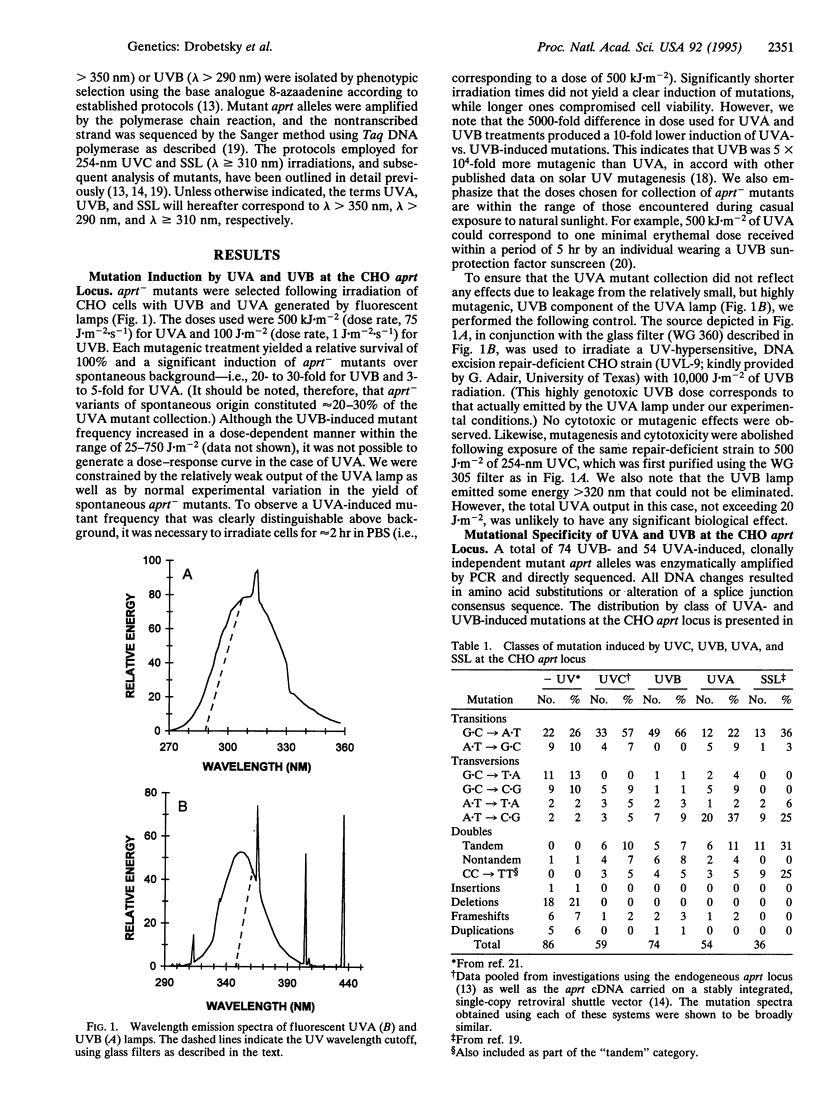
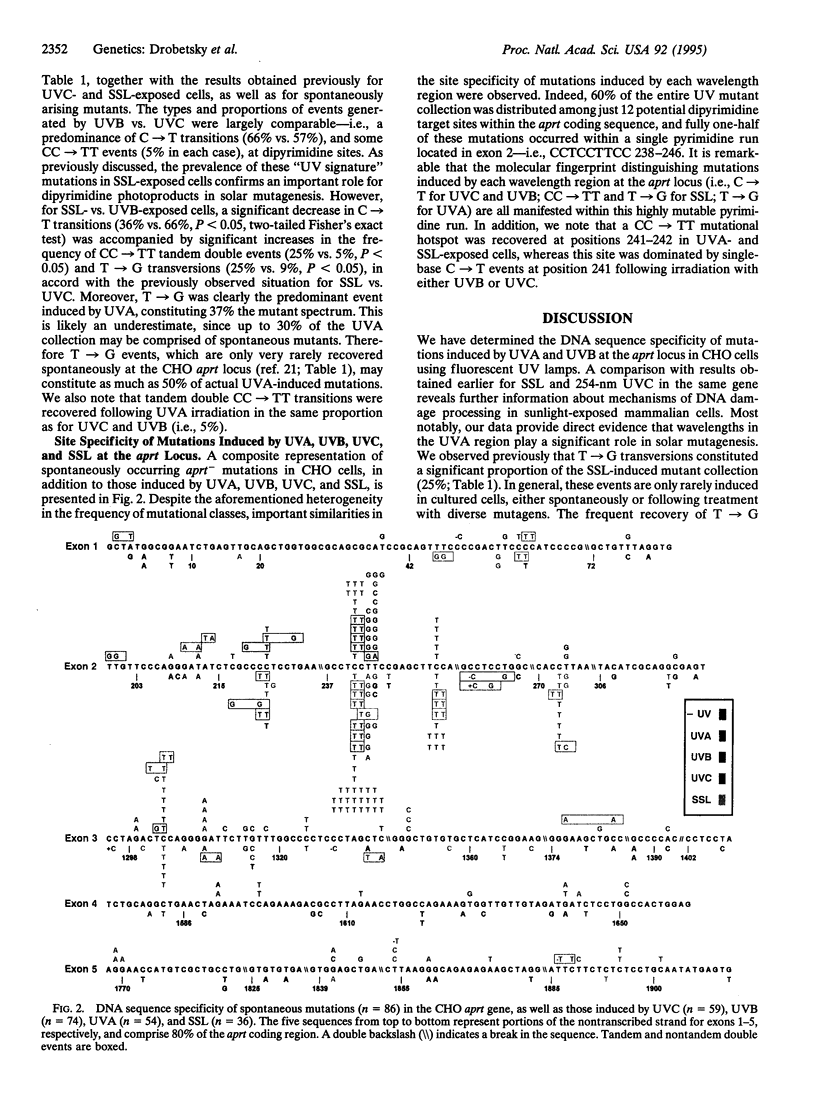
Abstract
It is well established that exposure to solar UVB (290-320 nm) gives rise to mutations in oncogenes and tumor suppressor genes that initiate the molecular cascade toward skin cancer. Although UVA (320-400 nm) has also been implicated in multistage photocarcinogenesis, its potential contribution to sunlight mutagenesis remains poorly characterized. We have determined the DNA sequence specificity of mutations induced by UVB (lambda > 290 nm), and by UVA (lambda > 350 nm), at the adenine phosphoribosyltransferase locus of Chinese hamster ovary cells. This has been compared to results previously obtained for stimulated sunlight (lambda > or = 310 nm) and 254-nm UVC in the same gene. We demonstrate that T-->G transversions, a generally rare class of mutation, are induced at high frequency (up to 50%) in UVA-exposed cells. Furthermore, this event comprises a substantial proportion of the simulated sunlight-induced mutant collection (25%) but is significantly less frequent (P < 0.05) in cells irradiated with either UVB (9%) or UVC (5%). We conclude that the mutagenic specificity of broad-spectrum solar light in rodent cells is not determined entirely by the UVB component and that UVA also plays an important role.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. D., Kunz B. A. Photoreactivation implicates cyclobutane dimers as the major promutagenic UVB lesions in yeast. Mutat Res. 1992 Jul;268(1):83–94. doi: 10.1016/0027-5107(92)90086-h. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruls W. A., Slaper H., van der Leun J. C., Berrens L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984 Oct;40(4):485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Glickman B. W. The specificity of UV-induced mutations at an endogenous locus in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Skandalis A., Glickman B. W. Perspectives on UV light mutagenesis: investigation of the CHO aprt gene carried on a retroviral shuttle vector. Somat Cell Mol Genet. 1989 Sep;15(5):401–409. doi: 10.1007/BF01534891. [DOI] [PubMed] [Google Scholar]
- Drobetsky E. A., Moustacchi E., Glickman B. W., Sage E. The mutational specificity of simulated sunlight at the aprt locus in rodent cells. Carcinogenesis. 1994 Aug;15(8):1577–1583. doi: 10.1093/carcin/15.8.1577. [DOI] [PubMed] [Google Scholar]
- Drobetsky E. A., Sage E. UV-induced G:C-->A:T transitions at the APRT locus of Chinese hamster ovary cells cluster at frequently damaged 5'-TCC-3' sequences. Mutat Res. 1993 Oct;289(2):131–138. doi: 10.1016/0027-5107(93)90062-k. [DOI] [PubMed] [Google Scholar]
- Freeman S. E., Gange R. W., Sutherland J. C., Matzinger E. A., Sutherland B. M. Production of pyrimidine dimers in DNA of human skin exposed in situ to UVA radiation. J Invest Dermatol. 1987 Apr;88(4):430–433. doi: 10.1111/1523-1747.ep12469778. [DOI] [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Quantitation of cyclobutane pyrimidine dimer formation in double- and single-stranded DNA fragments of defined sequence. Radiat Res. 1982 Jan;89(1):99–112. [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Amaudruz F., Tyrrell R. M. Determination of the spectrum of mutations induced by defined-wavelength solar UVB (313-nm) radiation in mammalian cells by use of a shuttle vector. Mol Cell Biol. 1988 Dec;8(12):5425–5431. doi: 10.1128/mcb.8.12.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. S., Haidle C. W., Robberson D. L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry. 1978 May 16;17(10):1890–1896. doi: 10.1021/bi00603a014. [DOI] [PubMed] [Google Scholar]
- Maki H., Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992 Jan 16;355(6357):273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- Phear G., Armstrong W., Meuth M. Molecular basis of spontaneous mutation at the aprt locus of hamster cells. J Mol Biol. 1989 Oct 20;209(4):577–582. doi: 10.1016/0022-2836(89)90595-0. [DOI] [PubMed] [Google Scholar]
- Reid T. M., Loeb L. A. Tandem double CC-->TT mutations are produced by reactive oxygen species. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3904–3907. doi: 10.1073/pnas.90.9.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E. Distribution and repair of photolesions in DNA: genetic consequences and the role of sequence context. Photochem Photobiol. 1993 Jan;57(1):163–174. doi: 10.1111/j.1751-1097.1993.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Grist E., Thompson K., Woodhead A. D. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Woodhead A. D. Temporal changes in the incidence of malignant melanoma: explanation from action spectra. Mutat Res. 1994 May 1;307(1):365–374. doi: 10.1016/0027-5107(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Sterenborg H. J., van der Leun J. C. Tumorigenesis by a long wavelength UV-A source. Photochem Photobiol. 1990 Mar;51(3):325–330. doi: 10.1111/j.1751-1097.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Tornaletti S., Pfeifer G. P. Slow repair of pyrimidine dimers at p53 mutation hotspots in skin cancer. Science. 1994 Mar 11;263(5152):1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- Tornaletti S., Rozek D., Pfeifer G. P. The distribution of UV photoproducts along the human p53 gene and its relation to mutations in skin cancer. Oncogene. 1993 Aug;8(8):2051–2057. [PubMed] [Google Scholar]
- Tyrrell R. M., Keyse S. M. New trends in photobiology. The interaction of UVA radiation with cultured cells. J Photochem Photobiol B. 1990 Mar;4(4):349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A., Leffell D. J., Kunala S., Sharma H. W., Gailani M., Simon J. A., Halperin A. J., Baden H. P., Shapiro P. E., Bale A. E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. G., Glickman B. W. Mutational analysis of the structure and function of the adenine phosphoribosyltransferase enzyme of Chinese hamster. J Mol Biol. 1991 Sep 5;221(1):163–174. doi: 10.1016/0022-2836(91)80212-d. [DOI] [PubMed] [Google Scholar]




