Abstract
Although it is now known that the human body is colonized by a wide variety of microbial populations in different parts (such as the mouth, pharynx and respiratory system, the skin, the gastro- and urogenital tracts), many effects of the complex interactions between the human host and microbial symbionts are still not completely understood. The dysbiosis of the gastrointestinal tract microbiota is considered to be one of the most important contributing factors in the development of many gastrointestinal diseases such as inflammatory bowel disease, irritable bowel syndrome and colorectal cancer, as well as systemic diseases like obesity, diabetes, atherosclerosis and non-alcoholic fatty liver disease. Fecal microbial transplantations appear to be promising therapies for dysbiosis-associated diseases; however, probiotic microorganisms have been growing in popularity due to increasing numbers of studies proving that certain strains present health promoting properties, among them the beneficial balance of the intestinal microbiota. Inflammatory bowel diseases and obesity are the pathologies in which there are more studies showing this beneficial association using animal models and even in human clinical trials. In this review, the association of the human gut microbiota and human health will be discussed along with the benefits that probiotics can confer on this symbiotic activity and on the prevention or treatment of associated diseases.
Keywords: Probiotics, Dysbiosis, Gut microbiota, Symbiosis, Treatment
Core tip: The human body is colonized by a wide variety of microorganisms that constantly interact with the host. The dysbiosis of the gut microbiota is considered to be one of the most important contributing factors in the development of gastrointestinal as well as systemic diseases. Many studies relate the health promoting properties of probiotic microorganisms with a beneficial balance of the host intestinal microbiota. In this review, the association of the human gut microbiota and human health will be discussed along with the benefits that probiotics can confer on this symbiotic activity and on the prevention or treatment of associated diseases.
INTRODUCTION
In February 2014, if one performed a MEDLINE search using the keywords “probiotics” crossed with “microbiota” or “microbiome”, the total hits would be over 1294 articles. From these, almost 75% (962) were published in the last 5 years (between 2009 and the beginning of 2014) showing that the association between probiotics and microbiota is not only recent but also is gaining the attention of scientists from around the worlds. The objective of this review is to give an overview of the most recent studies that have shown that the use of probiotics can modify the human microbiota and in turn can help in the prevention or treatment of a growing number of diseases that can be caused by a dysbiosis in the microbiota composition.
DEFINITIONS
Before going any further, it is important to clearly define the 2 terms that are going to be described in this review, probiotics and microbiota. The most commonly accepted definition of probiotics was published by the World Health Organization/Food and Agricultural Organization in 2001 that stated that probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit to the host”[1]. However, according to the International Scientific Association for Probiotics and Prebiotics (ISAPP), a non-profit scientific organization dedicated to advancing the science of probiotics and prebiotics, the term probiotic is commonly misused both commercially, when the term is featured on products with no substantiation of human health benefits, and scientifically, where the term has been used to describe bacterial components, dead bacteria or bacteria with uncharacterized health effects in humans (http://www.isapp.net/Portals/0/docs/ProbioticDefinitionClarification.pdf). The ISAPP does not provide a new definition for probiotics, it simply points out the important elements that are contained in the FAO/WHO definition. This being said, they clarify that a probiotic must: (1) be alive when administered; (2) have undergone controlled evaluation to document health benefits in the target host; (3) be a taxonomically defined microbe or combination of microbes (genus, species and strain level); and (4) be safe for its intended use.
Although the terms are sometimes used synonymously, “microbiome” and “microbiota” are terms that describe either the collective genomes of the microorganisms that reside in an environmental niche or the microorganisms themselves, respectively[2-4]. The term “microflora” is an equivalent term for “microbiota” that was used in the past and still appears in recent articles. The term “microbiota” is thus “the microscopic living organisms of a region” or “the microorganisms of a particular site, habitat, or geological period” according to the Dorland’s Medical Dictionary for Health Consumers (2007) and the Oxford Dictionary, respectively.
A keyword that has gained a lot of attention is “the human holobiont”. In this theory, humans did not evolve as a single species instead they evolved with a complex-associated microbiota, building a kind of “superorganism” or holobiont[5]. The human superorganism is a conglomerate of mammalian and microbial cells, with the latter estimated to outnumber the former by ten to one and the microbial genetic repertoire (microbiome) to be approximately 100-times greater than that of the human host[6]. The association between the host and its microbiota (also referred to as symbiote) provides a mutual beneficial relationship. It has recently been shown that the symbiote not only protects the host from pathogens but also decreases immune disorders by immunomodulation; while the host provides shelter and nutrients to the symbiote, the symbiote in turn also improve various body functions such as digestion to provide essential nutrients to the host[7].
Although it is now known that the human body is colonized by a wide variety of microbial populations in different parts of the human body (such as the mouth, pharynx and respiratory system, the skin, the gastro- and urogenital tracts), many effects of these complex interactions are still not completely understood. In this review, the association of the human gut microbiota and human health will be discussed along with the benefits that probiotics can confer on this symbiotic activity and on the prevention or treatment of associated diseases.
EFFECT OF THE HUMAN INTESTINAL MICROBIOTA ON HEALTH AND DISEASES
The human body has over 1014 microorganisms in the gastro-intestinal tract (GIT), literally 10 times more than the cells of the entire human body itself. In the past, it was thought that this microbiota was useful for the host because they could contribute nutrients and energy via the fermentation of non-digestible dietary components in the large intestine. Now, it is recognized that the microbiota is also extremely important to human health due to the emergence of studies that have shown that a dysbiosis of the GIT microbiota can cause diseases or that in certain diseases there is an observable change in the composition of this microbiota.
According to a recent review, a healthy microbiota is defined by high diversity and an ability to resist change under physiological stress; in contrast, microbiota associated with disease is defined by lower species diversity, fewer beneficial microbes and/or the presence of pathobionts[8]. In this review, diet-induced dysbiosis was described to be a contributing factor in the development of gastrointestinal diseases like inflammatory bowel disease, irritable bowel syndrome and colorectal cancer (CRC), as well as systemic diseases like obesity, diabetes, atherosclerosis and non-alcoholic fatty liver disease (NAFLD) (Figure 1).
Figure 1.
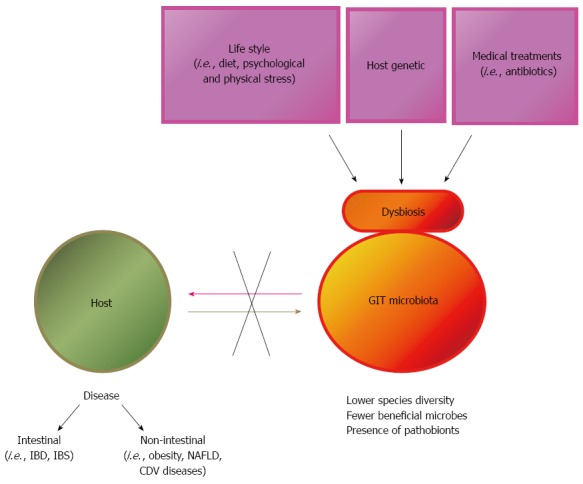
Causes of gastrointestinal tract microbiota dysbiosis and effect on host health. GIT: Gastrointestinal tract; NAFLD: Non-alcoholic fatty liver disease; IBD: Inflammatory bowel diseases; IBS: Irritable bowel syndrome; CDV: Canine distemper virus.
The close proximity of the GIT microbiota with the mucosa and gut lymphoid tissue helps explain why a balanced microbiota is likely to preserve mucosal health, whereas an unbalanced composition, as seen in dysbiosis, may increase the prevalence of diseases not only of the mucosa but also within the body due to the strong interactions with the gut immune system, the largest immune organ of the body[9]. Such abnormalities have been pinpointed as etiological factors in a wide range of diseases, including autoimmune disorders, allergy, irritable bowel syndrome, inflammatory bowel disease, obesity, and colon cancer. The intestinal mucosa is the body’s first line of defense against pathogenic and toxic invasions from food. After ingestion, orally administered antigens encounter the GALT (Gut Associated Lymphoid Tissue), which is a well-organized immune network that protects the host from pathogens and prevents ingested proteins from hyperstimulating the immune response through a mechanism called oral tolerance. The main mechanism of protection given by the GALT is humoral immune response mediated by secretory IgA (s-IgA) which prevents the entry of potentially harmful antigens, while also interacting with mucosal pathogens without potentiating damage. The stimulation of this immune response could thus be used to prevent certain infectious diseases that enter the host through the oral route. Numerous studies have shown that certain probiotic strains can increase s-IgA and modulate the production of cytokines (mediators produced by immune cells) that are involved in the regulation, activation, growth, and differentiation of immune cells and have recently been reviewed[10].
Inflammatory bowel diseases (IBD), such as Crohn’s disease (CD), ulcerative colitis (UC) or irritable bowel syndrome (IBS) can arise from the disruption of immune tolerance to the gut commensal microbiota, leading to chronic intestinal inflammation and mucosal damage in genetically predisposed hosts[11,12]. The gut microbiota composition and activity of IBD patients are abnormal, with a decreased prevalence of dominant members of the human commensal microbiota (i.e., Clostridium IXa and IV groups, Bacteroides, Bifidobacteria) and a concomitant increase in detrimental bacteria (i.e., Sulphate-reducing bacteria, Escherichia coli)[13]. Enterobacteria and Bacteroides species have been implicated as important factors in the observed dysbiosis and in the development and recurrence of IBD[14]. The observed dysbiosis is concomitant with defective innate immunity and bacterial killing (i.e., reduced mucosal defensins and IgA, malfunctioning phagocytosis) and overaggressive adaptive immune response (due to ineffective regulatory T cells and antigen presenting cells), which are considered the basis of IBD pathogenesis.
Changes in the equilibrium of the intestinal microbiota were also associated to the presence of CRC. A comparative study of the stool microbioma of healthy individuals and CRC patients showed that butyrate-producing bacterial species were under-represented in the CRC samples and this finding was correlated with proportionately lower amounts of butyrate and higher concentrations of acetate in stools of CRC patients, compared to the healthy individuals[15]. These results agree with the conception that butyrate is a microbial metabolite reported to have anti-tumorigenic effects, which were associated to the decrease of colonic inflammation, the reinforcement of the colonic barrier and the decreasing of oxidative stress[16]. Similar results were recently observed using a 1,2-dimethylhydrazine (DMH)-induced colon cancer model in rats. The animals from tumour group showed reduction of butyrate-producing bacteria such as Roseburia and Eubacterium in the gut microbiota. This experimental work also showed that DMH-induced carcinogenesis was associated to decrease of other beneficial species such as Ruminococcus and Lactobacillus in the gut microbiota of the rats[17]. New studies continue to show the differences in the intestinal microbiota between healthy individual and CRC patients. In this sense, it was described that a reduction of biodiversity and richness of microbial community, with increases of bacteroides was associated with colon cancer[18]. The analysis of the exact mechanisms by which these changes in the intestinal microbiota can be related to colon carcinogenesis are largely unknown. It was demonstrated that in CRC patients, in addition to the modification of intestinal metabolites, changes in the intestinal microbiota influence the host’s immune response. In this sense, it was demonstrated that IL-17C has an important role in microbiota-mediated tumorigenesis[19]. IL-17C was upregulated in human CRC samples and also in mouse models of CRC. IL-17C was induced in the intestinal epithelial cells by the dysregulated microbiota and promoted the survival of these cells, contributing to the tumorogenesis.
A detailed microbiota analysis of a well-characterized cohort of infants with food allergy (FA) showed that dysbiosis of fecal microbiota with several FA-associated key phylotypes, but not the overall microbiota diversity, may play a pathogenic role in FA[20]. In this study, the proportion of abundant Bacteroidetes, Proteobacteria, and Actinobacteria phyla were significantly reduced, while the Firmicutes phylum was highly enriched in the FA group.
Recent studies have suggested that an imbalance of the intestinal microbiota may be involved in the development of obesity and type 2 diabetes mellitus (T2DM). In a recent review it was stated that a high-fat diet may induce dysbiosis, which can result in a low-grade inflammatory state, obesity and other metabolic disorders and that modifying this diet can play a role in T2DM management due to positive intestinal microbiota modulation[21]. Also, a metagenome-wide association study analysis showed that patients with type 2 diabetes were characterized by a moderate degree of gut microbial dysbiosis, a decrease in the abundance of some universal butyrate-producing bacteria and an increase in various opportunistic pathogens, as well as an enrichment of other microbial functions conferring sulphate reduction and oxidative stress resistance[22].
In another study, it was suggested that the obesity epidemic in the United States may be partly driven by the mass exposure of Americans to foods containing low-residue antimicrobial agents that can alter the composition of the gut microbiota[23]. Studies that link microbiome modifying early life events to subsequent obesity risk provide some indirect evidence to support a causal role for gut microbiota in the pathogenesis of obesity[24]. Published data have proposed that dysbiosis of gut microbiota (at phyla, genus, or species level) affects host metabolism and energy storage stating that among the mechanisms involved, metabolic endotoxemia (higher plasma LPS levels), gut permeability and the modulation of gut peptides (GLP-1 and GLP-2) have been proposed as putative targets[25]. The mechanisms by which the gut microbiota affects metabolic disorders such as obesity, diabetes, and cardiovascular diseases have been proposed to be by two major routes: (1) the innate immune response to the structural components of bacteria [e.g., lipopolysaccharide (LPS)] resulting in inflammation; and (2) bacterial metabolites of dietary compounds (e.g., SCFA from fiber), which have biological activities that regulate host functions[26]. The concept of crosstalk, the biochemical exchange between host and microbiota, is also important to understand obesity since it maintains the metabolic health of the superorganism and whose dysregulation is a hallmark of the obese state[27].
Since the GIT and liver are connected by the portal venous system, the liver is thus more vulnerable to translocation of bacteria, bacterial products, endotoxin or secreted cytokines present in the GIT[8]. An obesogenic microbiota can alternate liver function by stimulating hepatic triglyceride and modulating systemic lipid metabolism that indirectly impact the storage of fatty acids in the liver[28]. A recent systematic database search was conducted and demonstrated that common mechanisms are involved in many of the local and systemic manifestations of NAFLD that can lead to an increased cardiovascular risk, and IBS, leading to microbial dysbiosis, impaired intestinal barrier and altered intestinal motility[29].
Studies in patients and animal disease models are shedding new light on the critical roles of the microbiota, metabolome and host responses in primary and recurrent Clostridium difficile (C. difficile) infection (CDI), which is the leading cause of antibiotic-associated diarrhea and pseudomembranous colitis in the healthcare setting[30]. In a recent study, culture-independent pyrosequencing was used to compare the distal gut microbiota for individuals with CDI, subjects with C. difficile-negative nosocomial diarrhea (CDN), and healthy control subjects[31]. This genomic analysis revealed significant alterations of organism lineages in both the CDI and CDN groups, which were accompanied by marked decreases in microbial diversity and species richness driven primarily by a paucity of phylotypes within the Firmicutes phylum. Normally abundant gut commensal organisms, including the Ruminococcaceae and Lachnospiraceae families and butyrate-producing C2 to C4 anaerobic fermenters, were significantly depleted in the CDI and CDN groups.
These examples of the effects of microbiota dysbiosis are just a few of the most recent studies published on the subject and show the immense lack of knowledge of the effect of the holobiont on human health. Correcting this dysbiosis is now the aim of many groups due to the diverse diseases that are directly or indirectly associated with this imbalance of the symbiotic microbiota.
PROS AND CONS OF FECAL TRANSPLANTATION ON THE INTESTINAL MICROBIOTA AND DISEASE
Fecal transplantation and synthetic microbiome transplants are being considered as promising therapies for dysbiosis-associated diseases.
Fecal microbial transplantation (FMT) is the process of transplantation of fecal bacteria from a healthy donor into a host with disease. Clinical criteria for inclusion and exclusion of both donor and recipient should be performed to limit the risk associated to this procedure and increase the chances of success[32]. Fecal transplantation represents a therapy with a high potential of success, and has been mostly studied in the treatment of chronic gastrointestinal infections[33]. The effectiveness of FMT was remarkable for recurrent C. difficile infection. Recently, it was reported that FMT was effective to improve clinical symptoms and eliminated fecal C. difficile toxins in a study of 27 patients with recurrent C. difficile infection who were given a single session of FMT[34]. This effect was associated to increased microbial diversity in all the patients and the effectiveness was also associated to the correction of the metabolism of bile salts that is disrupted in patients with recurrent C. difficile infection[35].
Considering that microbial dysbosis is associated to many intestinal and non-intestinal diseases, FMT was considered for treatment of different disorders, including IBS, IBD, insulin resistance, multiple sclerosis, obesity, and heart diseases[36]. However, its use remains controversial in patients with IBD[37]. There is a study showing the safety and positive clinical response after FMT in children and young adults with UC[38]. A totally different response was also reported where the case of a patient with UC (quiescent for more than 20 years) who was treated with FMT for a C. difficile infection and developed a flare of UC, indicating the need to be cautious in the use of this procedure in patients with IBD[39]. It was also suggested the value of characterizing not only the composition but also the temporal dynamics of the microbiota for a better understanding of FMT efficacy in the treatment of UC[40].
The current knowledge shows that FMT has a high potential to be used[41], but controlled trials of FMT in specific disorders and complemented by animal models of fecal transplantation, in which variables can be controlled and manipulated, are needed before FMT can be more accepted and applied clinically. Concerns over donor-derived infections (especially viral infection that are not normally detected) also exist, and it is difficult to quantify the true risk. The possibility to modify the transplantation of whole microbial communities from a healthy donor stool by another methodology has also recently been suggested in which specific fecal microorganisms grown in vitro could afterwards be transplanted[42]. The discovery of these commensal microorganisms will lead to the development of new probiotics that can replace FMT as applied today.
EFFECT OF PROBIOTIC ADMINISTRATION ON THE INTESTINAL MICROBIOTA AND DISEASE
Probiotic microorganisms have been growing in popularity due to increasing numbers of studies proving that certain strains present health promoting properties, among them the beneficial balance of the intestinal microbiota that can be also associated to other benefits to the host (Figure 2A). The most commonly used strains as probiotics are members of Lactobacilli, Enterococci and Bifidobacteria groups[43]. Lactic acid bacteria (LAB) represent a heterogeneous group of microorganisms that are present in the normal diet of many people and also in the gastrointestinal and urogenital tract of animals, and some of these claimed to be probiotics. Although most of the studies about probiotic have been mainly focused on bacteria, there are also many reports showing the potential of probiotic yeasts. In this context, Ianiro et al[44] reviewed the role of the “gut mycome”, and demonstrated that intestinal yeasts fulfill an important role in health maintenance. Selected yeast strains, especially from Saccharomyces boulardii were reported as probiotic, and their beneficial effects against different types of diarrhea were demonstrated using experimental animal models[45] and also in human trials[46,47]. Currently, many products containing LAB or other probiotic microorganisms are available on retail shelves throughout the world because of the increase consumer demand for healthier natural foods that can improve their overall well-being.
Figure 2.
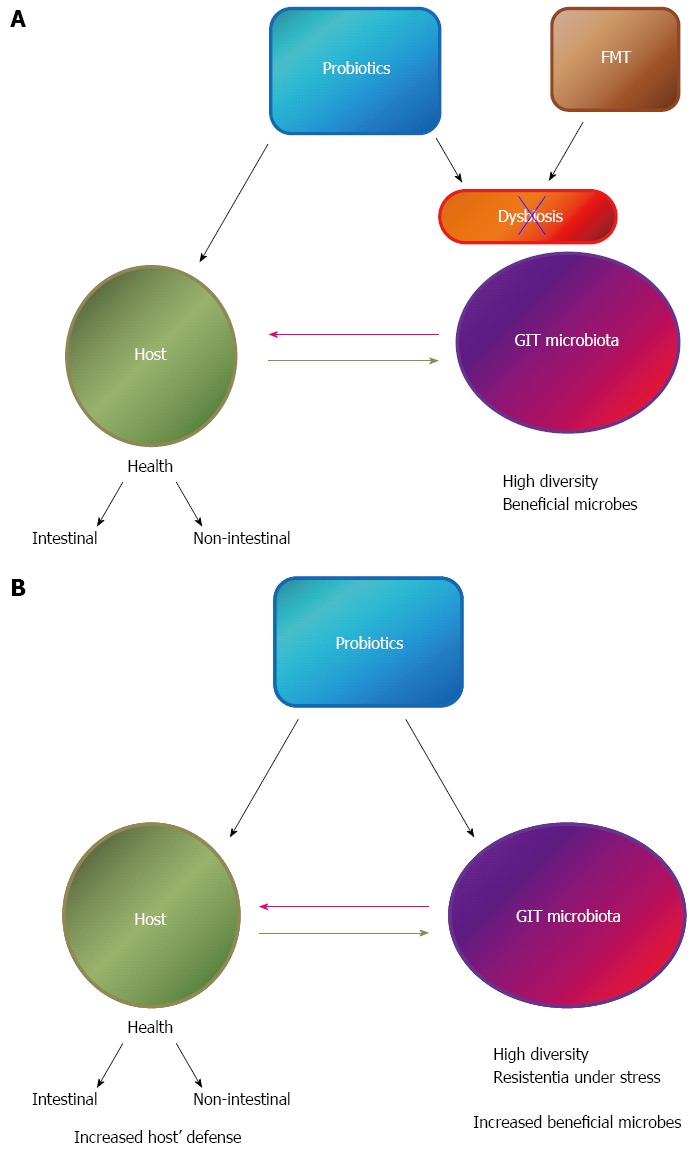
Effect of probiotic administration on gastrointestinal tract dysbiosis (A) or healthy individuals (B) and the interaction of the gastrointestinal tract microbiota with the host. GIT: Gastrointestinal tract; FMT: Fecal microbial transplantation.
EFFECTS OF PROBIOTICS ON INTESTINAL DISEASES
It has been shown that LAB and other probiotic microorganims can counteract inflammatory processes in the gut by stabilizing the microbial environment and the permeability of the intestinal barrier, and by enhancing the degradation of enteral antigens and altering their immunogenicity[48]. Lactobacillus reuteri (L. reuteri) was used to prevent colitis in IL-10 knock-out (KO) mice and to increase the number of lactobacilli in the gastrointestinal tract[49]. The normalization of Lactobacillus levels was obtained by oral administration of a prebiotic and rectal swabbing with L. reuteri to neonatal IL-10 KO mice. In a placebo-controlled trial, orally administered L. salivarius UCC118 reduced prevalence of colon cancer and mucosal inflammatory activity in IL-10 KO mice by modifying the intestinal microbiota in these animals with reduction in C. perfringens, coliforms, and enterococcus levels in the probiotic fed group[50]. The administration of yoghurt, with potential probiotic strains, decreased the inflammation by modulation of the host immune response in a trinitrobenzene sulphonic-induced mouse model of IBD. This effect was related to beneficial changes in the large intestine microbiota of the mice, with increases of bifidobacteria population[51].
The translation of the potential use of probiotics for IBD patients remains uncertain[52], and even when some authors reported their effectiveness against specific pathologies and the modification of GIT microbiota is one of the benefits attributed to them, there are only few reports where the fecal microbial composition of the patients was evaluated. A randomized, double-blind, placebo-controlled trial evaluated the effect of a probiotic mixture containing L. acidophilus, L. plantarum, L. rhamnosus, Bifidobacterium breve (B. breve), B. lactis, B. longum, and Streptococcus thermophilus in patients with IBS[53]. The fecal flora composition was analyzed by polymerase chain reaction denaturing gradient gel electrophoresis (DGGE) and it was reported that the therapeutic effect of this probiotic mixture was associated with the stabilization of intestinal microbiota. Another study showed that probiotic supplementation (Ecologic 825, Winclove, Amsterdam, the Netherlands) to patients with UC and severe pouchitis restored the mucosal barrier, which was correlated with the bacterial diversity of mucosal pouch microbiota[54].
Regarding the use of probiotic yeasts, the influence of the administration of Saccharomyces boulardii on the composition of the fecal microbiota was evaluated in a human microbiota-associated mouse model. The animals received antibiotic treatment that induced modifications in the intestinal microbiota. The administration of probiotic yeast was related with quicker return to the initial level for the Clostridium coccoides-Eubacterium rectale and Bacteroides-Porphyromonas-Prevotella groups, compared to the control animals without any special administration, and this effect was suggested as a possible mechanism by which S. boulardii affect beneficially human with antibiotic-associated diarrhea[55].
The use of probiotic S. boulardii was also examined in humans, but as was explained for probiotic bacteria, not many studies in human analyzed the modification of the intestinal microbiota. Regarding IBD patients, it was reported that S. boulardii was effective to reduce symptoms of disease and this was related to the improvement of intestinal microbiota composition[56]. S. boulardii was also evaluated for the treatment of diarrhea-predominant IBS and its effect was compared to mesalazine[57]. It was reported that all the treatments improved the symptoms of the patients; however, mesalazine alone or its combination with S. boulardii was more effective that the treatment with the probiotic yeast alone. A recent work demonstrated that probiotic S. boulardii, associated to conventional treatment improved the quality of life of patients with diarrhea-dominant IBS[58]. This effect was associated to an anti-inflammatory profile of cytokines in blood and tissues of patients that receive the probiotic compared to the placebo group.
EFFECTS OF PROBIOTICS ON NON-INTESTINAL DISEASES
The use of probiotics to beneficially affect the GIT microbiota was also evaluated in non-intestinal diseases (Figure 2B). Recent studies suggested that GIT microbiota might play a critical role in the development of obesity and LAB were pointed as candidate for an anti-obesity effect[59]. A review from 61 original articles showed that the main effect observed at the microbiota level (usually accompanied by weight loss) after probiotic or prebiotic administration in obese hosts was associated to increases in bifidobacteria populations[60].
Studies in diet induced obese mice showed that the supplementation of L. curvatus HY7601 and L. plantarum KY1032 reduced the obesity and modulated pro-inflammatory and fatty acid oxidation-related genes in the liver and adipose tissue; and this effect was associated to modulation of gut microbiota[61]. The relative abundance of 4 species belonging to the Ruminococcaceae and Lachnospiraceae families of the order Clostridiales and phylum Firmicutes were decreased by high fat diet and increased in mice receiving probiotic treatment. It was also observed that other GIT microbial species not associated with changes caused by high fat diet were affected in mice that received probiotics, standing out the relative abundance of endogenous Bifidobacterium pseudolongum.
VSL#3 is a mixture containing eight different strains of probiotic bacteria that was evaluated against different diseases, including the prevention and treatment of obesity and diabetes in several mouse models. This effect was associated to the modulation of the gut microbiota-short chain fatty acid (SCFA)-hormone axis[62]. VSL#3 supplementation induced changes in the microbiota that were associated with an increase in the levels of butyrate, and it was demonstrated in vitro that this SCFA stimulated the release of GLP-1 from intestinal cells. The hormone GLP-1 reduces food intake and improves glucose tolerance.
Recently, the beneficial effect of L. coryniformis CECT5711 was demonstrated in a high fat diet induced mouse model. Probiotic administration to obese mice induced marked changes in microbiota composition and reduced the metabolic endotoxemia by decrease of the LPS plasma level[63].
The effect of probiotics in humans was also observed; however, as was explained for other pathologies, there are not many articles that evaluate the intestinal microbiota. A clinical trial with the probiotic bacterium L. salivarius Ls-33 was conducted in obese adolescents to investigate the impact on fecal microbiota[64]. Ratios of Bacteroides-Prevotella-Porphyromonas group to Firmicutes belonging bacteria were significantly increased after administration of Ls-33; however, these changes were not related to effects on their metabolic syndrome.
A randomized, double-blind, placebo controlled study was conducted in order to evaluate the effects of probiotic capsule when combined with herbal medicine in treatment of obesity[65]. In this trial, each probiotic capsule contained viable cells Streptococcus thermophillus, L. plantarum, L. acidophilus, L. rhamnosus, B. lactis, B. longum, and B. breve. It was reported that probiotic administration prevented endotoxin production, which can lead to GIT microbiota dysbiosis associated with obesity. Gut B. breve population showed negative correlation with endotoxin level.
NAFLD is a disease linked to obesity and the beneficial role of probiotics was also reported[66]. Recently, it was shown that L. rhamnosus GG protected against NAFLD in a mice model[67]. The effect was associated to increase total bacterial numbers including the phyla Firmicutes and Bacteroidetes in the distal small intestine. This result was in concordance to the previous one that reported modulation of the microbiota in the small intestine with a concomitant anti-obesity effect in mice that received L. rhamnosus GG and L. sakei NR28[68].
The human GIT microbiota has also been related with a possible cardiovascular risk. GIT microbiota profiles were not only associated with metabolic diseases, but also the flux of metabolites derived from microbial metabolism of choline, phosphatidylcholine and l-carnitine that contribute directly to cardiovascular disease. In this sense, probiotics were reported among dietary strategies to modulate the GIT microbiota or their metabolic activities[69-71]. The improvement of disease biomarkers, especially plasma cholesterol levels, appears to be possible after probiotic administration to lower cardiovascular risk. In this sense, it was shown that the administration of a probiotic soy product containing Enterococcus faecium CRL 183 and L. helveticus 416 supplemented or not with isoflavones was associated with an improved cholesterol profile and inhibition of atherosclerotic lesion development in a rabbit model[72]. The authors reported that of Enterococcus spp., Lactobacillus spp. and Bifidobacterium spp. were negatively correlated with total cholesterol, non-HDL-cholesterol, and lesion size. The intake of the probiotic soy product increased significantly these bacterial species in the fecal microbiota.
EFFECTS OF PROBIOTICS IN HEALTHY HOSTS
There are also reports that showed the potential of probiotics in healthy hosts, maintaining a balanced microbiota, which, as was explained above, is an important key for health. The consumption of a probiotic product containing L. coryniformis CECT5711 and L. gasseri CECT5714 was analyzed in 30 children with no gastrointestinal pathology[73]. An increase in faecal lactobacilli counts was shown at the end of the experimental protocol, and these findings were associated to enhancing the defence against gastrointestinal aggressions and infections and enhancing the immune function with increase IgA concentration in faeces and saliva. A recent work reported a clinical trial that included 40 participants with no known digestive diseases. Laminaria japonica, a widely used ingredient in seaweed kimchi, and LAB of traditional fermented Korean food were given to volunteers and was related to increases in the number of some administered LAB species in their GIT microbiota[74].
CONCLUSION
The dysbiosis of the gastrointestinal tract microbiota is considered to be one of the contributing factors in the development of certain gastrointestinal and non-gastrointestinal diseases. Fecal transplantations appear to be promising therapies for dysbiosis-associated diseases; however, controlled trials of FMT in specific disorders are needed before FMT can be more accepted and applied clinically. The possibility to modify the traditional FMT by specific probioitc fecal microorganisms was also reported and would be a better alternative from a safety and therapeutic point of views.
Recent reports showed the potential of the administration of specific probiotic strains to improve the balance of the GIT microbiota that is altered in different diseases, being IBD and obesity the pathologies in which there are more studies showing this association using animal models and even in human clinical trials. The importance of probiotic consumption in healthy hosts was also demonstrated because its relationship with beneficial balance in GIT microbial populations, which is also associated to improved defense against gastrointestinal aggressions and infections and the enhancing of the host’s immune function.
However, as was explained for FMT, there are not enough human trials where the application of probiotics as biotherapeutic agents was evaluated in double-blinded large scale clinical trials. These assays are very important before the medical community will accept the addition of probiotic as supplements for specific patients with diseases associated to gut microbial dysbiosis as a viable alternative to FMT.
Footnotes
Supported by Consejo Nacional de Investigaciones Científicas y Técnicas No. CONICET and the Agencia Nacional de Promoción Científica y Tecnológica No. ANPCyT
P- Reviewer: Baryshnikova NV, Servin AL S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Food and Agriculture Organization/World Health Organization. Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina, 1 to 4 October. 2001. Available from: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf. [Google Scholar]
- 2.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 6.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8:435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhtiar SM, LeBlanc JG, Salvucci E, Ali A, Martin R, Langella P, Chatel JM, Miyoshi A, Bermúdez-Humarán LG, Azevedo V. Implications of the human microbiome in inflammatory bowel diseases. FEMS Microbiol Lett. 2013;342:10–17. doi: 10.1111/1574-6968.12111. [DOI] [PubMed] [Google Scholar]
- 8.Chan YK, Estaki M, Gibson DL. Clinical consequences of diet-induced dysbiosis. Ann Nutr Metab. 2013;63 Suppl 2:28–40. doi: 10.1159/000354902. [DOI] [PubMed] [Google Scholar]
- 9.Vipperla K, O’Keefe SJ. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr Clin Pract. 2012;27:624–635. doi: 10.1177/0884533612452012. [DOI] [PubMed] [Google Scholar]
- 10.Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54:938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- 11.Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself. 2010;1:299–309. doi: 10.4161/self.1.4.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med. 2014;68:122–133. doi: 10.1016/j.freeradbiomed.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011;17:557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Q, Jin Z, Wu W, Gao R, Guo B, Gao Z, Yang Y, Qin H. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One. 2014;9:e90849. doi: 10.1371/journal.pone.0090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huipeng W, Lifeng G, Chuang G, Jiaying Z, Yuankun C. The differences in colonic mucosal microbiota between normal individual and colon cancer patients by polymerase chain reaction-denaturing gradient gel electrophoresis. J Clin Gastroenterol. 2014;48:138–144. doi: 10.1097/MCG.0b013e3182a26719. [DOI] [PubMed] [Google Scholar]
- 19.Song X, Gao H, Lin Y, Yao Y, Zhu S, Wang J, Liu Y, Yao X, Meng G, Shen N, et al. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2014;40:140–152. doi: 10.1016/j.immuni.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80:2546–2554. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallucca F, Porrata C, Fallucca S, Pianesi M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev. 2014;30 Suppl 1:48–54. doi: 10.1002/dmrr.2518. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 23.Riley LW, Raphael E, Faerstein E. Obesity in the United States - dysbiosis from exposure to low-dose antibiotics? Front Public Health. 2013;1:69. doi: 10.3389/fpubh.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan JL, Walker WA. Early gut colonization and subsequent obesity risk. Curr Opin Clin Nutr Metab Care. 2012;15:278–284. doi: 10.1097/MCO.0b013e32835133cb. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris K, Kassis A, Major G, Chou CJ. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes. 2012;2012:879151. doi: 10.1155/2012/879151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scalera A, Di Minno MN, Tarantino G. What does irritable bowel syndrome share with non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:5402–5420. doi: 10.3748/wjg.v19.i33.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peniche AG, Savidge TC, Dann SM. Recent insights into Clostridium difficile pathogenesis. Curr Opin Infect Dis. 2013;26:447–453. doi: 10.1097/01.qco.0000433318.82618.c6. [DOI] [PubMed] [Google Scholar]
- 31.Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, Wang GP. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Bella S, Drapeau C, García-Almodóvar E, Petrosillo N. Fecal microbiota transplantation: the state of the art. Infect Dis Rep. 2013;5:e13. doi: 10.4081/idr.2013.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pamer EG. Fecal microbiota transplantation: effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014;7:210–214. doi: 10.1038/mi.2013.117. [DOI] [PubMed] [Google Scholar]
- 34.Dutta SK, Girotra M, Garg S, Dutta A, von Rosenvinge EC, Maddox C, Song Y, Bartlett JG, Vinayek R, Fricke WF. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014;12:1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306:G310–G319. doi: 10.1152/ajpgi.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452–1459. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 38.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 39.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 40.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 41.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 42.Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014;146:1573–1582. doi: 10.1053/j.gastro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82:279–289. [PubMed] [Google Scholar]
- 44.Ianiro G, Bruno G, Lopetuso L, Beghella FB, Laterza L, D’Aversa F, Gigante G, Cammarota G, Gasbarrini A. Role of yeasts in healthy and impaired gut microbiota: the gut mycome. Curr Pharm Des. 2014;20:4565–4569. doi: 10.2174/13816128113196660723. [DOI] [PubMed] [Google Scholar]
- 45.Martins FS, Dalmasso G, Arantes RM, Doye A, Lemichez E, Lagadec P, Imbert V, Peyron JF, Rampal P, Nicoli JR, et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar Typhimurium protects mice and modifies T84 cell response to the infection. PLoS One. 2010;5:e8925. doi: 10.1371/journal.pone.0008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burande MA. Comparison of efficacy of Saccharomyces boulardii strain in the treatment of acute diarrhea in children: A prospective, single-blind, randomized controlled clinical trial. J Pharmacol Pharmacother. 2013;4:205–208. doi: 10.4103/0976-500X.114603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shan LS, Hou P, Wang ZJ, Liu FR, Chen N, Shu LH, Zhang H, Han XH, Han XX, Cai XX, et al. Prevention and treatment of diarrhoea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Benef Microbes. 2013;4:329–334. doi: 10.3920/BM2013.0008. [DOI] [PubMed] [Google Scholar]
- 48.de Moreno de Leblanc A, Del Carmen S, Zurita-Turk M, Santos Rocha C, van de Guchte M, Azevedo V, Miyoshi A, Leblanc JG. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011;2011:892971. doi: 10.5402/2011/892971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isolauri E, Salminen S, Ouwehand AC. Microbial-gut interactions in health and disease. Probiotics. Best Pract Res Clin Gastroenterol. 2004;18:299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 51.O’Mahony L, Feeney M, O’Halloran S, Murphy L, Kiely B, Fitzgibbon J, Lee G, O’Sullivan G, Shanahan F, Collins JK. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15:1219–1225. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 52.Chaves S, Perdigon G, de Moreno de LeBlanc A. Yoghurt consumption regulates the immune cells implicated in acute intestinal inflammation and prevents the recurrence of the inflammatory process in a mouse model. J Food Prot. 2011;74:801–811. doi: 10.4315/0362-028X.JFP-10-375. [DOI] [PubMed] [Google Scholar]
- 53.Sinagra E, Tomasello G, Cappello F, Leone A, Cottone M, Bellavia M, Rossi F, Facella T, Damiani P, Zeenny MN, et al. Probiotics, prebiotics and symbiotics in inflammatory bowel diseases: state-of-the-art and new insights. J Biol Regul Homeost Agents. 2013;27:919–933. [PubMed] [Google Scholar]
- 54.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 55.Persborn M, Gerritsen J, Wallon C, Carlsson A, Akkermans LM, Söderholm JD. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38:772–783. doi: 10.1111/apt.12451. [DOI] [PubMed] [Google Scholar]
- 56.Makuuchi M, Takayama T, Kosuge T, Yamazaki S, Yamamoto J, Hasegawa H, Takayasu K. The value of ultrasonography for hepatic surgery. Hepatogastroenterology. 1991;38:64–70. [PubMed] [Google Scholar]
- 57.Avalueva EB, Uspenskiĭ IuP, Tkachenko EI, Sitkin SI. [Use of Saccharomyces boulardii in treating patients inflammatory bowel diseases (clinical trial)] Eksp Klin Gastroenterol. 2010;(7):103–111. [PubMed] [Google Scholar]
- 58.Bafutto M, Almeida JR, Leite NV, Costa MB, Oliveira EC, Resende-Filho J. Treatment of diarrhea-predominant irritable bowel syndrome with mesalazine and/or Saccharomyces boulardii. Arq Gastroenterol. 2013;50:304–309. doi: 10.1590/S0004-28032013000400012. [DOI] [PubMed] [Google Scholar]
- 59.Tsai YT, Cheng PC, Pan TM. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl Microbiol Biotechnol. 2014;98:1–10. doi: 10.1007/s00253-013-5346-3. [DOI] [PubMed] [Google Scholar]
- 60.da Silva ST, dos Santos CA, Bressan J. Intestinal microbiota; relevance to obesity and modulation by prebiotics and probiotics. Nutr Hosp. 2013;28:1039–1048. doi: 10.3305/nh.2013.28.4.6525. [DOI] [PubMed] [Google Scholar]
- 61.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, Garrido-Mesa N, Rodríguez-Cabezas ME, Olivares M, Gálvez J, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci (Lond) 2014;127:33–45. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- 64.Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, Jakobsen M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32:935–940. doi: 10.1016/j.clnu.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Lee SJ, Bose S, Seo JG, Chung WS, Lim CY, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: A randomized double-blind controlled clinical trial. Clin Nutr. 2013:Epub ahead of print. doi: 10.1016/j.clnu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Kelishadi R, Farajian S, Mirlohi M. Probiotics as a novel treatment for non-alcoholic Fatty liver disease; a systematic review on the current evidences. Hepat Mon. 2013;13:e7233. doi: 10.5812/hepatmon.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ji YS, Kim HN, Park HJ, Lee JE, Yeo SY, Yang JS, Park SY, Yoon HS, Cho GS, Franz CM, et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef Microbes. 2012;3:13–22. doi: 10.3920/BM2011.0046. [DOI] [PubMed] [Google Scholar]
- 69.Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54:175–189. doi: 10.1080/10408398.2011.579361. [DOI] [PubMed] [Google Scholar]
- 70.Taga ME, Walker GC. Pseudo-B12 joins the cofactor family. J Bacteriol. 2008;190:1157–1159. doi: 10.1128/JB.01892-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuohy KM, Fava F, Viola R. ‘The way to a man’s heart is through his gut microbiota’--dietary pro- and prebiotics for the management of cardiovascular risk. Proc Nutr Soc. 2014;73:172–185. doi: 10.1017/S0029665113003911. [DOI] [PubMed] [Google Scholar]
- 72.Cavallini DC, Suzuki JY, Abdalla DS, Vendramini RC, Pauly-Silveira ND, Roselino MN, Pinto RA, Rossi EA. Influence of a probiotic soy product on fecal microbiota and its association with cardiovascular risk factors in an animal model. Lipids Health Dis. 2011;10:126. doi: 10.1186/1476-511X-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lara-Villoslada F, Sierra S, Boza J, Xaus J, Olivares M. [Beneficial effects of consumption of a dairy product containing two probiotic strains, Lactobacillus coryniformis CECT5711 and Lactobacillus gasseri CECT5714 in healthy children] Nutr Hosp. 2007;22:496–502. [PubMed] [Google Scholar]
- 74.Ko SJ, Kim J, Han G, Kim SK, Kim HG, Yeo I, Ryu B, Park JW. Laminaria japonica combined with probiotics improves intestinal microbiota: a randomized clinical trial. J Med Food. 2014;17:76–82. doi: 10.1089/jmf.2013.3054. [DOI] [PubMed] [Google Scholar]


