Abstract
Background
Tuberculosis (TB) is a cause of 1.2–1.5 million deaths worldwide, including deaths from TB among HIV positive people. Determining the extent of immune cells belonging to cell mediated immunity and haematological parameters is critical to maximize the potential benefit of anti-tubercular treatment and case management.
Materials and Methods
Comparative cross sectional study was conducted to determine the white blood cell (WBC) count, CD4, CD8, haemoglobin (Hgb), red blood cell (RBC) count, mean corpuscular haemoglobin (MCHC), mean corpuscular volume (MCV) between newly diagnosed TB patients and apparently healthy controls (HCs).
Results
From consecutively enrolled 108 TB patients, pulmonary TB (PTB) accounted for 48(44.4%), TB lymphadenitis accounted for 48(44.4%), and disseminated/miliary TB accounted for 12(11.1%). Analysis of variance revealed that mean ± SD of CD4 count of male TB patients (650 ± 224cells/µl) was significantly lower than male control group (883 ± 256 cells/µl) (p= 0.001). In a similar manner, the mean CD4 count of female TB patients (793 ± 332cells/µl) was lower than female control group (975 ± 300 cells/µl) (p=0.001). There was no statistically significant difference in CD8 counts between cases and controls for both genders. Forty (37.0%) TB patients had developed anaemia of whom 22(55%) were among PTB, 13(32.5%) from tuberculous lymphadenitis and 5(20%) from disseminated TB. Morphologically, from all anaemia among TB patients, normocytic normochromic anaemia accounted for 15(37.5%) followed by normocytic hypochromic anaemia 13(30.4%).
Conclusion
CD4 lymphopenia was significant among TB patients. Granulocyte count was increased. Mild anaemia was found major haematological abnormality among newly diagnosed TB patients.
Keywords: Tuberculosis, CD4 count, CD8 count, anaemia, haemoglobin
Introduction
TB was declared a global emergency in 1993. In that year an estimated 7–8 million cases and 1.3–1.6 million deaths occurred (1). Despite the availability of effective treatment, TB remains a major global health problem. After about a decade in 2010, there were an estimated 8.5–9.2 million cases and 1.22–1.59 million deaths including deaths from Human Immunodeficiency Virus (HIV)-associated TB (2). Hospital data of the Ministry of health in Ethiopia show that TB is the leading cause of morbidity, the third cause of hospital admission, and the first cause of hospital death in Ethiopia (3). According to WHO Global Report of 2011, Ethiopia ranked 7th among the high TB burden countries in the world, with an estimated incidence of all forms of TB of 261/100,000 population/year. The estimated prevalence of all forms of TB reported was 394/100,000 population (2).
M. tuberculosis targets macrophages which are important effector cells in the immune system, as its preferred habitat. Whereas resting macrophages fail to harm M. tuberculosis, activated macrophages can control the growth of the microbe, although sterile eradication is seldom achieved. Several different T-cell populations are required for the successful control of the pathogen. This dynamic interplay underlying protection is the reason for the long-term persistence of M. tuberculosis (4). CD4 lymphocytopenia is a well-defined risk factor for the development of active TB in patients infected with HIV. TB may be also associated with CD4 and CD8 lymphopenia even in patients without HIV virus infection (5).
CD4 T cells play central roles in the function of the immune system: They help B cells make antibody, enhance and maintain responses of CD8 T cells, regulate macrophage function, orchestrate immune responses against a wide variety of pathogenic microorganisms, and regulate/suppress immune responses both to control autoimmunity and to adjust the magnitude and persistence of responses. CD4 T cells are important mediators of immunologic memory, and when their numbers are diminished or their functions are lost, the individual becomes susceptible to a wide range of infectious disorders including TB (6).
The protective and pathologic response to M. tuberculosis is complex and multifaceted, involving many components of the immune system. A clear picture of the network of immune responses to this pathogen, as well as an understanding of the effector functions of these components is essential to the design and implementation of effective treatments for TB (7). It is essential to determine immunological and haematological parameters at the baseline of anti-TB treatment for further consideration of supportive care and other treatment options that might be required for some patients to enhance the anti-TB treatment outcome.
Materials and Methods
A Comparative cross-sectional study was conducted between TB patients and apparently healthy controls at Jimma University Specialized Hospital TB clinic from January-2012 to May-2012. One hundred eight newly diagnosed TB patients and 116 HIV negative apparently healthy controls in the age group of 15–55 years were included in the study consecutively. The diagnosis of TB was made according to the national guideline. The cases were reportedly naive to anti-TB treatment. Newly diagnosed TB patients who have already started anti-TB treatment, receiving any kind of immunosuppressive drugs, known or suspected history of other chronic disease, pregnant women and HIV positive individuals were excluded. Apparently healthy controls were recruited from blood donors 15–55 years of age.
Data was collected during enrolment using pre-structured questionnaire. Four milliliters of blood specimen was collected in pre-labelled evacuated tubes (BD vacutainer, Oxford, UK) from each study participant. CD4, CD8 and CD4/ CD8 ratio counts were performed on BD- FACS Count System (Becton Dickinson Biosciences, San Jose California, USA) and CBC was done on Cell-Dyn 1800 system (Abbott Diagnostic, Illinois, USA). Data from the laboratory investigation and questionnaire were compiled and analyzed using SPSS 16.0. Mean values were calculated and compared using one way ANOVA test for TB patients and apparently healthy controls. Significant differences were evaluated between groups by Post Hoc Tukey test. P value < 0.05 was considered statistically significant. The study was approved by the Ethical Review Board of Jimma University, College of Public Health and Medical sciences, Department of Medical Laboratory Science and Pathology. The objective of the study was explained to the patients and all the study subjects (cases and controls) were included after written consent was obtained.
Results
A total of 108 newly diagnosed TB patients and 116 healthy controls were enrolled in this study. Cases accounted for 45(41.7%) males and 63(58.3%) females, and healthy controls account for 54(46.6%) males and 62(53.4%) females. There was no statistically significant difference between the mean age (p=0.094) and sex distribution (p=0.599) of the TB patients and healthy controls. Distribution of age, sex, educational status and body mass index (BMI) among newly diagnosed TB patients is shown in (Table 1).
Table 1.
Distribution of age, sex, educational status, and body mass index among newly diagnosed TB patients
| Type of TB | ||||
| TBL | DTB | P TB | Total | |
| Age category | ||||
| 16–20 | 9(8.3%) | 2(1.9%) | 13(12%) | 24(22.2%) |
| 21–25 | 8(7.4%) | 3(2.8%) | 13(12%) | 24(22.2%) |
| 26–30 | 11(10.2%) | 0 | 11(10.2%) | 22(20.4%) |
| 31–35 | 7(6.4%) | 1(0.9%) | 2(1.9%) | 10(9.2%) |
| 36–40 | 6(5.6%) | 1(0.9%) | 1(0.9%) | 8(7.4%) |
| 41–45 | 2(1.9%) | 0 | 4(3.7%) | 6(5.6%) |
| 46–50 | 4(3.7%) | 4(3.7%) | 0 | 8(7.4%) |
| 51–55 | 1(0.9%) | 1(0.9%) | 4(3.7%) | 6(5.6%) |
| Total | 48(44.4%) | 12(11.1%) | 48(44.4%) | 108(100%) |
| Gender (sex) | ||||
| Male | 18(16.7%) | 4(3.7%) | 23(21.3%) | 45(41.7%) |
| Female | 30(27.8%) | 8(7.4%) | 25(23.1%) | 63(58.3%) |
| Total | 48(44.4%) | 12(11.1%) | 48(44.4%) | 108(100%) |
| Educational Status | ||||
| Illiterate | 27(25.0%) | 7(6.5%) | 13(12%) | 47(43.5%) |
| Only read and Write | 1(0.9%) | 0 | 1(0.9%) | 2(1.9%) |
| 1–4th | 3(2.8%) | 1(0.9%) | 6(5.6%) | 10(9.3%) |
| 5–8th | 8(7.4%) | 2(1.9%) | 9(8.3%) | 19(17.6%) |
| 9–12th + | 9(8.3%) | 2(1.9%) | 19(17.6%) | 30(27.8%) |
| Total | 48(44.4%) | 12(11.1%) | 48(44.4%) | 108(100%) |
| BMI | ||||
| <18.5 kg/m2 | 20(18.5%) | 7(6.5%) | 33(30.6%) | 60(55.6%) |
| 18.5–255 kg/m2 | 28(25.9%) | 5(3.6%) | 15(13.9%) | 48(44.4%) |
| Total | 48(44.4%) | 12(11.1%) | 48(44.4%) | 108(100%) |
TBL= tuberculous lymphadenitis, DTB= disseminated TB, PTB=pulmonary TB
From the total of 108 cases, PTB accounts 48(44.4%), TB lymphadenitis (TBL) 48(44.4%) and disseminated/miliary TB (DTB) accounts 12(11.1%) (Table 1). Compared with male healthy controls (6.83 ± 2.17 ×103cells/µl) male TB patients had significantly high mean absolute WBC count (8.35±3.3 ×103cells/µl) (p=007). Female TB patients also had significantly high mean absolute WBC count (8.62±2.89 ×103cells/µl) than female healthy controls (6.67±1.5×103cells/µl) (p=0.001). The mean absolute granulocytes count (AGC) of both male and female TB patients were significantly higher than healthy controls (p=0.001). Absolute lymphocytes counts of male TB patients were significantly lower compared with male healthy controls (p=0.008). But, there was no statistically significant difference between female TB patients and female healthy controls (p=0.101) (Table 2). Mean CD4 count of male TB patients (650 ± 224cells/µl) were significantly lower than male healthy controls (883 ± 256 cells/µl) (p=001). Similarly, the mean CD4 count of female TB patients (793±332 cells/µl) is lower than the mean CD4 count of female healthy controls (975 ± 300 cells/µl) (p=0.002). But, in case of CD8 count, there was no statistically significant difference between cases and controls in both genders (Table 2).
Table 2.
Comparison of mean values of immunological and hematological parameters in the newly diagnosed TB patients versus healthy control
| Male | Female | |||||
| TB patient mean ± SD |
HC mean ± SD |
P-value | TB patient mean ± SD |
HC mean ± SD |
P-value | |
| WBC × 103cells/µl | 8.35± 3.3 | 6.83 ± 2.17 | 0.007 | 8.62 ± 2.89 | 6.67 ± 1.5 | 0.001 |
| AGC ×103cells/µl | 5.62 ± 2.97 | 3.9 ± 1.85 | 0.001 | 5.65 ±2.67 | 3.66 ± 1.22 | 0.001 |
| ALC × 103cells /µl | 1.90 ± 0.52 | 2.2 ± 0.58 | 0.008 | 2.07 ±0.81 | 2.28 ± 0.55 | 0.101 |
| CD4cells /µl | 650 ± 224 | 883 ±256 | 0.001 | 793±332 | 975±300 | 0.002 |
| CD8 cells/µl | 612± 266 | 675 ±277 | 0.256 | 640±290 | 712±260 | 0.217 |
| CD4/CD8 ratio | 1.17 ± 0.44 | 1.41 ± 0.46 | 0.009 | 1.59 ± 0.95 | 1.44 ±0.43 | 0.268 |
| RBC × 106cells /µl | 4.95 ±1.58 | 5.81 ± 0.74 | 0.001 | 4.74 ±0.99 | 5.24±0.74 | 0.001 |
| Hgb g/dl | 13.3 ± 4.6 | 17.1 ± 2.2 | 0.001 | 12.5±3.6 | 14.9 ± 2.2 | 0.001 |
| MCV fl | 88.0 ± 14 | 94 ±5.8 | 0.001 | 87 ±14.7 | 93.2±7.2 | 0.001 |
| MCHC g/dl | 32.09 ± 2.18 | 33.34±0.82 | 0.001 | 32 ± 2.6 | 33 ± 0.8 | 0.001 |
| PLT × 103cells /µl | 455.9 ± 329 | 315 ±124 | 0.001 | 442± 317 | 340±144 | 0.001 |
HC= healthy controls
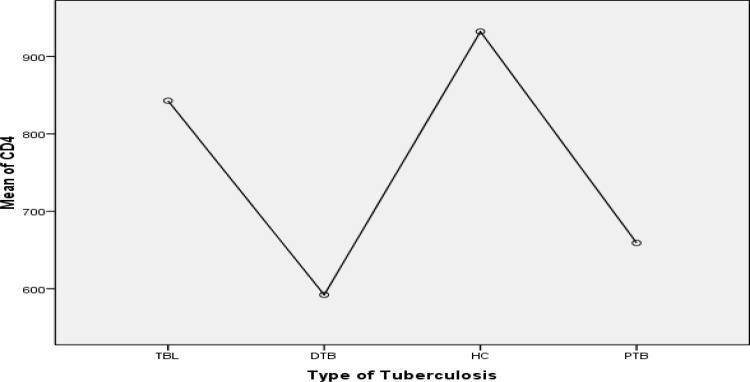
Further analysis using Post Hoc Tukey test showed that mean CD4 cells count of DTB group was significantly lower than TBL (p=0.034) and healthy control group (p<0.001) but showed no statistically significant difference with PTB (0.884). Mean CD4 cells count of PTB also lower than TBL (p=0.009) and healthy control group (p=0.001) (Fig. 1). Of the total 108 TB patients 24(22.2%) had low CD4 count (<500cells/µl), of whom 5(4.6%) had severe CD4 lymphocytopenia (<300cells/µl).
Figure 1.
Comparson of mean CD4 count between different tuberculosis group; TBL (Tuberculoous lymphadnitis), DTB (Dissiminated TB), PTB (Pulmonary TB) and healthy control (HC)
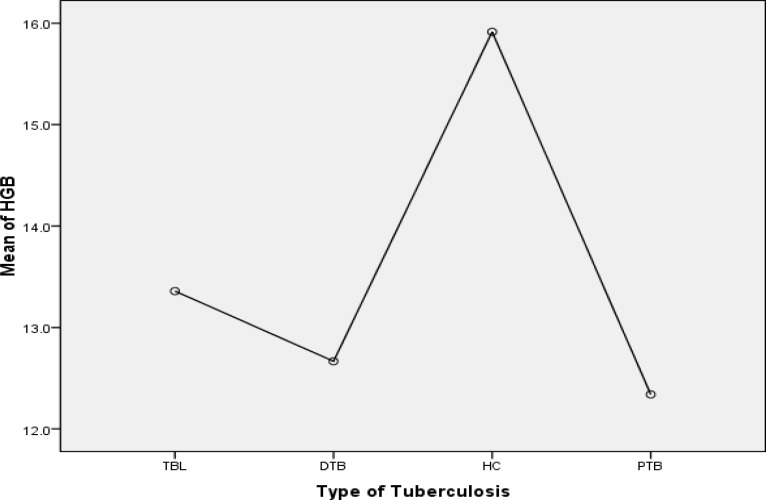
Mean ± SD of RBC counts, haemoglobin (Hgb), MCV, MCH and MCHC values of TB patients were significantly lower than those male and female healthy controls (p=0.001). Platelet counts and red cell distribution width (RDW) were significantly higher in both sexes among cases than controls (p=0.001) (Table 2). Further analysis by Post Hoc Tukey test showed mean Hgb values of all groups of TB patients were lower than those of control group (p=0.001). Mean Hgb values of PTB group were also lower than TBL (p=0.028) and no statistical significant difference between PTB and DTB cases were observed (p=0.941) (Fig. 2). From cases, 40(37.0%) were found anaemic of whom 38(95.0%) had moderate anaemia and 2(5.0%) severe anaemia. From these 40 anaemic cases, 13(32.5%) were among TBL, 5(12.5%) among DTB and 22(55.0%) among PTB. The RBC morphology and RBC indices in our study showed that 15(37.5%) of cases developed normocytic normochromic anaemia followed by normocytic hypochromic anaemia, 13(32.6%), (Table 3). From a total of 53(49.1%) thrombocytosis, PTB accounted for 26(49.1%), TBL for 21(39.6%) and DTB for 6(11.3%).
Figure. 2.
Comparson of mean Hemoglobine (Hgb) value between diffirent tuberculosis group; TBL (Tuberculous lymphadnitis), DTB (Dissiminated TB) PTB (Pulmonary TB) and healthy control (HC)
Table 3.
Classification of anemia, red cell morphology and indices among newly diagnosed TB patients
| MCHC(pg) | Total | |||
| Hypochromic | Normochromic | |||
| MCV (fl) | Microcytic | 8(20%) | 2(5%) | 10(25%) |
| Normocytic | 13(32.5 %) | 15(37.5%) | 28(70%) | |
| Macrocytic | 2(5%) | 0 | 2(5%) | |
| Total | 23(57.5%) | 17(42.5%) | 40(100%) | |
MCV= mean corpuscular volume, fl= femtoliter
Discussion
M. tuberculosis has a variety of surface molecules that interact with the innate host response. The interaction of the bacterial surface molecules along with the auto-regulation of the immune response by several mechanisms results in less optimal conditions for control of bacterial growth (4). M. tuberculosis is a classic example of a pathogen for which the protective response relies on cell mediated immunity. . Both CD4 and CD8 T-cells are important for successful immunity to TB (8).
In our finding, the mean CD4 count of both male and female TB patients was significantly lower than that of the respective healthy control. Absolute CD8 count has no significant difference between TB patients and healthy controls in both sexes. CD4/CD8 ratio in male TB patients was decreased relative to male healthy controls but in female TB patients, there is no significant difference in CD4/CD8 ratio with female healthy controls. The finding of our study is comparable to a study conducted in Pune, India, among 39 male patients who were HIV negative but PTB positive. The study showed that CD4 counts were significantly lower, CD8 values were normal in patients with PTB when compared with values obtained in normal blood donors. The CD4/CD8 ratio was significantly lower in patients with TB (9).
Another similar study conducted in Tan Tock Seng Hospital in Singapore on 60 patients showed that patients with TB had a lower median absolute lymphocyte count, lower CD8 count and a trend towards a lower CD4 count when compared to controls (10). A study done in Turkey on 75 active PTB patients has shown reduced percentage of circulating CD4 T cells and CD8 T cells compared with healthy individuals (11). A study from United States conducted on 85 HIV negative patients also reported comparable findings (12). The low mean CD4 count among TB patient than healthy controls in this study is also comparable with the finding of other studies (5, 10, 12–16).
In our study, the CD4 count of TB patients is compared against the type of TB. Lower CD4 count was recorded among cases of disseminated TB than tuberculous lymphadenitis but no significant difference was observed with PTB. Similar to study from Guangzhou Chest Hospital, China, our study revealed that CD4 count from patients of disseminated TB was lower than patients with PTB (16). But, a study done in Tehran showed that mean CD4 count of disseminated TB was lower than all other types of TB (15). In another study done on PTB in Turkey and in E. Tornu Hospital, Argentina, introduced that mean CD4 count significantly decreased in patients than control groups (11, 17). Similar with other previous studies (9, 17), the difference in mean CD8 count between the cases and control was not statistically significant in our finding.
Mean RBC counts, Hgb, MCV, MCH and MCHC values were significantly lower than the corresponding control group for both males and females. From 40(37%) study subjects who developed anaemia, 15(32.6%) had normocytic normochromic anaemia followed by 13(32.5%) those who normocytic hypochromic anaemia. Other similar studies have reported lower haemoglobin levels among TB patients (13, 18–21). However, study conducted on adult patients diagnosed with TB at Seoul National University Hospital, Korea among 880 patients, anaemia was identified in 281 patients (31.9%) at the time of diagnosis [19] which was lower than the present study. It also showed that Normocytic normochromic anaemia was most common and identified in 202 (71.9%) patients and followed by microcytic hypochromic anaemia 26(9.1%) (19). On the other hand, a study conducted in India reported normocytic normochromic anaemia as the most common abnormality observed in all cases, groups and subgroups (DTB/MTB 84%, PTB 86%) (18). The variation might be due to the difference in the stage of the disease during diagnosis, geographic, nutrition and other cultural differences that may directly or indirectly be related to anaemia.
The platelet counts of newly diagnosed TB patients among PTB patients were higher as compared to healthy controls. A study conducted at India Institute of Medical Sciences, New Delhi, on 32 DTB and 23 PTB indicated that thrombocytopenia was more common in patients with disseminated/miliary TB, whereas thrombocytosis was more common in patients with PTB (18). A study in Sao Paulo State University, Brazil, on 80 PTB patients revealed that platelet count values were higher in those with less clinical disease duration (22). This was, because of the fact that, at the beginning of the TB process, there was strong pro-inflammatory cytokine activity (IFN-γ & TNF-α) which stimulates expression of acute-phase proteins and thrombocytosis.
This study demonstrated that the mean CD4 count is significantly lower in newly diagnosed TB patients when compared with apparently healthy control for both male and female. The mean CD8 count is comparable among cases and control groups. Haematological parameters like RBC count, Hgb, MCV and MCHC were significantly lower when compared with healthy controls. Morphologically normocytic normochromic anaemia is a common haematological abnormality among TB patients. Increased thrombocytosis was observed among newly diagnosed treatment naive TB patients.
Our study was not without pitfalls. First, apparently healthy controls were recruited depending only from the blood bank information. Second, due to logistic reasons, in-depth characterization of immune cells to appreciate their functional status was not performed.
Acknowledgements
This study was funded by Jimma University, Collage of Public Health and Medical Sciences Research Program. We acknowledge the support of the Jimma University Specialized Hospital Laboratory and TB Clinic workers. The authors are thankful to all the study participants for their trust and collaboration.
References
- 1.WHO declares tuberculosis a global emergency. Sozial-und Präventivmedizin/ Social and Preventive Medicine. 1993;38(4):251–252. [PubMed] [Google Scholar]
- 2.WHO, author. Global Tuberculosis Control. 2011 [Google Scholar]
- 3.MOH, author. Monthly HIV Care and ART Update. Addis Ababa, Ethiopia: 2008. [Google Scholar]
- 4.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1(1):20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aska A, Al-Anazi AR, Al-Subaei SS, et al. CD4+ T-lymphopenia in HIV negative tuberculous patients at King Khalid University Hospital in Riyadh, Saudi Arabia. Eur J Med Res. 2011;16(6):285–288. doi: 10.1186/2047-783X-16-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu JYH, and PW E. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn J L, Flynn C J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Cooper A M. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uppal SS TS, Verma S, Dhot PS. Comparison of CD4 and CD8 Lymphocyte Counts in HIV-Negative Pulmonary TB Patients With Those in Normal Blood Donors and the Effect of Anti-tubercular Treatment: Hospital-Based Flow Cytometric Study. Cytometry Part B (Clinical Cytometry) 2004;61B:20–26. doi: 10.1002/cyto.b.20018. [DOI] [PubMed] [Google Scholar]
- 10.Villacian JSTG, Paton Teo LF, The NI. effect of infection with Mycobacterium tuberculosis on T-cell activation and proliferation in patients with and without HIV co-infection. J Infect. 2005;51(5):408–412. doi: 10.1016/j.jinf.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Deveci F, Akbulut H, Celik L, et al. Lymphocyte subpopulations in pulmonary tuberculosis patients. Mediators Inflamm. 2006;2:89070. doi: 10.1155/MI/2006/89070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones B E, Oo MM, Taikwel EK, et al. CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clin Infect Dis. 1997;24(5):988–991. doi: 10.1093/clinids/24.5.988. [DOI] [PubMed] [Google Scholar]
- 13.Kony SJ, Hanne AA, Larouze B, et al. Tuberculosis-associated severe CD4+ T-lymphocytopenia in HIV-seronegative patients from Dakar. SIDAK Research Group. J Infect. 2000;41(2):167–171. doi: 10.1053/jinf.2000.0721. [DOI] [PubMed] [Google Scholar]
- 14.Pessaran Z FF, Oreizi F, Ghavaminejad A, Kiani A, Siadat ZD. Immunophenotypic Characterization of Peripheral Blood T-Lymphocytes and Their Subpopulations in Tuberculosis Patients before and after Treatments. Iran J Allergy Asthma Immunol. 2005;4(1):23–26. [PubMed] [Google Scholar]
- 15.Davoudi S, Rasoolinegad M, Younesian M, et al. CD4+ cell counts in patients with different clinical manifestations of tuberculosis. Braz J Infect Dis. 2008;12(6):483–486. doi: 10.1590/s1413-86702008000600008. [DOI] [PubMed] [Google Scholar]
- 16.Li D X, Zhang TT, Tan SY, et al. The clinical significance of changes in CD4+ T cell counts by peripheral blood from patients with pulmonary tuberculosis after antitubercular treatment. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26(7):679–681. [PubMed] [Google Scholar]
- 17.Pilheu JA, De Salvo MC, Gonzalez J, et al. CD4+ T-lymphocytopenia in severe pulmonary tuberculosis without evidence of human immunodeficiency virus infection. Int J Tuberc Lung Dis. 1997;1(5):422–426. [PubMed] [Google Scholar]
- 18.Singh KJAG, Sharma SK, Saxena R, Chaudhary VP, Anant M. Significance of haematological manifestations in patients with tuberculosis. J Assoc Physicians India. 2001;49(788):790–794. [PubMed] [Google Scholar]
- 19.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028–1032. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Omar IAAARM, Shah AH. Hematological abnormalities in Saudis suffering from pulmonary tuberculosis and their response to the treatment. Research Journal of Pharmacology. 2009;3(4):78–85. [Google Scholar]
- 21.Saathoff EVE, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Anemia in adults with tuberculosis is associated with HIV and anthropometric status in Dar es Salaam, Tanzania. International Journal of Tuberculosis and Lung Disease. 2011;15(7):925–932. doi: 10.5588/ijtld.10.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliva V M, Cezario GAG, Cocato RA, Marchondes-Machado J, et al. Pulmonary tuberculosis: hematology, serum biochemistry and the relation with the disease duration. Journal of Venomous Animals and Toxins including Tropical Diseases. 2008;14:71–81. [Google Scholar]