Abstract
Cell cycle progression is regulated by members of the cyclin-dependent kinase (CDK), Polo and Aurora families of protein kinases. The levels of expression and localization of the key regulatory kinases are themselves subject to very tight control. There is increasing evidence that crosstalk between the mitotic kinases provides for an additional level of regulation. We have previously shown that Aurora B activates Polo kinase at the centromere in mitosis, and that the interaction between Polo and the chromosomal passenger complex (CPC) component INCENP is essential in this activation. In this report, we show that Polo kinase is required for the correct localization and activity of the CPC in meiosis and mitosis. Study of the phenotype of different polo allele combinations compared to the effect of chemical inhibition revealed significant differences in the localization and activity of the CPC in diploid tissues. Our results shed new light on the mechanisms that control the activity of Aurora B in meiosis and mitosis.
Keywords: chromosomal passenger complex, Aurora kinases, Polo-like kinases, mitosis, meiosis, Drosophila
2. Introduction
Cell cycle progression is regulated by reversible phosphorylation [1]. Protein kinases and phosphatases control the correct levels of phosphorylation of key substrates to ensure smooth progression of the cell cycle [1,2]. Frequently, these substrates are themselves kinases or phosphatases that form part of complex regulatory networks involving multiple feedback loops [1]. A group of highly conserved protein kinases is responsible for the overall control of these regulatory networks. This group includes the families of cyclin-dependent kinases (CDKs) [3], Aurora kinases [4,5] and Polo-like kinases (Plks) [6]. Misexpression of these protein kinases is linked to aneuploidy and carcinogenesis, making them very attractive targets for the development of new anti-cancer therapies [7–10]. The levels and activity of the master regulatory kinases must therefore be very tightly regulated.
Regulation of the mitotic kinases occurs at multiple levels including modulation of their expression, proteolysis and targeting to different subcellular locations. Additionally, their enzymatic activity is regulated by specific cofactors and by the level of phosphorylation of activation segments—either by autophosphorylation or by the action of other kinases and phosphatases (for reviews, see [5,6,11]). There is increasing evidence of crossregulation among CDKs, Polo-like and Aurora kinases. Polo-like kinase 1 (Plk1) modulates CDK1 activity through phosphorylation of several CDK1 regulators: promoting accumulation in the nucleus of Cdc25C [12], the degradation of Wee1 [13] and inhibition of Myt1 [14]. CDK1 acts as a priming kinase regulating the docking of Plk1 to its substrates (i.e. Bub1 [15] and BubR1 [16]). Aurora A and its interactor Bora are responsible for the activation of Plk1 at the centrosome at the G2–M transition [17,18] and in human cells contribute to its activation later in mitosis [19]. CDK1 phosphorylation of Bora enhances binding to Plk1 [20,21] and therefore promotes activation by Aurora A. Conversely, Plk1 regulates degradation of both Bora [18,21] and Aurora A [22]. We recently demonstrated that Aurora B is the kinase responsible for the activation of Polo kinase at the centromere [23] in mitosis, and that the chromosomal passenger complex (CPC) component INCENP is essential for this activation. Importantly, we showed that this regulatory mechanism plays an essential role in diploid tissues in vivo and not only in cultured aneuploid cell lines.
In this study, we have analysed the regulation of the CPC by Polo kinase in meiosis and mitosis in Drosophila. Using different combinations of polo mutant alleles, we show that Polo kinase is required for the correct localization and activity of the CPC at all stages of male meiosis as well as in larval neuroblast mitoses. This analysis reveals differences in the regulation of the centromeric localization of the CPC by Polo kinase between the two meiotic divisions. In addition, we show that chemical inhibition of Polo kinase activity in neuroblasts phenocopies the CPC defects in localization and activity observed in polo mutants. Interestingly, analysis of the neuroblast mitoses revealed significant differences between the phenotypes resulting from the depletion of Polo kinase protein in comparison with the inhibition of its kinase activity.
3. Material and methods
3.1. Drosophila strains
Fly strains were grown at 25°C in standard Drosophila medium. The following stocks were used: Canton-S. polo1/TM6C. w; polo9/TM6C. w; polo10/TM6C. Immunostaining of testes and third instar larval neuroblasts was performed as described previously [24]. For drug treatment, larval neuroblasts were dissected and treated with either dimethylsulfoxide (DMSO) or 100 nM BI 2536 for 2 h before being processed for immunostaining as described previously [23].
3.2. Antibodies
Primary antibodies and dilutions for immunofluorescence analysis were as follows: mouse monoclonal B512 anti-αTubulin (SIGMA, 1 : 2000); rabbit polyclonal anti-INCENP Rb-801, Rb-803 [25], 1 : 500; mouse monoclonal anti- Plk1T210Ph (Abcam ab39068, 1 : 100) and rabbit polyclonal anti-Histone3Ser10Ph (Upstate, 1 : 500). Secondary antibodies were obtained from Jackson Immunoresearch.
3.3. Drosophila cell culture, drug treatment and immunofluorescence
Drosophila cell lines were grown in Express-Five medium (GIBCO). The AC5-Polo-GFP cell line was described previously [26]. Cells exponentially growing were seeded on Con-A treated coverslips and treated with either DMSO or 100 nM BI 2536 for 2 h before being processed for immunostaining as described previously [23,25]. Imaging was performed using an Olympus IX-71 microscope controlled by Delta Vision SoftWorx (Applied Precision, Issequa, WA, USA). Image stacks were deconvolved, quick-projected, and saved as tiff images to be processed using Adobe PhotoShop.
Signal intensities were measured using the softWoRx Data Inspector tool; average background was subtracted; data were plotted using Prism software.
4. Results
4.1. Polo kinase is required for the correct localization of the chromosomal passenger complex in meiosis I
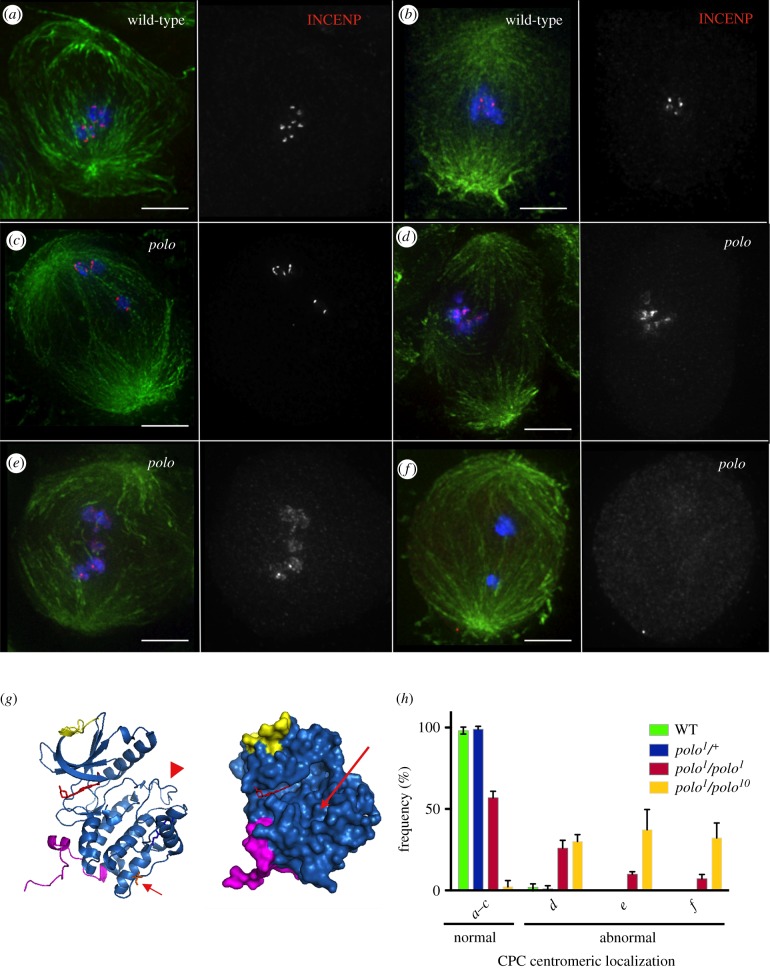
In order to investigate the role of Polo kinase in the regulation of the CPC in meiosis, we decided to study the distribution of the CPC component INCENP in Drosophila spermatogenesis in different polo mutant allelic combinations. In prometaphase and in metaphase I wild-type spermatocytes, the CPC concentrates at centromeres (figure 1a,b). The centromeric levels of the complex in metaphase I appear considerably reduced compared to prometaphase I.
Figure 1.
The CPC is mislocalized in polo mutant meiosis I. In wild-type spermatocytes the CPC concentrates at the centromeres in prometaphase I (a) and metaphase I (b). In all polo mutant combinations, we observe differences in the CPC localization in spermatocytes. The different phenotypic categories observed are shown in (c–f). These phenotypes range from normal localization (c), slight dispersion to surrounding heterochromatin (d), diffuse localization all over the chromatin (e) or reduction/absence of CPC signal (f). Green, α-tubulin; red, INCENP; blue, DNA; scale bars, 1 µm. (g) Predicted effect of the polo1 mutation on kinase function. Left, structure of human Plk1 kinase domain (PDB code 2OWB). Arrowhead points to the T-loop; arrow points to the residue mutated in polo1 (valine 242). Right, surface view of the kinase domain. Long arrow points to substrate-binding groove, formed by subdomains VIII and IX. (h) Quantification of the percentages of each phenotypic category (as shown in c–f) in the different polo mutant allelic combinations studied.
In polo mutants, we observe the following patterns of CPC localization (figure 1c–f): INCENP is found either normally concentrated at centromeres (figure 1c), slightly dispersed on the region surrounding the centromeres (figure 1d), completely dispersed all over the chromatin (figure 1e) or highly reduced/absent from chromatin (figure 1f). These phenotypic categories were quantified in different allelic combinations of polo mutations that result in a decrease of either the levels or the activity of the kinase.
The original polo1 mutation is a point mutation resulting in a substitution of valine 242 in the kinase domain by glutamic acid (figure 1g, left panel, arrow). Mapping this mutation in the structure of human PLK1 kinase domain (PDB code 2OWB; figure 1g, left, arrow) shows that this substitution would disrupt hydrophobic contacts with the first helix of kinase subdomain XI. We predict that such disruption may alter the relative positions of subdomains X, where valine 242 sits, and IX, which in turn would affect the substrate-binding groove, formed by subdomains VIII and IX (figure 1g, right, arrow). Subdomain VIII is also important for the stability of the kinase domain: first, via a nearly invariant ion pair with subdomain XI, secondly, by its direct interaction with the activation loop (figure 1g, left, arrowhead). As a result of this substitution, the Polo1 mutant kinase is predicted to have reduced enzymatic activity and be compromised in its substrate recognition. The expression levels of the mutant kinase are similar to wild-type [27]. By contrast, the polo9 and polo10 mutations are P-element insertions in the upstream regulatory region of the gene that result in a dramatic reduction of Polo expression levels [28].
Our analysis shows that the reduction in kinase activity caused by the polo1 mutation results in CPC localization defects (figure 1h). However in over 50% of homozygous polo1 mutant spermatocytes, the CPC appears normally localized at centromeres (in some instances even when bivalents are missegregating, figure 1c). A further reduction of protein levels in the polo1/polo10 (and polo1/polo9, data not shown) results in a significant increase in the percentage of spermatocytes showing abnormal CPC localization (figure 1h).
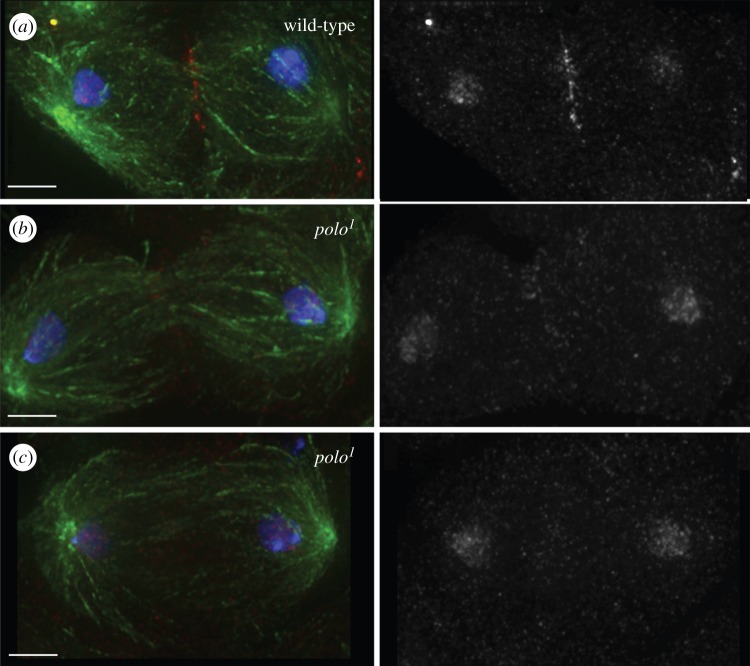
A considerable proportion (31%) of homozygous polo1 mutant cysts undergoing meiosis I shows defects in late anaphase and cytokinesis [24]. These include defects in the formation of both the spindle midzone and the contractile ring. As the CPC is critically involved in the regulation of events in late mitosis and cytokinesis, we wanted to find out if the phenotypes found in polo1 mutants are at least in part a consequence of a disruption of CPC localization or function. The dynamic localization of the CPC shows important differences in meiosis: at the metaphase I to anaphase I transition part of the CPC transfers to the central spindle as it does in mitosis, but a subset of the CPC remains associated with chromatin ([29] and figure 2). Analysis of late stages of meiosis I in polo1 mutants revealed that the CPC does not transfer to microtubules correctly at the metaphase to anaphase transition (figure 2b,c). This defective CPC localization is independent of the degree of disruption of the spindle midzone, as visualized by staining for microtubules (cf. figure 2b,c).
Figure 2.
The CPC is mislocalized in late meiosis I in polo1 mutants. (a) Wild-type telophase I spermatocyte showing correct transfer of the CPC to the central spindle. (b,c) polo1/polo1mutant spermatocyte showing defective CPC transfer to the midzone in the presence (b) or the absence (c) of normal central spindle. Green, tubulin; red, INCENP; blue, DNA; scale bars, 5 µm.
4.2. Polo kinase function is required for the correct localization of the chromosomal passenger complex in meiosis II
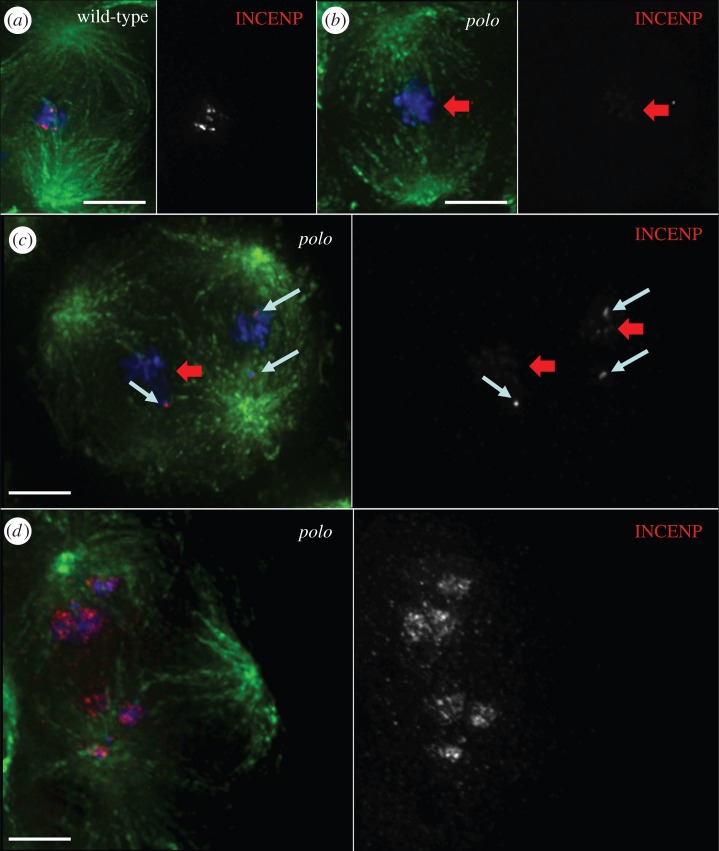
A small percentage of homozygous polo1 mutant spermatocytes complete meiosis I and proceed to meiosis II. Spermatocytes that fail cytokinesis in meiosis I form multipolar spindles in meiosis II (figure 3c,d). In wild-type spermatocytes, the localization of the CPC in prometaphase and metaphase II resembles that in the first meiotic division, with the CPC concentrating at centromeres (figure 3a). As spermatocytes within each cyst undergo meiosis in a slightly asynchronous way, we are able to observe consecutive stages of the meiotic division side by side in single cysts. In polo1 mutant cysts in prometaphase II, the CPC localizes properly to the centromeres of unaligned chromosomes (figure 3c, white arrows), but it is virtually undetectable on chromosomes aligned at the equatorial plate (figure 3c, red arrows). In these cells, once the spermatocytes reach metaphase II, the CPC is virtually undetectable on chromosomes (figure 3b). We conclude that Polo kinase activity is not required for the initial targeting and concentration of the CPC to the centromeres in meiosis II, but it is required for the stable localization of the complex, possibly in a tension-dependent manner. In some instances, we also observe the CPC dispersed all over the chromatin in prometaphase and metaphase II, similarly to what occurs in meiosis I (figure 3d).
Figure 3.
Tension-dependent CPC mislocalization in polo mutant meiosis II. In wild-type spermatocytes, the CPC concentrates at the centromeres in metaphase II (a). In polo mutant spermatocytes, we observe defects in CPC localization (b–d). In polo1/polo1 metaphase II spermatocytes the CPC is frequently absent from aligned chromosomes (b,c, red arrows) while it is normally concentrated at centromeres of unaligned chromosomes (c, white arrows). (d) In polo1/polo10 spermatocytes, the predominant phenotype is a diffuse localization of the CPC all over the chromatin. Green, α-tubulin; red, INCENP; blue, DNA; scale bars, 5 µm.
4.3. Polo kinase is required for the correct localization and activity of the chromosomal passenger complex in Drosophila larval neuroblast mitoses
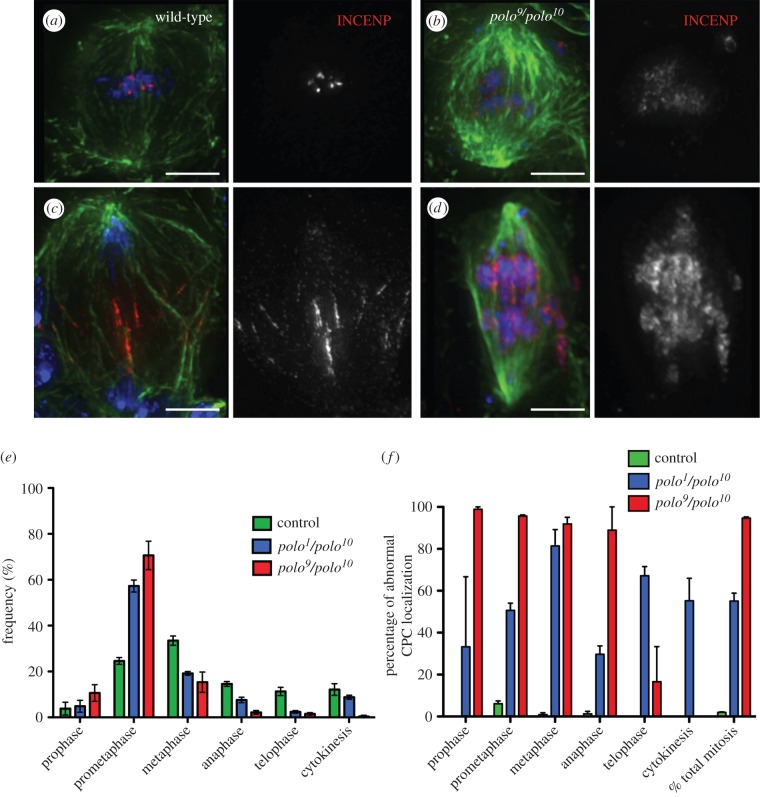
In order to analyse the consequences of further depletion of the Polo kinase protein levels in CPC function, we studied mitosis in polo9/polo10mutants. Flies carrying this allelic combination are late larval lethal. Thus, it is not possible to study meiotic phenotypes, since the testes are not yet mature. Instead we studied the mitotic phenotypes in third instar larval neuroblasts in polo mutants. Wild-type neuroblasts are actively dividing, with a mitotic index of 1.8 ± 0.21% (n = 1000 per experiment). The mitotic index is significantly higher in polo9/polo10and polo1/polo10 neuroblasts (26.7 ± 1.9% and 8.6 ± 0.26%, respectively, n = 1000 per experiment). In most mitotic figures analysed, the CPC shows an abnormal pattern of localization and is dispersed all over the chromatin (figure 4b,d; electronic supplementary material, figure S1).
Figure 4.
Localization of the CPC is abnormal in polo mutant neuroblast mitoses. In wild-type third instar larval neuroblasts, the CPC concentrates at the centromeres in metaphase (a) and transfers to the spindle microtubules and cortex in anaphase (c). In polo9/polo10 mutants, INCENP is dispersed on the chromatin in mitosis (b,d). Green, α-tubulin; red, INCENP; blue, DNA; scale bars, 5 µm. (e) Distribution of cells in the different stages of mitosis and cytokinesis. (f) Frequency of cells showing abnormal CPC localization in different stages of mitosis and cytokinesis (error bars = standard deviation from triplicate experiments).
Analysis of the distribution of mitotic phases shows a significant increase in the proportion of neuroblasts in prometaphase. This is accompanied by a decrease in the frequency of cells in later mitotic stages (up to 10 times less from 38% in wild-type to 3.9% in polo9/polo10, figure 4e). This distribution is a consequence of the characteristic prometaphase delay in polo mutants. Defects in CPC localization are observed in all stages of mitosis with high frequency, especially in polo9/polo10 where 95% of cells in mitosis show abnormal INCENP distribution (figure 4f). Compared to in polo9/polo10, in polo1/polo10 mutants there is a slightly higher frequency of neuroblasts in anaphase (figure 4e). In these cells, the CPC remains associated with segregating chromatids and does not transfer normally to the central spindle microtubules (electronic supplementary material, figure S1d), similarly to what we observe in meiosis I. These abnormal mitoses exhibit totally depleted or barely undetectable levels of PoloPh-Thr182, the active form of the kinase (electronic supplementary material, figure S2).
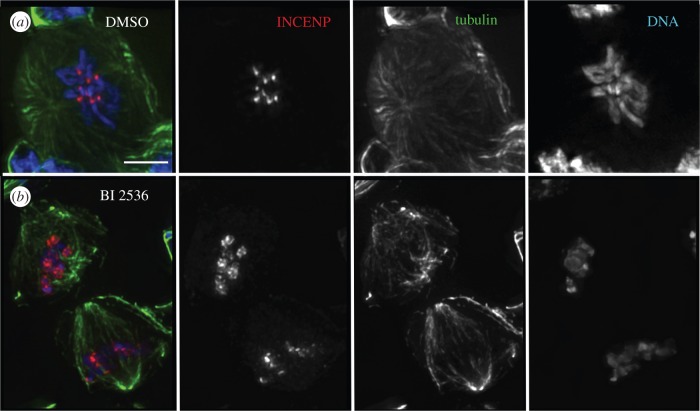
We next questioned whether the observed defects in CPC localization had an impact on the activity of Aurora B kinase. We monitored this by quantification of the levels of phosphorylation of the Aurora B substrate Histone3 Serine10—H3Ser10Ph (and H3Ser28Ph, data not shown). In order to assess the difference between the effects of depleting Polo protein levels in polo mutants versus those resulting from inhibition of its kinase activity (in which the protein is still present), we compared the levels of H3Ser10Ph in wild-type neuroblasts treated with the Plk1 inhibitor BI 2536 with those observed in neuroblasts from various combinations of polo mutants (figures 5 and 6; electronic supplementary material, figures S3 and S4). BI 2536 treatment of wild-type neuroblasts results in an elevated mitotic index compared to wild-type (18 ± 1.2%; n = 1000 per experiment). Drug treatment also phenocopies the CPC mislocalization phenotypes observed in polo mutants (figure 5). Thus, the phenotype observed after treatment with inhibitor has a much higher penetrance than that observed in polo1/polo1 neuroblasts (data not shown). This is most probably explained by the residual enzymatic activity in the Polo1 mutant kinase.
Figure 5.
Treatment with Plk1 inhibitor BI 2536 phenocopies polo mutant phenotype in neuroblast mitoses. (a) Control (DMSO-treated) third instar larval neuroblasts show normal localization of the CPC, whereas BI 2536-treated ones (b) show CPC mislocalization phenotypes similar to those observed in polo mutants. Green, α-tubulin; red, INCENP; blue, DNA; scale bars, 5 µm.
Figure 6.
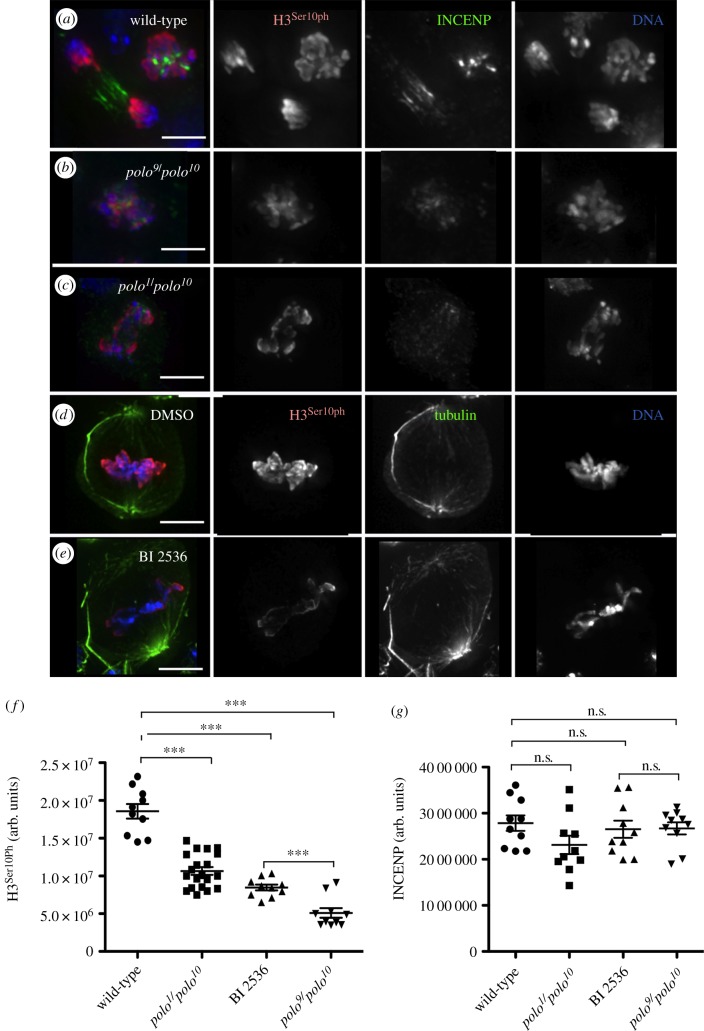
Aurora B activity is reduced in polo mutant and BI 2536-treated neuroblast mitoses. (a–c) Levels of phosphorylation of Histone3-Ser10 in neuroblast mitoses. (a) wild-type, (b) polo9/polo10mutants, (c) polo1/polo10 mutants. Green, INCENP; red, PhosphoHistone3-Ser10; blue, DNA. (d–e) Levels of phosphorylation of Histone3-Ser10 in DMSO (d) and BI 2536-treated (e) neuroblasts. Green, tubulin; red, PhosphoHistone3 Ser10; blue, DNA; scale bars, 5 µm. (f–g) Quantification of the levels of Histone 3-Ser10 phosphorylation (f) and INCENP (g) in polo mutant combinations and BI 2536-treated neuroblasts. In (f), t-test comparing wild-type with each of three experiments shows differences are significant (***p < 0.0001); difference between BI 2536-treated and polo9/polo10 is also significant (***p < 0.0003).
Although in all experimental conditions we observe a reduction of H3Ser10Ph levels compared to the levels of the wild-type (figure 6f), the biggest reduction is found in the polo9/polo10mutant (figure 6b). Interestingly, the level of phosphorylation in polo9/polo10 mutant neuroblasts is significantly lower than that in the BI 2536-treated neuroblasts (p = 0.0003, figure 6f; electronic supplementary material, figure S4). Thus, as far as the activity of Aurora B is concerned, the reduction of Polo protein levels in the mutant neuroblasts has a stronger effect than inhibition of Polo kinase activity.
4.4. Polo does not require its own kinase activity for localization to centromeres in Drosophila
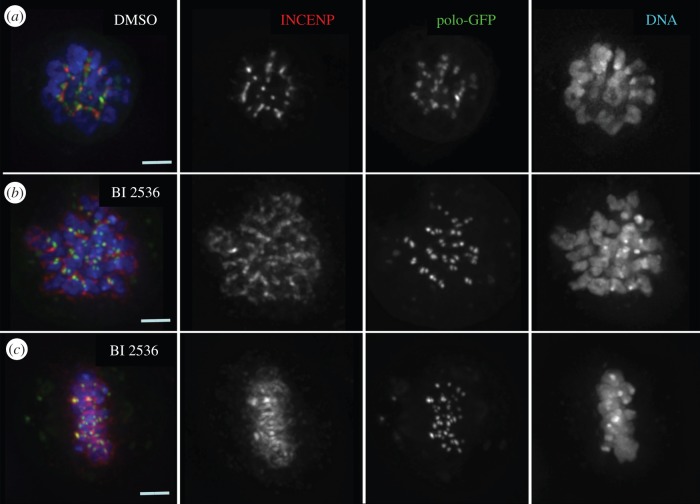
In order to understand better the different effects observed as a consequence of either lowering the levels of Polo or inhibiting its kinase activity, we analysed the localization of the kinase in DMel2 cultured cells treated with the BI 2536 inhibitor. Similarly to what we observed in dividing neuroblasts, the distribution of the CPC is abnormal in inhibitor-treated cells (figure 7). Interestingly, and in contrast to what has been described in human cells, Polo kinase localizes normally to the kinetochore in DMel2 cells (figure 7b,c).
Figure 7.
Polo kinase does not require its own kinase activity for kinetochore localization. D-Mel2 cells stably expressing Polo-GFP were treated with either DMSO (a) or the Plk1 inhibitor BI 2536 (b,c). Red, INCENP; green, Polo-GFP; blue, DNA; scale bars, 5 µm.
5. Discussion
Members of the CDK, Polo and Aurora kinase families are frequently involved in the regulation of the same cell cycle events, and even act upon the same substrates. Coordination of the activities of these highly conserved kinases is therefore essential for the smooth progression of cell division. One way that the cell accomplishes this coordination is by making the activity of one key regulatory kinase dependent on another one. For example, activation of CDK1 is accomplished by a bistable system that depends on feedback loops that both activate CDC25 and inactivate WEE1 (reviewed in [30]). Plk1 is responsible for the activation of this bistable switch [31]. In turn, CDK1 is frequently the priming kinase that allows binding of Plk1 to its substrates in mitosis through its polo-box domain [32]. In addition, the activation of Plk1 at the G2–M transition depends on Aurora A-Bora [17,18]. In a recent study, we reported that the activation of Polo kinase at the centromere depends on Aurora B kinase activity and is mediated by the CPC component INCENP [23].
Here, we show that the localization and activity of the CPC depends on Polo kinase in male meiosis and neuroblast mitosis in Drosophila. A similar dependence was reported previously in tissue culture cells [33]. Here for the first time, we were able to study the regulation of the CPC by Polo kinase in all phases of male meiosis. This was made possible thanks to the use of the weaker polo1 allele (either homozygous or in combination with stronger polo alleles). A recent study examining the effect of chemical inhibition of Polo kinase in Drosophila spermatocytes showed a much stronger phenotype than that of polo1 mutants [34]. In that study, BI 2536-treated spermatocytes were blocked in a prometaphase-like state with condensed bivalents that did not divide. Our analysis showed that Polo is essential not only for the correct centromeric localization of the CPC in both meiotic divisions but also for transfer of the CPC to the central spindle in anaphase. Similar phenotypes were also observed in neuroblast mitoses (figure 4; electronic supplementary material, figure S1).
Analysis of different combinations of polo alleles allowed us to compare the effect of depletion of Polo protein levels versus inhibition of its kinase activity on CPC localization and activity. Quantification of the different CPC localization phenotypes in meiosis I revealed that partial inhibition of the kinase activity in polo1 homozygotes resulted in a majority of spermatocytes showing mild (figure 1d) or no defects (figure 1c). Further decreasing the level of Polo kinase (polo1/polo10) resulted in a significant increase in the proportion of cells showing the most extreme CPC mislocalization phenotype, with the complex spread all over the chromatin. It is tempting to predict that if we could analyse the CPC meiotic localization in polo9 and polo10 mutants we would find an even higher proportion of this extreme phenotype. This was indeed the case when we analysed the mitotic phenotype in polo9/polo10 neuroblasts (see below).
Contrary to what was described for BI 2536-treated human cells [35], where cells are delayed in prophase with monopolar spindles, inhibitor-treated and polo mutant Drosophila neuroblasts form bipolar spindles but exhibit a prometaphase delay. This is true for all allelic combinations studied and also for BI 2536-treated neuroblasts (figure 5 and data not shown).
Altogether these results indicate a significant difference between the kinase inhibition and depletion phenotypes. Additionally, in Polo kinase depleted (polo9/polo10) neuroblasts 95% of the mitotic cells show the CPC dispersed all over the chromatin, a much higher proportion than is found in BI 2536-treated neuroblasts. As we have shown that inactive Polo kinase retains the ability to target to the centromere in Drosophila (figure 7), we propose that the mutant kinase could also retain some capacity to dock the CPC, thereby resulting in a relatively more stable centromeric localization of the complex.
Cytokinesis requires both Polo and Aurora B kinase function [6,11,36,37]. Plk1 is required for the regulation of cytokinesis in all species studied [11,24,38,39]. It has been proposed that Plk1 inhibitory phosphorylation of PRC1 prevents premature midzone assembly [40]. Although Plk1 is known to act in part through the activation of the GTPase RhoA at the actomyosin ring [39,41,42], the roles and substrates of Plk1 in cytokinesis are not completely characterized. The CPC regulates abscission, the last stage of cytokinesis [43–45]. Our results show that the CPC does not transfer to the central spindle normally in anaphase in both mitosis and meiosis of polo mutants. In flies and human cells, subito/MKLP2 binds to Aurora B and INCENP and is required for the correct localization of the CPC to the central spindle microtubules in anaphase [46,47]. Plk1 binds and phosphorylates MKLP2, negatively regulating its microtubule bundling activity. However, phosphorylation by Plk1 is not required for the localization of MKLP2 to the spindle midzone [46–48]. Our results indicate that if the CPC depends on Polo for its correct positioning at the spindle midzone, this must occur through an alternative pathway. Additionally, our results suggest that at least part of the Polo requirement in cytokinesis could be explained by its role in CPC localization and function. However, at this point we cannot exclude that the CPC mislocalization phenotype might be a secondary consequence of central spindle defects in the polo mutants.
The intricate web of interactions between CDK1, Polo and the CPC is critical throughout mitosis. Here, we have shown that not only do these kinases regulate one another by adjusting their activity levels, but they also have a role in regulating mitotic progression by ensuring that the kinases are active in the right place at the right time.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank David M. Glover and Pier Paolo D'Avino for providing reagents. We would also like to help Mrs Emma Peat for help with Drosophila crosses.
Funding statement
This work was funded by the Wellcome Trust, of which W.C.E. is a Principal Research Fellow (grant no. 073915). The Wellcome Trust Centre for Cell Biology is supported by core grant nos. 077707 and 092076.
References
- 1.Novak B, Kapuy O, Domingo-Sananes MR, Tyson JJ. 2010. Regulated protein kinases and phosphatases in cell cycle decisions. Curr. Opin. Cell Biol. 22, 801–808. (doi:10.1016/j.ceb.2010.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollen M, Gerlich DW, Lesage B. 2009. Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol. 19, 531–541. (doi:10.1016/j.tcb.2009.06.005) [DOI] [PubMed] [Google Scholar]
- 3.Malumbres M, Barbacid M. 2005. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641. (doi:10.1016/j.tibs.2005.09.005) [DOI] [PubMed] [Google Scholar]
- 4.Barr AR, Gergely F. 2007. Aurora-A: the maker and breaker of spindle poles. J. Cell Sci. 120, 2987–2996. (doi:10.1242/jcs.013136) [DOI] [PubMed] [Google Scholar]
- 5.Carmena M, Ruchaud S, Earnshaw WC. 2009. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 21, 796–805. (doi:10.1016/j.ceb.2009.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archambault V, Glover DM. 2009. Polo-like kinases: conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10, 265–275. (doi:10.1038/nrm2653) [DOI] [PubMed] [Google Scholar]
- 7.Taylor S, Peters JM. 2008. Polo and Aurora kinases: lessons derived from chemical biology. Curr. Opin. Cell Biol. 20, 77–84. (doi:10.1016/j.ceb.2007.11.008) [DOI] [PubMed] [Google Scholar]
- 8.Malumbres M, Barbacid M. 2009. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166. (doi:10.1038/nrc2602) [DOI] [PubMed] [Google Scholar]
- 9.Strebhardt K. 2010. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug. Discov. 9, 643–660. (doi:10.1038/nrd3184) [DOI] [PubMed] [Google Scholar]
- 10.Lens SM, Voest EE, Medema RH. 2010. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer 10, 825–841. (doi:10.1038/nrc2964) [DOI] [PubMed] [Google Scholar]
- 11.Petronczki M, Lenart P, Peters JM. 2008. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev. Cell 14, 646–659. (doi:10.1016/j.devcel.2008.04.014) [DOI] [PubMed] [Google Scholar]
- 12.Toyoshima-Morimoto F, Taniguchi E, Nishida E. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3, 341–348. (doi:10.1093/embo-reports/kvf069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl Acad. Sci. USA 102, 11 663–11 668. (doi:10.1073/pnas.0500410102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278, 25 277–25 280. (doi:10.1074/jbc.C300126200) [DOI] [PubMed] [Google Scholar]
- 15.Qi W, Tang Z, Yu H. 2006. Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol. Biol. Cell 17, 3705–3716. (doi:10.1091/mbc.E06-03-0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA. 2007. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 21, 2205–2219. (doi:10.1101/gad.436007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macurek L, et al. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455, 119–123. (doi:10.1038/nature07185) [DOI] [PubMed] [Google Scholar]
- 18.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. 2008. Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658. (doi:10.1126/science.1157425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruinsma W, Macurek L, Freire R, Lindqvist A, Medema RH. 2014. Bora and Aurora-A continue to activate Plk1 in mitosis. J. Cell Sci. 127, 801–811. (doi:10.1242/jcs.137216) [DOI] [PubMed] [Google Scholar]
- 20.Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA. 2006. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev. Cell 11, 147–157. (doi:10.1016/j.devcel.2006.06.002) [DOI] [PubMed] [Google Scholar]
- 21.Chan EH, Santamaria A, Sillje HH, Nigg EA. 2008. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma 117, 457–469. (doi:10.1007/s00412-008-0165-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Leuken R, Clijsters L, van Zon W, Lim D, Yao X, Wolthuis RM, Yaffe MB, Medema RH, van Vugt MATM. 2009. Polo-like kinase-1 controls Aurora A destruction by activating APC/C-Cdh1. PLoS ONE 4, e5282 (doi:10.1371/journal.pone.0005282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmena M, et al. 2012. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol. 10, e1001250 (doi:10.1371/journal.pbio.1001250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmena M, Riparbelli MG, Minestrini G, Tavares AM, Adams R, Callaini G, Glover DM. 1998. Drosophila polo kinase is required for cytokinesis. J. Cell Biol. 143, 659–671. (doi:10.1083/jcb.143.3.659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams RR, Maiato H, Earnshaw WC, Carmena M. 2001. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865–880. (doi:10.1083/jcb.153.4.865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Avino PP, Archambault V, Przewloka MR, Zhang W, Lilley KS, Laue E, Glover DM. 2007. Recruitment of Polo kinase to the spindle midzone during cytokinesis requires the Feo/Klp3A complex. PLoS ONE 2, e572 (doi:10.1371/journal.pone.0000572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavares AA, Glover DM, Sunkel CE. 1996. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J. 15, 4873–4883. [PMC free article] [PubMed] [Google Scholar]
- 28.Donaldson MM, Tavares AA, Ohkura H, Deak P, Glover DM. 2001. Metaphase arrest with centromere separation in polo mutants of Drosophila. J. Cell Biol. 153, 663–676. (doi:10.1083/jcb.153.4.663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick TD, Satinover DL, MacIsaac F, Stukenberg PT, Earnshaw WC, Orr-Weaver TL, Carmena M. 2006. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev. Cell 11, 57–68. (doi:10.1016/j.devcel.2006.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindqvist A, Rodriguez-Bravo V, Medema RH. 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185, 193–202. (doi:10.1083/jcb.200812045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobjois V, Jullien D, Bouche JP, Ducommun B. 2009. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim. Biophys. Acta 1793, 462–468. (doi:10.1016/j.bbamcr.2008.12.015) [DOI] [PubMed] [Google Scholar]
- 32.Park JE, Soung NK, Johmura Y, Kang YH, Liao C, Lee KH, Park CH, Nicklaus MC, Lee KS. 2010. Polo-box domain: a versatile mediator of polo-like kinase function. Cell Mol. Life Sci. 67, 1957–1970. (doi:10.1007/s00018-010-0279-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moutinho-Santos T, Conde C, Sunkel CE. 2012. POLO ensures chromosome bi-orientation by preventing and correcting erroneous chromosome-spindle attachments. J. Cell Sci. 125, 576–583. (doi:10.1242/jcs.092445) [DOI] [PubMed] [Google Scholar]
- 34.Riparbelli MG, Gottardo M, Glover DM, Callaini G. 2014. Inhibition of Polo kinase by BI2536 affects centriole separation during Drosophila male meiosis. Cell Cycle 13, 2064–2072. (doi:10.4161/cc.29083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters J-M. 2007. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315. (doi:10.1016/j.cub.2006.12.046) [DOI] [PubMed] [Google Scholar]
- 36.Carmena M. 2008. Cytokinesis: the final stop for the chromosomal passengers. Biochem. Soc. Trans. 36, 367–370. (doi:10.1042/BST0360367) [DOI] [PubMed] [Google Scholar]
- 37.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. 2012. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803. (doi:10.1038/nrm3474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkura H, Hagan IM, Glover DM. 1995. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9, 1059–1073. (doi:10.1101/gad.9.9.1059) [DOI] [PubMed] [Google Scholar]
- 39.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, Jallepalli PV. 2007. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl Acad. Sci. USA 104, 4383–4388. (doi:10.1073/pnas.0701140104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu CK, Ozlu N, Coughlin M, Steen JJ, Mitchison TJ. 2012. Plk1 negatively regulates PRC1 to prevent premature midzone formation before cytokinesis. Mol. Biol. Cell 23, 2702–2711. (doi:10.1091/mbc.E12-01-0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petronczki M, Glotzer M, Kraut N, Peters JM. 2007. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell 12, 713–725. (doi:10.1016/j.devcel.2007.03.013) [DOI] [PubMed] [Google Scholar]
- 42.Wolfe BA, Takaki T, Petronczki M, Glotzer M. 2009. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 7, e1000110 (doi:10.1371/journal.pbio.1000110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capalbo L, Montembault E, Takeda T, Bassi Z, Glover DM, D'Avino PP. 2012. The chromosomal passenger complex controls the function of ESCRT-III Snf7 proteins during cytokinesis. Open Biol. 2, 120070 (doi:10.1098/rsob.120070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. 2012. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science 336, 220–225. (doi:10.1126/science.1217180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmena M. 2012. Abscission checkpoint control: stuck in the middle with Aurora B. Open Biol. 2, 120095 (doi:10.1098/rsob.120095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166, 167–172. (doi:10.1083/jcb.200403084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cesario JM, Jang JK, Redding B, Shah N, Rahman T, McKim KS. 2006. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J. Cell Sci. 119, 4770–4780. (doi:10.1242/jcs.03235) [DOI] [PubMed] [Google Scholar]
- 48.Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Bar FA.2003. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863–875. (doi:10.1083/jcb.200306009) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.