Abstract
Objective
To conduct a systematic review and meta-analysis examining the effectiveness of behavioural interventions targeting diet, physical activity or smoking in low-income adults.
Design
Systematic review with random effects meta-analyses. Studies before 2006 were identified from a previously published systematic review (searching 1995–2006) with similar but broader inclusion criteria (including non-randomised controlled trials (RCTs)). Studies from 2006 to 2014 were identified from eight electronic databases using a similar search strategy.
Data sources
MEDLINE, EMBASE, PsycINFO, ASSIA, CINAHL, Cochrane Controlled Trials, Cochrane Systematic Review and DARE.
Eligibility criteria for selecting studies
RCTs and cluster RCTs published from 1995 to 2014; interventions targeting dietary, physical activity and smoking; low-income adults; reporting of behavioural outcomes.
Main outcome measures
Dietary, physical activity and smoking cessation behaviours.
Results
35 studies containing 45 interventions with 17 000 participants met inclusion criteria. At postintervention, effects were positive but small for diet (standardised mean difference (SMD) 0.22, 95% CI 0.14 to 0.29), physical activity (SMD 0.21, 95% CI 0.06 to 0.36) and smoking (relative risk (RR) of 1.59, 95% CI 1.34 to 1.89). Studies reporting follow-up results suggested that effects were maintained over time for diet (SMD 0.16, 95% CI 0.08 to 0.25) but not physical activity (SMD 0.17, 95% CI −0.02 to 0.37) or smoking (RR 1.11, 95% CI 0.93 to 1.34).
Conclusions
Behaviour change interventions for low-income groups had small positive effects on healthy eating, physical activity and smoking. Further work is needed to improve the effectiveness of behaviour change interventions for deprived populations.
Keywords: PUBLIC HEALTH, PREVENTIVE MEDICINE, SOCIAL MEDICINE
Strengths and limitations of this study.
This was a comprehensive systematic review with meta-analysis to examine the effects of behavioural interventions in a deprived proportion of the population, namely those with a low income.
We updated a previous review on this topic and focused exclusively on evidence from randomised controlled trials, which are often termed ‘the gold standard’ of research.
Applying meta-analysis enabled us to summarise the data quantitatively and estimate pooled effect sizes, which could be compared to those for interventions from other population groups.
We searched for studies where participants were described as ‘low income’ as this is a financially and socially relevant indicator of deprivation, but relevant papers not using this term may have been missed.
The majority of the studies were conducted in the USA, potentially limiting generalisability and did not tend to describe their intervention content comprehensively, making it difficult to further explore ‘what works’ for people with a low income.
Introduction
Health outcomes are strongly correlated with social position in societies across the western world: individuals from deprived backgrounds die younger and experience a greater proportion of their lives with a disability.1–5 In the most deprived areas of England, for example, life expectancy is approximately 8 years less, and disability-free life expectancy 15 years less than in the least deprived areas.1 Among several deprivation indicators, a person's individual or household income is widely recognised as being strongly positively correlated with health outcomes.3 The social gradient in health is predicted to steepen further2 despite policy efforts aimed at maximising equality.3–5
Behaviours linked to health, particularly healthy eating, physical activity and smoking, show a similar social gradient to health outcomes. Consumption of tobacco, a poor diet and a lack of physical activity are major risks to premature morbidity and mortality.6 7 People of lower socioeconomic status are more likely to smoke,5 be sedentary8 and eat a poor diet9 compared with those of higher socioeconomic status. These behaviours have been suggested as mediators of the link between social position and health outcomes.10–12
Changing health behaviours
Given the potential improvements that changes in behaviour can bring to health, health research and clinical practice devotes considerable time and effort to behavioural interventions. For instance, stopping smoking increases life expectancy at any age and halves the risk of cardiovascular disease within 1 year.13 Experts agree that major improvements in public health will be brought about through behaviour changes in the population.7 14 15 Targeting behaviour change efforts at people at the lower end of the income spectrum is seen as a major means to reducing health inequalities. Gruer et al (ref 12, p.5) for instance argued that “the scope for reducing health inequalities related to social position […] is limited unless many smokers in lower social positions can be enabled to stop smoking.”
Health behaviour change in low-income populations
Existing behaviour change support for those disadvantaged by income may not be fit for purpose.14 Evidence suggests that people from low-income groups are more difficult to identify and successfully recruit to general population interventions.16–18 Moreover, it has been suggested that low-income populations may achieve poorer behaviour change outcomes following interventions compared with more affluent participants, resulting in poorer health outcomes19–21 and potentially leading to intervention-generated inequalities.22
In studies targeted at the whole population rather than specific subgroups, Michie et al23 have argued that observed differences in outcomes between socioeconomic groups may reflect baseline differences in health behaviours, and that the interventions themselves may be effective across the socioeconomic spectrum. In their review of interventions targeted specifically at those disadvantaged by income, examining controlled studies (with or without random allocation) published between 1995 and 2006, they found 13 relevant studies with 17 available comparisons. Approximately half of interventions were reported as effective relative to controls, but no meta-analysis was performed to estimate an overall effect size. At present, there is a lack of evidence on the effectiveness of interventions specifically targeting health behaviour change in low-income individuals.24 25
The aim of the current systematic review is to build on Michie et al's23 work by (A) providing an updated review including studies published since 2006, (B) including only randomised controlled trials (RCTs) and (C) applying meta-analysis to estimate intervention effect sizes. We investigated whether studies of interventions targeted at participants from low-income groups are effective in changing diet, physical activity or smoking behaviour.
Methods
Eligibility criteria
A protocol for this review is not publicly available; however, this article does reflect the relevant components of the PRISMA checklist for the reporting of systematic reviews. The article was submitted with a copy of the checklist confirming this.
Studies included in this review had to meet the following inclusion criteria:
Population: Adults aged 18 years and over, of low income and from the general population. Studies were considered to target a low-income group if they explicitly referred to their participants as ‘low income’. General population was defined as not belonging to a specific clinical group, such as those with diabetes or cardiovascular disease. Pregnant and overweight individuals were not considered to belong to a clinical group and were therefore included.
Interventions: Interventions targeting a change in smoking, eating and/or physical activity behaviours. Studies could target a single behaviour or multiple behaviours in any combination.
Study design: Published RCTs and cluster RCTs (cRCTs). Control condition could be no intervention, a less intense intervention or an intervention with different content.
Outcomes: Behavioural outcomes relevant to smoking cessation, healthy eating and physical activity with no restrictions on length of follow-up. Self-reported individual-level behaviour, more ‘objective’ measures of behaviour and measures of behavioural change were all included, as in Michie et al.23 Studies were excluded if reported data were unsuitable for meta-analysis.
Date: 1995–2014: Studies published from 1995 to 2006 were identified by screening Michie et al,23 the primary search included studies published between January 2006 and July 2014. We chose to focus on studies published within the previous two decades to ensure relevance to current financial, social, health and healthcare climates.
Language: English language: in line with Michie et al's23 review.
Search strategy
We used studies from 1995 to 2006 which had been identified by Michie et al's23 review rather than running the search again because the previous review's search criteria were similar but broader than our own and should therefore include all articles relevant to the current review. Specific search strategies were created (see online supplementary file 1) to search for studies published since Michie et al's23 review of 1995–2006 papers. We searched eight databases: MEDLINE, EMBASE, PsycINFO, ASSIA, CINAHL, Cochrane Controlled Trials, Cochrane Systematic Reviews and DARE Electronic Databases. Search strategies were based on Michie et al23 and included three components: low-income population terms (eg, low-income, poverty, social class or socioeconomic status), terms for the three targeted health behaviours (eg, physical activity, diet, smoking cessation, lifestyle, health behaviour or weight reduction) and intervention-relevant terms (eg, behaviour/behaviour change, health program, intervention, health promotion or program evaluation). The specific strategies were iteratively created and tailored to each database's reference terms with an experienced NHS Clinical Librarian (PM). One author (ERB) initially ran the final searches on 1 December 2011 (January 2006–December 2011) and updated the search using the same search terms in the same databases on 10 July 2014 (December 2011–July 2014). In addition to the primary search, we checked the bibliography of each included study.
Study selection
One author (ERB) used the current review's inclusion criteria to screen the full texts of the 13 studies published between 1995 and 2006 included in Michie et al.23 For the studies published from 2006 onwards ERB, NM and SUD initially screened titles and abstracts, and obtained potentially relevant studies for full-text screening. If no abstract was available the full text was scanned at this first screening stage. If no full text was retrieved, or screening information was missing, ERB contacted the corresponding study author requesting further information. NM and ERB double screened a random sample of 10% of titles and abstracts from the studies from 2006 onwards which they had not previously screened (n=257), agreement with the primary screener was 96%. Later in the screening process, NM screened a random sample of 10% of full-text articles assessed (n=12), agreement was 92%. The small number of disagreements were resolved through discussion.
Data collection process
Data were extracted using a prespecified and piloted data extraction form based on Davidson et al's26 criteria, including study design, target behaviour, participants, recruitment strategies, intervention content and outcome data. Risk of bias in individual studies was assessed based on standard criteria adapted from Avenell et al.27 Where published online supplementary materials were available they were used to assist data extraction (these are referred to in online supplementary table S1), and if information was missing, the corresponding author was contacted. When interventions targeted more than one behaviour, then data were extracted for the different behaviours separately. ERB, SUD, NM and MJ jointly extracted the outcome data.
Data were extracted for all reported time points. The primary outcome was behaviour or behaviour change following the end of the intervention. For the dichotomous smoking outcomes proportions were extracted (eg, per cent of sample reporting smoking abstinence for the past 7 days). For continuous diet and physical activity outcomes means and SDs were extracted (eg, mean portions of fruit and vegetables consumed per week). Where there was a choice of outcome measures, the outcome chosen was the primary behavioural outcome measure specified by the authors, measured by the most objective means (eg, accelerometer data were preferred to self-reported minutes of physical activity) and adjusted for baseline differences if this had been seen as necessary by the authors.
Synthesis of results
Data from included studies were meta-analysed in RevMan (V.5.2) using random effect models. For outcomes where a reduction (eg, mean percentage calories in fat) signifies a change in a healthy direction, data were reverse-scored before being entered for meta-analysis. For continuous diet and physical activity outcomes, standardised mean differences (SMD) were calculated using Hedges’ g28 to express the difference between the means for the intervention and control groups in SD units. For dichotomous smoking outcomes, we calculated relative risk (RR) of smoking abstinence and applied the Cochran-Mantel-Haenszel test.29
Where studies had multiple comparisons (several intervention arms or reported outcomes for different behaviours) or were cRCTs, we adjusted participant numbers in line with Cochrane recommendations where possible.30 We conducted meta-analyses for the three behaviours separately at two time points: the most proximal time point postintervention and the longest follow-up time point where reported. A 95% CI was used and p<0.05 was taken as significant. We assessed variation in effect size between studies using the I2 statistic, with an I2 >50% interpreted as indicating the presence of heterogeneity.27 Following Cochrane Handbook recommendations,30 we compared independent subgroups of studies differing for two clinically relevant characteristics: interventions targeting women only versus a mixed sex sample, and interventions targeting a single behaviour versus multiple behaviours. Publication bias was assessed by visually inspecting funnel plots.
Results
Study selection
A flow diagram is presented in figure 1. We identified 3939 references from the database search (including the updated search: numbers for this search are given in figure 1) along with the 13 studies identified in Michie et al's23 review. After removing 1383 duplicates and excluding 2439 references on the basis of title and abstract screening 130 full texts were screened, of which 120 full texts were successfully retrieved, as 8 articles had no full text and 2 were irretrievable. Full-text screening initially led to the inclusion of 32 studies. Three further studies were identified from title screening reference sections, so that 35 studies with 45 comparisons met inclusion criteria.25 31–71
Figure 1.
Study selection flow diagram (italics signify numbers from July 2014 updated search).
Study characteristics
Participant identification and recruitment
Studies initially identified low-income participants through their place of residence (ie, living within an identified deprived area), by belonging to certain ethnic groups identified by the authors as suffering income inequality, being registered on a financial support programme, through belonging to a health clinic serving disadvantaged groups, by their employment (working in a manual workplace) or by an indicator of income (eg, quintile on the electoral role). Online supplementary table S1 describes how each study defined its study population as ‘low income’. Twenty-three studies reported having measured participants’ income as part of the study. Varying thresholds and income groupings were applied, but most commonly, incomes below US$15–US$20 000 (approximately £8840–11 800) per year were considered ‘low’ and most studies reported that the majority of participants were in this category. Of the remaining 12 studies, 8 recruited participants from financial support programmes which required beneficiaries’ earnings to be equivalent or near to official US poverty levels (which vary over time and depending on the individual's household size), 2 reported that the majority of participants held a manual, low wage occupation and the final 2 studies reported that participants’ neighbourhoods had a high proportion of residents living in poverty.
Following initial identification, participants were recruited through face-to-face contact, via letter, telephone, via media advertisement or most commonly a mixture of methods. Face-to-face opportunities described were door-to-door neighbourhood recruitment, organisation of a community health fair, invitation at medical or social services appointments, or through presentations at schools or other community groups. Telephone calls were usually a follow-up method of contact. Media advertisements included posters in community venues, newspaper, radio and television advertisements. In the majority of cases, it was the study investigators who initiated these recruitment activities. Timeframe of recruitment varied from 1 day to over 2 years. Techniques used to engage low-income groups in participating were poorly specified: those most commonly reported were offers of material incentives (eg, vouchers for signing up), prompts and cues (eg, a fridge magnet with the study telephone number) or social support to facilitate participation (eg, advising about crèche facilities).
Study design and participant characteristics
The characteristics of the 35 included studies are summarised in online supplementary table S1. The majority (k=30) were conducted in the USA; the remaining studies were from the UK (k=3), Australia (k=1) and Chile (k=1). Twenty-eight studies were RCTs; seven were cRCTs. Studies took place in community (k=22), healthcare (k=12) or workplace (k=1) settings. Seven studies tested a dietary intervention, 7 studies tested a physical activity intervention, 15 studies tested a smoking intervention, and the remaining 6 tested interventions for multiple behaviours (5 tested diet and physical activity interventions, 1 tested diet and smoking interventions). Three studies had multiple intervention arms for one behaviour. In total, this yielded 16 interventions for the dietary meta-analysis, 12 interventions for physical activity meta-analysis and 17 for smoking meta-analysis. Each study randomised between 27 and 2549 participants, yielding a total of exactly 17 000 participants across the 35 studies. Of the 34 studies specifying participants’ sex, 19 targeted women exclusively and no study sampled only men. Women formed 72.4% of all participants. Mean average age of participants was 38.6, this ranged from 22.0 to 66.2 across study subgroups.
Intervention content
The content of interventions varied from provision of tailored self-help materials, to individual counselling or group programmes, but was often complex and poorly described (see online supplementary table S1). Control groups in the intervention tended to receive usual care, a less intense version of the intervention or an inactive version (eg, non-tailored materials). Intervention duration varied from a single episode to 2 years; the mode duration was 3 months. The intervention facilitator was described in 18 studies. In 13 studies this was either a routine healthcare provider such as a nurse or general medical practitioner, or a ‘non-routine’ healthcare provider such as a psychologist, dietician or smoking counsellor. Of the remaining five studies, the facilitator was a peer educator in three studies and a study administrator in two.
Intervention outcomes
Twenty-one studies assessed the behavioural outcome using self-report; 14 studies included an objective measure relating to behaviour such as biochemically confirmed smoking cessation. For dietary interventions, the primary outcome was fruit and vegetables consumed, grams of fat, dietary risk assessment score (which estimates saturated fat and cholesterol intake) or calories from fat consumed per day. For physical activity, studies reported a wider range of outcomes including mean number of minutes or hours of moderate physical activity per week, metres walked in 6 min, or metabolic equivalent minutes of activity per week. Smoking studies reported the number of participants who were abstinent from smoking, such as for the past 7 days, postpartum or for the previous 6 months. Studies differed in the delay between end of the intervention and most proximal assessment: this ranged from a few hours up to 8 months. Fourteen studies included follow-up data beyond the end of intervention time point. Overall 19.8% participants did not complete final assessments.
Risk of bias within studies
Online supplementary table S2 details the risk of bias assessment of the included studies. Risk of bias was variable. The majority of studies did not describe random allocation concealment procedures, provided numbers but not reasons for dropouts, did not mention blinding of any party and stated having used intention-to-treat analyses. There is therefore some risk of bias particularly during randomisation and surrounding blinding.
Quantitative data synthesis: effectiveness of interventions
Diet
Study outcomes are included in online supplementary table S3. The 16 dietary interventions were found to have an SMD of 0.22 (95% CI 0.14 to 0.29, I2=48%; figure 2). Eight dietary interventions provided longer term follow-up data, for 6–12 months postbaseline with combined SMD of 0.16 (95% CI 0.08 to 0.25, I2=41%).
Figure 2.
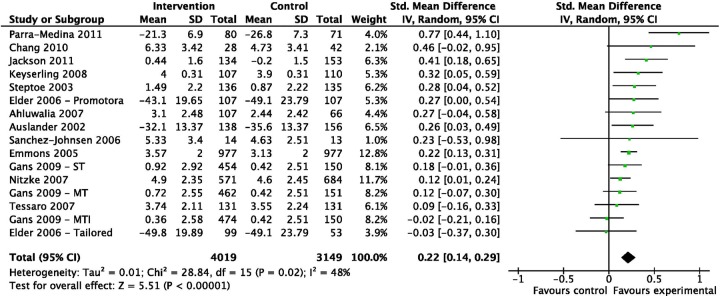
Standardised mean differences immediately postintervention for studies focusing on dietary change (ordered by effect size).
Physical activity
Twelve physical activity interventions yielded an SMD of 0.21 (95% CI 0.06 to 0.36, I2=76%; figure 3). Three interventions provided longer term follow-up data 6–8 months postbaseline with a combined SMD of 0.17 (95% CI −0.02 to 0.37, I2=0%).
Figure 3.
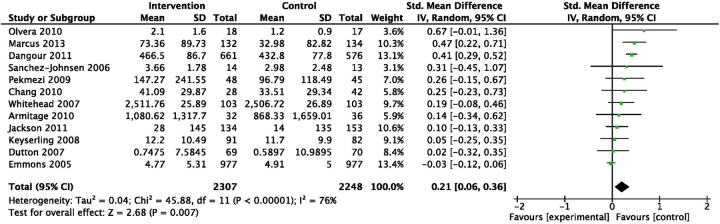
Standardised mean differences immediately postintervention for studies focusing on physical activity change (ordered by effect size).
Subgroup analyses for heterogeneity suggested SMDs were not different (p=0.48) in four interventions targeting women only (SMD 0.14, 95% CI 0.00 to 0.27, I2=0%) compared with eight with a mixed sex sample (SMD 0.24, 95% CI −0.02 to 0.49, I2=90%). Effects were larger (p<0.001) in seven interventions targeting physical activity only (SMD 0.32, 95% CI 0.18 to 0.45, I2=32%) than five interventions targeting multiple behaviours including physical activity (SMD 0.00, 95% CI −0.07 to 0.08, I2=0%).
Smoking
Seventeen smoking interventions were found to have a RR of smoking abstinence of 1.59 (95% CI 1.34 to 1.89, I2=40%; figure 4). Ten interventions provided longer term follow-up data for 3–12 months postbaseline. Positive intervention effects were not maintained; RR of smoking abstinence was 1.11 (95% CI 0.93 to 1.34, I2=15%).
Figure 4.
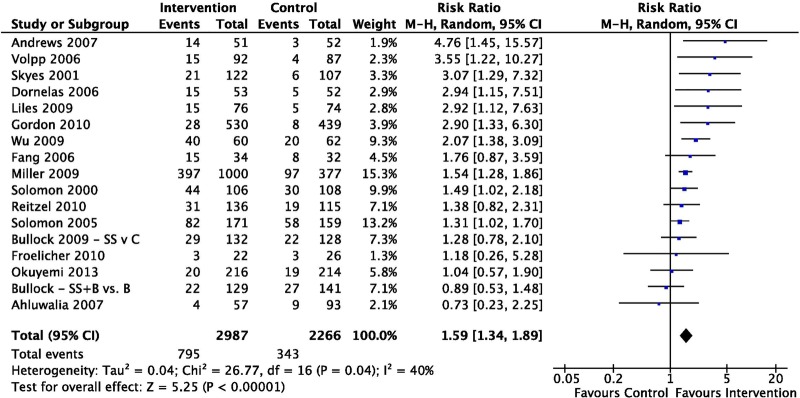
Relative risk of smoking abstinence immediately postintervention for studies focusing on smoking interventions (ordered by effect size).
Publication bias
Visual inspection of funnel plots showed little evidence of publication bias.
Discussion
Summary of evidence
We systematically reviewed the effectiveness of interventions targeted at changing the diet, physical activity or smoking of low-income groups. The review updates and extends a previous narrative review23 by including recently published studies; incorporating RCTs only and applying meta-analysis to examine intervention effect.
We identified 35 studies containing 45 dietary, physical activity and smoking interventions.25 31–71 Studies used a wide range of methods to identify and engage low-income participants. Most studies were conducted in the USA, contained mostly women and were often delivered by a healthcare professional. The quality of studies was variable with some risk of bias identified.
Our meta-analysis estimated a postintervention SMD of 0.22 for diet, 0.21 for physical activity interventions and a RR of smoking abstinence of 1.59 for smoking interventions. This means that the interventions had small positive effects on behaviour relative to controls.72 For studies reporting follow-up data, the small positive effects were maintained for diet (SMD 0.16) but not physical activity (SMD 0.17) or smoking cessation (RR 1.11). However long-term effects are based on a small subset of studies. Our exploration of the variation between physical activity interventions suggested that studies which focused on a single behaviour were more effective.
Implications of findings
We found small intervention effects on the behaviour of low-income groups compared with controls. For healthy eating, this was equivalent to intervention groups eating just under half a portion of fruit and vegetables more than controls each day. Similar reviews not targeting low-income participants tend to report larger effects: four such reviews targeting adults in the general population73–75 or obese adults with additional risk factors76 reported larger effects for diet (SMD 0.31),75 physical activity (SMD 0.28–0.32)73 75 76 and smoking (RR 2.17) interventions.74 Although true comparison is not possible unless the same interventions were compared in different population groups, this does suggest that interventions may be less effective for low-income populations. If other population groups benefit more from current interventions, even than those specifically targeted at low-income groups, then we can expect an overall gradual widening of health inequalities, as has been reported.2 Clearly research with more effective interventions is needed, including RCTs conducted in the UK, to increase our understanding of ‘what works’ for low-income groups.
Our analysis of the variation in physical activity studies showed a trend towards studies being more effective if they target a single behaviour than two behaviours. In addition, only one smoking study targeted both smoking and diet31 32 and this was the study with the lowest overall effect size. This resonates with the argument that human self-regulation draws on limited resources77 78 which may be best applied to one behaviour change target at a time. In contrast, physical activity studies including women only did not seem to vary widely in effectiveness from those with a mixed sex sample. Nevertheless there may be other unexplored sources of heterogeneity including other aspects of the delivery of interventions, such as those in the TIDIER checklist79 or use of techniques from the recently published Behaviour Change Technique taxonomy v1.80
Limitations
This study was a systematic but not exhaustive review, for instance not including informally published reports or ‘grey literature’, which tend not to be indexed within conventional databases. It limited its scope to RCTs and cluster RCTs to gather the highest quality evidence available, but some authors argue that reviewers should include less well-controlled studies because they often have enhanced external validity.81 In common with similar reviews82 methodological quality of studies was variable: for example, few studies blinded participants, facilitators or outcome assessors to treatment group. However, blinding of treatment condition in behavioural interventions is notoriously difficult: this is a criticism common to many similar reviews.83
Definitions of and thresholds for ‘low income’ varied somewhat between studies, reflecting the fact that there is no one agreed-on ‘cut-off’ for low income. We specified that the term ‘low income’ had to be used to refer to participants for studies to be included, since this is a relevant deprivation indicator in our financial and social context, perhaps more so than others such as education level. However, relevant papers not using this term may have been missed, particularly studies from some settings (eg, perhaps a church setting) where income may have been less likely to have been measured than others (eg, the workplace). Nevertheless, our review did identify studies using a wide range of concepts to target low socioeconomic status, such as area of residence, belonging to certain ethnic groups, belonging to a health clinic serving disadvantaged groups, as well as concepts directly linked to low income, such as indicator of income. Therefore, using the term ‘low income’ allowed us to implement a clear, objective and replicable criterion for including studies in the review, while also allowing us to capture studies considering low socioeconomic status in a variety of ways.
Additionally, the majority of studies were conducted in the USA, limiting generalisability to the UK context, although effect sizes for the UK studies fell within the typical range. Interventions were generally poorly specified. Categorisation or coding of control group content was not possible, even though studies show that this may vary substantially and influence intervention outcomes.84 Our review is also limited in scope to studies written in the English language. A final caveat for our findings is that while we excluded a study where the authors advised us that the data were zero-inflated,85 this may have been true of other studies.
Conclusions
This systematic review with meta-analysis of randomised controlled interventions to improve the diet, physical activity or smoking behaviour of low-income groups found small positive effects of interventions on behaviour compared with controls, which persisted over time only for diet. Despite research highlighting the urgent need for effective behaviour change support for people from low-income groups to assist in reducing health inequalities,10–12 this review suggests that our current interventions for low-income groups are positive, but small, risking ‘intervention-generated inequalities’.22 Policy makers and practitioners alike should seek improved interventions for disadvantaged populations to change health behaviours in the most vulnerable people and reduce health inequalities.
Supplementary Material
Acknowledgments
The authors are grateful for the assistance of Mr Paul Manson, NHS Grampian Clinical Librarian, in the design of search strategies. They would also like to sincerely thank Professor Susan Michie, University College London, Dr Linda Leighton-Beck, NHS Grampian Keep Well Programme Director and Mrs Dorothy Ross-Archer, NHS Grampian Keep Well Programme Manager. Finally, they are also very grateful to the study authors who kindly provided additional data or advice for the review.
Footnotes
Contributors: ERB and MJ had the original idea for the paper and designed the review method and analyses. ERB, SUD, NM and MJ participated in study selection and data extraction. ERB and SUD conducted statistical analysis. ERB, SUD, NM and MJ participated in writing the manuscript. ERB is the guarantor for the study.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ERB is an employee of NHS Grampian. SUD is an employee of University of Stirling. NM is a PhD student at the University of Aberdeen. MJ is an emeritus professor at of University of Aberdeen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Office for National Statistics. Inequality in Disability-free life expectancy by area deprivation: England, 2002–05 and 2006–09 2012. http://www.ons.gov.uk/ons/dcp171778_265133.pdf (accessed 14 Feb 2014).
- 2.Marmot M, Atkinson T, Bell J et al. Fair society, healthy lives: the Marmot Review: strategic review of health inequalities in England post-2010. London: The Marmot Review, 2010. [Google Scholar]
- 3.Adler N, Boyce W, Chesney M et al. Socioeconomic inequalities in health: no easy solution. J Am Med Assoc 1993;269:3140–5. [PubMed] [Google Scholar]
- 4.Department of Health. Choosing health. London: Stationery Office, 2004. (White paper) [Google Scholar]
- 5.Scottish Government. Equally well: report of the ministerial task force on health inequalities. Edinburgh: The Stationery Office, 2008. [Google Scholar]
- 6.Mokdad A, Marks J, Stroup D et al. Actual causes of death in the United States, 2000. JAMA 2004;291:1238–45. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organisation. The world health report 2002. Geneva: World Health Organisation, 2002. [Google Scholar]
- 8.Stamatakis E. Obesity, eating and physical activity. In: Bajekal M, Osborne V, Yar M, Meltzer M eds. Focus on health London. Office for National Statistics/Palgrave Macmillan, 2006:47–61. [Google Scholar]
- 9.Drewnowski A, Specter S. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004;79:6–16. [DOI] [PubMed] [Google Scholar]
- 10.Whitley E, Batty GD, Hunt K et al. The role of health behaviours across the life course in the socioeconomic patterning of all-cause mortality: the west of Scotland twenty-07 prospective cohort study. Ann Behav Med 2014;47:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart C, Gruer L, Watt G. Cause specific mortality, social position, and obesity among women who had never smoked: 28 year cohort study. BMJ 2011;342:d3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruer L, Hart CL, Gordon DS et al. Effect of tobacco smoking on survival of men and women by social position: a 28 year cohort study. BMJ 2009;338:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doll R, Peto R, Boreham J et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.House of lords: Science and technology select sub-committee. 2nd Report of session 2010–12: behaviour change. London: HMSO, 2011. [Google Scholar]
- 15.Wanless D. Securing good health for the whole population: final report. London: Stationery Office, 2004. [Google Scholar]
- 16.Anderson A. Dietary interventions in low-income women: issues for UK Policy. Nutr Bull 2007;32:15–20. [Google Scholar]
- 17.Marcus B, Williams D, Dubbert P et al. Physical activity intervention studies: what we know and what we need to know. A scientific statement from the American Heart Association council on nutrition, physical activity, and metabolism (subcommittee on physical activity); council on cardiovascular diseases in the young; and the interdisciplinary working group on quality of care and outcomes research. Circulation 2006;114:2739–52. [DOI] [PubMed] [Google Scholar]
- 18.Shah LM, Arora V, King A et al. The presence of tobacco cessation programs is not sufficient for low-income hospitalized smokers. Arch Intern Med 2009;169:902–3. [DOI] [PubMed] [Google Scholar]
- 19.Hiscock R, Judge K, Bauld L. Social inequalities in quitting smoking: what factors mediate the relationship between socioeconomic position and smoking cessation? J Public Health 2011;33:39–47. [DOI] [PubMed] [Google Scholar]
- 20.Niederdeppe J, Fiore MC, Baker TB et al. Smoking-cessation media campaigns and their effectiveness among socioeconomically advantaged and disadvantaged populations. Am J Public Health 2008;98:916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesterman J, Judge K, Bauld L et al. How effective are the English smoking treatment services in reaching disadvantaged smokers? Addiction 2005;100:36–45. [DOI] [PubMed] [Google Scholar]
- 22.White M, Adams J, Heywood P. How and why do interventions that increase health overall widen inequalities within populations? In: Barbones S. ed. Health, inequality and public health. Bristol: Policy Press, 2009:65–81. [Google Scholar]
- 23.Michie S, Jochelson K, Markham WA et al. Low-income groups and behaviour change interventions: a review of intervention content, effectiveness and theoretical frameworks. J Epidemiol Community Health 2009;63:610–22. [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Health and Clinical Excellence (NICE). Behaviour change at population, community and individual levels (Public Health Guidance 6). London: NICE, 2007. [Google Scholar]
- 25.Armitage CJ, Arden MA. A volitional help sheet to increase physical activity in people with low socioeconomic status: a randomised exploratory trial. Psychol Health 2010;25:1129–45. [DOI] [PubMed] [Google Scholar]
- 26.Davidson K, Goldstein M, Kaplan R et al. Evidence-based behavioural medicine: what is it and how do we achieve it? Ann Behav Med 2003;26:161–71. [DOI] [PubMed] [Google Scholar]
- 27.Avenell A, Broom J, Brown T et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8:1–182. [DOI] [PubMed] [Google Scholar]
- 28.Hedges L. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Behav Stat 1981;6:107–28. [Google Scholar]
- 29.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 30.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- 31.Ahluwalia JS, Nollen N, Kaur H et al. Pathways to health: cluster-randomized trial to increase fruit and vegetable consumption among smokers in public housing. Health Psychol 2007;26:214–21. [DOI] [PubMed] [Google Scholar]
- 32.Okuyemi KS, James AS, Mayo MS et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav 2007;34:43–54. [DOI] [PubMed] [Google Scholar]
- 33.Auslander W, Haire-Joshu D, Houston C et al. A controlled evaluation of staging dietary patterns to reduce the risk of diabetes in African-American women. Diabetes Care 2002;25:909–14. [DOI] [PubMed] [Google Scholar]
- 34.Chang MW, Nitzke S, Brown R. Design and outcomes of a mothers in motion behavioral intervention pilot study. J Nutr Educ Behav 2010;42(3 Suppl):S11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang MW, Brown R, Nitzke S. Participant recruitment and retention in a pilot program to prevent weight gain in low-income overweight and obese mothers. BMC Public Health 2009;9:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elder J, Ayala G, Campbell N et al. Long-term effects of a communication intervention for Spanish-dominant Latinas. Am J Prev Med 2006;31:159–66. [DOI] [PubMed] [Google Scholar]
- 37.Emmons K, Stoddard A, Flotcher R et al. Cancer prevention among working class, multiethnic adults: results of the healthy directions-health centers study. Am J Public Health 2005;95:1200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gans KM, Risica PM, Strolla LO et al. Effectiveness of different methods for delivering tailored nutrition education to low-income, ethnically diverse adults. Int J Behav Nutr Phys Act 2009;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson RA, Stotland NE, Caughey AB et al. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns 2011;83:203–9. [DOI] [PubMed] [Google Scholar]
- 40.Keyserling TC, Samuel Hodge CD, Jilcott SB et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: the North Carolina enhanced WISEWOMAN project. Prev Med 2008;46:499–510. [DOI] [PubMed] [Google Scholar]
- 41.Jilcott SB, Keyserling TC, Samuel-Hodge CD et al. Linking clinical care to community resources for cardiovascular disease prevention: the North Carolina Enhanced WISEWOMAN project. J Womens Health 2006;15:569–83. [DOI] [PubMed] [Google Scholar]
- 42.Nitzke S, Kritsch K, Boeckner L et al. A stage-tailored multi-modal intervention increases fruit and vegetable intakes of low-income young adults. Am J Health Promot 2007;22:6–14. [DOI] [PubMed] [Google Scholar]
- 43.Nitzke S, Kritsch K, Lohse B et al. Extension and research professionals join forces to address a critical nutrition issue. JOE 2004;42. [Google Scholar]
- 44.Parra-Medina D, Wilcox S, Salinas J et al. Results of the heart healthy and ethnically relevant lifestyle trial: a cardiovascular risk reduction intervention for African American women attending community health centers. Am J Public Health 2011;101:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Johnsen LA, Stolley MR, Fitzgibbon ML. Diet, physical activity, and breast health intervention for Latina women. Hispanic Health Care Int 2006;4:101–10. [Google Scholar]
- 46.Steptoe A, Perkins-Porras L, McKay C et al. Behavioural counselling to increase consumption of fruit and vegetables in low-income adults: randomised trial. BMJ 2003;326:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessaro I, Rye S, Parker L et al. Effectiveness of a nutrition intervention with rural low-income women. Am J Health Behav 2007;31:35–43. [DOI] [PubMed] [Google Scholar]
- 48.Dangour AD, Albala C, Allen E et al. Effect of a nutrition supplement and physical activity program on pneumonia and walking capacity in Chilean older people: a factorial cluster randomized trial. PLoS Med 2011;8:e1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dangour A, Albala C, Aedo C et al. A factorial-design cluster randomised controlled trial investigating the cost-effectiveness of a nutrition supplement and an exercise programme on pneumonia incidence, walking capacity, and body mass index in older people living in Santiago, Chile: the CENEX study protocol. Nutr J 2007;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutton GR, Davis Martin P, Welsch MA et al. Promoting physical activity for low-income minority women in primary care. Am J Health Behav 2007;31:622–31. [DOI] [PubMed] [Google Scholar]
- 51.Marcus BH, Dunsiger SI, Pekmezi DW et al. The seamos saludables study: a randomized controlled physical activity trial of Latinas. Am J Prev Med 2013;45:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olvera NN, Bush JA, Sharma SV et al. BOUNCE: a community-based mother-daughter healthy lifestyle intervention for low-income Latino families. Obesity 2010;18(Suppl 1):S102–4. [DOI] [PubMed] [Google Scholar]
- 53.Olvera NN, Knox B, Scherer R et al. A healthy lifestyle program for Latino daughters and mothers: the BOUNCE overview and process evaluation. Am J Health Ed 2008;39:283–95. [Google Scholar]
- 54.Pekmezi DW, Neighbors CJ, Lee CS et al. A culturally adapted physical activity intervention for Latinas: a randomized controlled trial. Am J Prev Med 2009;37:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitehead D, Bodenlos JS, Cowles ML et al. A stage-targeted physical activity intervention among a predominantly African-American low-income primary care population. Am J Health Promot 2007;21:160–3. [DOI] [PubMed] [Google Scholar]
- 56.Andrews JO, Felton G, Wewers ME et al. The effect of a multi-component smoking cessation intervention in African-American women residing in public housing. Res Nurs Health 2007;30:45–60. [DOI] [PubMed] [Google Scholar]
- 57.Andrews JO, Felton G, Wewers ME et al. Sister to sister: a pilot study to assist African American women in subsidized housing to quit smoking. South Online J Nurs Res 2005;6:2–23. [Google Scholar]
- 58.Bullock L, Everett KD, Mullen PD et al. Baby BEEP: a randomized controlled trial of nurses’ individualized social support for poor rural pregnant smokers. Matern Child Health J 2009;13:395–406. [DOI] [PubMed] [Google Scholar]
- 59.Dornelas EA, Magnavita J, Beazoglou T et al. Efficacy and cost-effectiveness of a clinic-based counseling intervention tested in an ethnically diverse sample of pregnant smokers. Patient Educ Couns 2006;64:342–9. [DOI] [PubMed] [Google Scholar]
- 60.Fang CY, Ma GX, Miller SM et al. A brief smoking cessation intervention for Chinese and Korean American smokers. Prev Med 2006;43:321–4. [DOI] [PubMed] [Google Scholar]
- 61.Froelicher ES, Doolan D, Yerger VB et al. Combining community participatory research with a randomized clinical trial: the protecting the hood against tobacco (PHAT) smoking cessation study. Heart Lung 2010;39:50–63. [DOI] [PubMed] [Google Scholar]
- 62.Gordon JS, Andrews JA, Albert DA et al. Tobacco cessation via public dental clinics: results of a randomized trial. Am J Public Health 2010;100:1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liles S, Hovell MF, Matt GE et al. Parent quit attempts after counseling to reduce children's secondhand smoke exposure and promote cessation: main and moderating relationships. Nicotine Tob Res 2009;11:1395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller CL, Sedivy V. Using a quitline plus low-cost nicotine replacement therapy to help disadvantaged smokers to quit. Tob Control 2009;18:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okuyemi KS, Goldade K, Whembolua GL et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108:1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reitzel LR, Vidrine JI, Businelle MS et al. Preventing postpartum smoking relapse among diverse low-income women: a randomized clinical trial. Nicotine Tob Res 2010;12:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solomon LJ, Scharoun GM, Flynn BS et al. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Prev Med 2000;31:68–74. [DOI] [PubMed] [Google Scholar]
- 68.Solomon LJ, Marcy TW, Howe KD et al. Does extended proactive telephone support increase smoking cessation among low-income women using nicotine patches? Prev Med 2005;40:306–13. [DOI] [PubMed] [Google Scholar]
- 69.Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self-help programme for smokers in London, UK. Health Promot Int 2001;16:255–60. [DOI] [PubMed] [Google Scholar]
- 70.Volpp KG, Gurmankin Levy A, Asch DA et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev 2006;15:12–18. [DOI] [PubMed] [Google Scholar]
- 71.Wu D, Ma GX, Zhou K et al. The effect of a culturally tailored smoking cessation for Chinese American smokers. Nicotine Tob Res 2009;11:1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 73.Foster C, Hillsdon M, Throrogood M et al. Interventions for promoting physical activity. Cochrane Database Syst Rev 2013;(1):14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemmens V, Oenema A, Knut I et al. Effectiveness of smoking cessation interventions among adults: a systematic review of reviews. Eur J Cancer Prev 2008;17:535–44. [DOI] [PubMed] [Google Scholar]
- 75.Michie S, Abraham C, Whittington C et al. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690–701. [DOI] [PubMed] [Google Scholar]
- 76.Dombrowski S, Sniehotta F, Avenell A et al. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev 2012;6:7–32. [Google Scholar]
- 77.Baumeister R, Bratslavsky E, Muraven M et al. Ego depletion: is the active self a limited resource? J Pers Soc Psychol 1998;74:1252–65. [DOI] [PubMed] [Google Scholar]
- 78.Vohs K, Heatherton T. Self-regulatory failure: a resource-depletion approach. Psychol Sci 2000;11:249–54. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann T, Glasziou P, Boutron I et al. Better reporting of interventions: the Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 80.Michie S, Richardson M, Johnston M et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. [DOI] [PubMed] [Google Scholar]
- 81.Glasgow R, Green L, Klesges L et al. External validity: we need to do more. Ann Behav Med 2006;31:105–8. [DOI] [PubMed] [Google Scholar]
- 82.Agency for Healthcare Research and Quality. Efficacy of interventions to modify dietary behavior related to cancer risk. Evidence Report/Technology Assessment No. 25 Rockville: Agency for Healthcare Research and Quality, 2000. [Google Scholar]
- 83.Boutron I, Moher D, Altman D et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–309. [DOI] [PubMed] [Google Scholar]
- 84.deBruin M, Viechtbauer W, Hospers HJ et al. Standard care quality determines treatment outcomes in control groups of HAART-Adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychol 2009;28:668–74. [DOI] [PubMed] [Google Scholar]
- 85.Hovell MF, Mulvihill M, Buono MJ et al. Culturally tailored aerobic exercise intervention for low-income Latinas. Am J Health Promot 2008;22:155–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



