection) the tripartite cocktail (25 μg of cPB10/mouse) at indicated times (hours). Survival was monitored for five days.
Finally, to assess the therapeutic potential of the tripartite cocktail, mice were challenged with 10 x LD50 ricin and then administered the cocktail at hourly intervals thereafter at amounts equivalent to 25 μg cPB10 per mouse (Figure 3C). In agreement with what we reported recently for cPB10 alone (Sully et al., 2014), the tripartite cocktail was able to fully rescue mice from toxin-induced death if administered within 4 h following challenge. It should be underscored that the mouse model is particularly stringent and that the actual therapeutic window in humans may in fact be greater than 4 h depending on the dose.
In summary, we have generated a cocktail of chimeric mAbs against three putative biothreat toxins derived from common, readily accessible plant (ricin) and bacteria (ETX and SEB). Due to their excellent safety profile and efficacy, mAbs are a rapidly growing class of therapeutic drugs (Reichert et al., 2005). Moreover, passive immunization with antibodies has been shown to be effective against a wide variety of toxins (Froude et al., 2011; Wang et al., 2013). We therefore envision that the chimeric mAbs (or fully humanized derivatives) could be used as a means of providing passive immunity to first responders, laboratory staff or military personnel in the event that they may be at risk of toxin exposure. As alluded to above, future improvements to the cocktail may include humanization of the mAbs and the engineering of point mutations in the Fc gamma chain constant regions that result in extended serum half-life with the possibility of using the cocktail as a prophylactic and provide passive protection for greater than six months (Robbie et al., 2013; Zalevsky et al., 2010). Such a cocktail would constitute a significant resource within the public health and biodefense community.
We also envision the possibility that the cocktail could be used as a post-exposure therapeutic, although it is important to underscore that in this study we have only examined the potential of the tripartite cocktail to rescue mice following ricin challenge. We did not investigate whether the combination of cPB10, c19F1 and c4D7 had any therapeutic activity against SEB or ETX. Indeed, rescuing an individual following C. perfringens ETX exposure may be a particularly formidable challenge considering that the toxin exerts its effects on host cells virtually instantaneously. In the ETX-challenge model employed in this study, control mice succumbed to intoxication with 12 h, indicating that the therapeutic window (in rodents, at least) is likely to be very narrow. However, as is the case for ricin and SEB, the actual therapeutic potential of any antibody against ETX is going to be dependent on the amount and route (i.e., systemic versus mucosal) of toxin exposure. From the results of our limited in vitro and in vivo analysis of the combination of cPB10, c19F1 and c4D7 antibodies, we can only speculate that the tripartite cocktail has therapeutic utility in humans in an actual clinical setting.
Supplementary Material
Figure S1. Structures of ricin, SEB and C. perfrigens ETX. Biological assembly images from the Protein Data Bank (PDB) (Berman et al., 2000). The images correspond to the following PBD identifiers: (A) ricin, 2AAI; (B) SEB, 3SEB; (C) ETX, 1UYJ.
HIGHLIGHTS.
Ricin, staphylococcal enterotoxin (SEB), and Clostridium perfringens epsilon toxin (ETX) are biothreat toxins
We developed a cocktail of chimeric monoclonal antibodies (mAbs) that neutralizes all three toxins
Chimeric mAbs were expressed using a robust plant-based platform
The tripartite cocktail also passively protected mice against ricin, SEB, and ETX in relevant challenge models
These studies represent a major advancement towards a broad antitoxin antibody-based therapeutic
Ethical Statement.
With this letter I attest that the manuscript entitled “A Tripartite Cocktail of Chimeric Monoclonal Antibodies Passively Protects Mice against Ricin, Staphylococcal Enterotoxin B and Clostridium perfringens Epsilon Toxin” to be submitted to Toxicon is an original study that has not previously been submitted to this (or any other) journal. In submitting this article we (the authors) have adhered to the ethical guidelines as described by the publisher: http://www.elsevier.com/publishingethics and http://www.elsevier.com/ethicalguidelines.
ACKNOWLEDGEMENTS
The authors thank Drs. Yuri Gleba and Victor Klimyuk for access to the Icon Genetics’ magnICON system and Dr. Herta Steinkellner for access to the transgenic N. benthamiana. This work was supported by NIH grants U01AI082276 (LZ), R01AI098774 (LZ). E.K.S. was supported by a Biodefense and Emerging Infectious Diseases (BD-EID) training grant from the National Institutes of Health (T32 AI055429; principal investigator, K. A. McDonough).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- NIAID Strategic Plan for Biodefense Research - 2007 Update. 2007 [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froude JW, 2nd, Stiles B, Pelat T, Thullier P. Antibodies for biodefense. MAbs. 2011:3. doi: 10.4161/mabs.3.6.17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JP, Beingesser J, Bohorov O, Bohorova N, Goodman C, Kim D, Pauly M, Velasco J, Whaley K, Zeitlin L, Roy CJ, Uzal FA. Prevention and treatment of Clostridium perfringens epsilon toxin intoxication in mice with a neutralizing monoclonal antibody (c4D7) produced in Nicotiana benthamiana. Toxicon. 2014;88C:93–98. doi: 10.1016/j.toxicon.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci U S A. 2006;103:14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer PJ, Clough NE. Development of monoclonal antibodies suitable for use in antigen quantification potency tests for clostridial veterinary vaccines. Developments in biological standardization. 1999;101:85–94. [PubMed] [Google Scholar]
- Hiatt A, Pauly M. Monoclonal antibodies from plants: A new speed record. Proceedings of the National Academy of Sciences. 2006;103:14645–14646. doi: 10.1073/pnas.0607089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, Devi VS, Stavale E, Warfield KL, Zeitlin L, Roy CJ, Sidhu SS, Aman MJ. Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J Biol Chem. 2012;287:25203–25215. doi: 10.1074/jbc.M112.364075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence. 2013;4:759–773. doi: 10.4161/viru.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay CD, Griffiths GD. Addressing bioterrorism concerns: options for investigating the mechanism of action of Staphylococcus aureus enterotoxin B. Human & experimental toxicology. 2013;32:606–619. doi: 10.1177/0960327112458941. [DOI] [PubMed] [Google Scholar]
- Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Morici LA, Roy CJ. Mucosal Vaccines for Biodefense. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattix ME, Hunt RE, Wilhelmsen CL, Johnson AJ, Baze WB. Aerosolized staphylococcal enterotoxin B-induced pulmonary lesions in rhesus monkeys (Macaca mulatta). Toxicol Pathol. 1995;23:262–268. doi: 10.1177/019262339502300304. [DOI] [PubMed] [Google Scholar]
- O'Hara JM, Brey RN, Mantis NJ. Comparative Efficacy in Mice of Two Lead Candidate Ricin Toxin A Subunit (RTA) Vaccine. Clinical and Vaccine Immunology. 2013 doi: 10.1128/CVI.00098-13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57:6147–6153. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SL, Li J, Uzal FA, McClane BA. Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin. PLoS One. 2011;6:e22053. doi: 10.1371/journal.pone.0022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles BG, Barth G, Barth H, Popoff MR. Clostridium perfringens epsilon toxin: a malevolent molecule for animals and man? Toxins (Basel) 2013;5:2138–2160. doi: 10.3390/toxins5112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnology Journal. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim do H, Pauly MH, Velasco J, Hiatt E, Morton J, Swope K, Roy CJ, Zeitlin L, Mantis NJ. Chimeric Plantibody Passively Protects Mice against Aerosolized Ricin Challenge. Clin Vaccine Immunol. 2014;21:777–782. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future microbiology. 2014;9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DJ, Mantis NJ. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin's enzymatic subunit. Toxicon. 2012;60:874–877. doi: 10.1016/j.toxicon.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XZ, Coljee VW, Maynard JA. Back to the future: recombinant polyclonal antibody therapeutics. Current opinion in chemical engineering. 2013;2:405–415. doi: 10.1016/j.coche.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe DN, Florence W, Bryant P. Current biodefense vaccine programs and challenges. Human vaccines & immunotherapeutics. 2013:9. doi: 10.4161/hv.24063. [DOI] [PubMed] [Google Scholar]
- Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Structures of ricin, SEB and C. perfrigens ETX. Biological assembly images from the Protein Data Bank (PDB) (Berman et al., 2000). The images correspond to the following PBD identifiers: (A) ricin, 2AAI; (B) SEB, 3SEB; (C) ETX, 1UYJ.