Abstract
Obesity and its associated diseases are a worldwide epidemic disease. Usual weight loss cures - as diets, physical activity, behavior therapy and pharmacotherapy - have been continuously implemented but still have relatively poor long-term success and mainly scarce adherence. Bariatric surgery is to date the most effective long term treatment for morbid obesity and it has been proven to reduce obesity-related co-morbidities, among them nonalcoholic fatty liver disease, and mortality. This article summarizes such variations in gut hormones following the current metabolic surgery procedures. The profile of gut hormonal changes after bariatric surgery represents a strategy for the individuation of the most performing surgical procedures to achieve clinical results. About this topic, experts suggest that the individuation of the crosslink among the gut hormones, microbiome, the obesity and the bariatric surgery could lead to new and more specific therapeutic interventions for severe obesity and its co-morbidities, also non surgical.
Keywords: Obesity, Bariatric surgery, Gut hormones, Nonalcoholic fatty liver disease, Microbiome
Core tip: It is important to emphasize the role of the major peptides released by the enteroendocrine system, which promote satiety and modulate energy homeostasis and utilization, as well as those that control fat absorption and intestinal permeability. Bariatric surgery could be the most effective treatment for obesity and co-morbidities, often within days after surgery, independently of weight loss and it is currently the only therapy available for obesity which results in long-term, sustained weight loss. We hypothesize that gut hormones might play a role in induction and long-term maintenance of weight loss, could determine the improvement of obesity-related co-morbidities and could help to identify new drug targets and improved surgical techniques.
INTRODUCTION
Obesity epidemic in the United States (US), as well as all over the world, is steadily rising[1,2]. According to National Health and Nutrition Examination Survey in the 2007-2008, a percentage as high as of US population was found to be obese [body mass index (BMI) ≥ 30 kg/m2], of which about 5.7% was found to be of severe obesity (BMI ≥ 40 kg/m2)[1,2]. Obesity rates have also been advancing worldwide, and more than 700 million people will be obese by 2015, as predicted by the World Health Organization.
OBESITY RISK
Obesity is associated to comorbidities as hypertension, hyperlipidemia, heart failure, type 2 diabetes mellitus (T2DM), obstructive sleep apnea, thromboembolic disease, osteoarthrosis, gastroesophageal reflux disease, asthma, polycystic ovary disease, nonalcoholic fatty liver disease (NAFLD), as well as several cancers[1,3]. Metabolic syndrome, which involves the combination of risk factors for cardiovascular disease such as insulin resistance, visceral obesity, dyslipidemia, glucose intolerance, and hypertension, has often been associated with more severe liver[4]. Insulin resistance and chronic low-grade inflammation appear to be key points of this anomalous condition.
In addition, a number of other features contribute to the obesity development, including social factors, such as the accessibility of calorie-dense foods (elevated energy intake) and the sedentary lifestyle (reduced energy expenditure), as well as genetic and epigenetic predispositions[1,5].
MECHANISM OF ENERGY HOMEOSTASIS: DEFENDING A WEIGHT SET POINT
Body weight remains usually constant during the life. This condition manifests an overall balance between energy intake and expenditure. Kennedy, 50 years ago, proposed a negative feedback system of peripheral fat on food intake[6]. The adipose tissue role in the complex systems of energy homeostasis has been increasingly understood over the last decade.
Central control of feeding
Humans take food in discrete episodes during the day. The energy intake is determined on the basis of frequency and caloric amount of each meal. Hunger and food-seeking behaviour before a meal are stimulated by various short-term polypeptide hormonal signals from the periphery (orexigen hormones, e.g., ghrelin etc.), whereas other peripheral stimuli promote satiety and/or meal cessation [anorectics; e.g., glucagon-like peptide-1 (GLP-1), peptide tyrosine tyrosine 3-36 (PYY3-36), cholecystokinin (CCK), oxyntomodulin (OXM), and several others) (Figure 1)[7]. The efficacy of short-term signals to defend against deviations from a set weight point can be modulated by long-term signals related to total body energy stores (e.g., insulin, leptin, ghrelin, etc.)[1,8].
Figure 1.

Humoral signals implicated in the physiological regulation of food intake. Diagram summarising the major signalling pathways which converge on the hypothalamus and brainstem in order to regulate food intake. ARC: Arcuate nucleus; NPY/AgRP: Neuropeptide Y and agouti-related peptide; POMC/CART: Proopiomelanocortin and cocaine- and amphetamine-regulated transcript; DVC: Dorsal vagal complex; DVN: Dorsal motor nucleus of vagus; NTS: Nucleus of the tractus solitarius; AP: Area postrema; GLP-1: Glucagon-like peptide-1; CCK: Cholecystokinin; PP: Pancreatic polypeptide; PYY: Peptide YY; OXM: Oxyntomodulin.
Numerous important areas of the brain have been associated with the regulation of energy balance. Insulin, leptin, and PYY inhibit, while ghrelin stimulate the arcuate nucleus (ARC) of the hypothalamus that contains populations of neuropeptide Y/Agouti-related protein (NPY/AgRP)-expressing neurons[9]. Orexigenic neurons, identified in the lateral hypothalamic area and perifornical area, are stimulated by NPY/AgRP. Increased feeding and weight gain are derived by stimulation of NPY/AgRP neurons[10]. The ARC includes neurons that can express the proopiomelanocortin and cocaine- and amphetamine-regulated transcript. Leptin stimulate these cells, while NPY/AgRP neurons inhibit[11]. In rodent and human researches, the reduction of food intake and weight loss derived by treatment with leptin. The ARC of the hypothalamus and the lateral hypothalamic area acquire and integrate information about overall energy stores from the periphery and relay this information to higher centers, such as the ventral tegmental area of the mesolimbic system, which is involved in the hedonic experience of feeding, as reported by Harvey et al[1].
Peripheral control of feeding
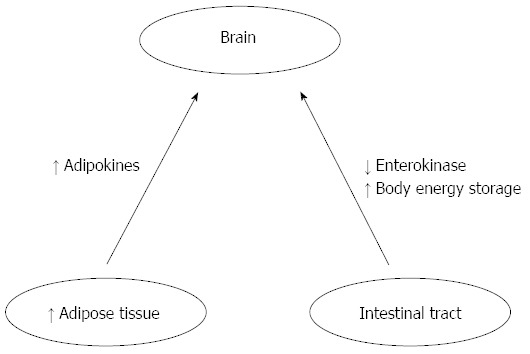
Hormonal, nutritional, and neuronal afferent feedback from the periphery to the central nervous system (CNS) depend on ability to increase or decrease calorie intake in response to changes in energy needs. Adipokines, including leptin and adiponectin, which are hormonal signals from adipose tissue provide feedback signals to the brain (adipose-brain axis), whereas feedback signals from the intestinal tract (the gut-brain axis) include polypeptide hormones (enterokinase) and neuronal signals via the vagus and other autonomic afferents, as reported by Harvey et al[1] (Figure 2). Basal levels of insulin and leptin proportionally develop following the increase of the fat mass, providing a long-term signal of body energy stores to the brain. On the contrary, basal ghrelin levels are inversely related to the body energy storage. Short-term fluctuations in numerous gut hormones including ghrelin, insulin GLP-1, PYY3-36, and CCK, as well as vagal signals regulate mealtime, as reported by Harvey et al[1].
Figure 2.
Peripheral feedback signals.
HORMONAL CHANGES AFTER BARIATRIC SURGERY
Bariatric surgery consists of various interventions that have been subdivided as purely restrictive, purely malabsorptive, or combined restrictive and malabsorptive.
The number of bariatric interventions (i.e., metabolic surgery) for the treatment of obesity is in exponential increase. This is partly due to the most effective and durable weight loss; in addition, a good deal of improvement of co-morbidities after surgery compared with medical treatments such as diet and physical activity[12], or actually applicable pharmacologic[13] or endoscopic[14] treatments was observed. Actually, bariatric surgery is greatly approved by patients and physicians because the techniques are minimally invasive and well finished and standardized[15].
Each bariatric technique results in multiple effects, according to the intervention on the gastrointestinal anatomy (Figure 3). For example, sleeve gastrectomy (SG) has been formerly considered exclusively restrictive with the capacity of the stomach greatly reduced and the absorptive surface of the small unchanged. Nevertheless, the largest production of ghrelin, an appetite-stimulating hormone, occurs in the fundus and body of the stomach, and the ghrelin reduced production following SG probably plays an important role in decreasing appetite.
Figure 3.

Gastric banding: an adjustable gastric band is used to divide the stomach into a small proximal compartment taken down a few centimeters distal to the compartment (pouch) and a larger distal compartment (residual stomach). Roux-en-Y gastric bypass: The stomach is gastric inlet. The jejunum is divided 50 cm beyond the ligament of Treitz, and its aboral end is connected to the small gastric pouch. Some 150 cm distal to this point, the other end of the small bowel is sewn to a loop that has been pulled up to meet it (so-called Roux-en-Y reconstruction). Surgical effects: restriction, with an additional malabsorptive component. Sleeve gastrectomy: More than 80% of the stomach is resected, and the gastric remnant is tubularized, with an initial filling volume of less than 100 mL. Surgical effects: Restrictive and hormonal mechanisms. Biliopancreatic diversion duodenal switch (DS): The stomach is reduced in size as in sleeve gastrectomy. Duodenum is divided distal to the pylorus, and the jejunum is divided 250 cm proximal to the ileocecal valve and anastomosed to the duodenum. The other end is connected to the ileum 100 cm proximal to the ileocecal valve. Mechanism of effect: A combination of restrictive gastric surgery with a considerable degree of malabsorption.
Bypass-type interventions, like Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion, with or without duodenal switch (BPD/DS), are usually regarded as a combination of restrictive and malabsorptive outcomes: bypassing or removing a large part of the stomach, these interventions divert chyme away from the duodenum and proximal small bowel rapidly presenting nutrients to the distal gut, as reported by Harvey et al[1]. The adjustable gastric band (AGB), which places a constrictive ring around the gastric inlet and is usually considered to be an exclusively restrictive intervention, results in prolonged satiety; the mechanism is yet partially understood[1,16].
Reduction caloric intake and gut hormone expression modifications are determinants for weight loss and improvement of the metabolic derangements associated with obesity. Therefore, there are two considerations that supported this hypothesis. Firstly, following bariatric surgery, while patients are in a state of negative energy balance and are quickly losing weight, they do not complain of feeling hungry, but rather have decreased appetite and precociously satiety[1,17]. Secondly, following different types of metabolic surgery, patients with T2DM show a rapid improvement in glycemic control, occurring after surgery; before significant weight loss can to appear.
Here we will consider actual knowledge of the role of specific hormones believed to be essential in energy homeostasis and the expression of which is modified after bariatric surgery (Table 1).
Table 1.
Anatomic and physiologic changes due to bariatric surgery
| Stomach | Duodenum | Proximal intestine | Distal intestinal | |
| RYGB | Decreased capacity to 15-30 mLChyme bypasses 90% of stomachDecreased gastric transit time | Bypassed by chyme | Proximal jejunum bypassed by chymeRapid exposure of distal jejunum to chymeBile mixes with chyme in proximal ileum | Earlier exposure to chyme |
| AGB | Restrictive band around gastric cardiaDirect pressure on vagus nerves | No alterations | No alterations | No alterations |
| BPD/DS | Decreased capacity to 60-150 mL85%-90% of stomach removedAccelerated gastric emptying by 2-fold to 3-fold | Bypassed by chyme | Chyme bypasses jejunum Rapid exposure of ileum to chymeBile mixes with chyme in distal ileum | Rapid exposure to chime |
| SG | Decreased capacity to 60-150 mL85%-90% of stomach removedAccelerated gastric emptying by 2-fold to 3-fold | More rapid exposure to chyme | Possibly earlier exposure to chyme | Possibly earlier exposure to chyme |
AGB: Adjustable gastric band; BPD/DS: Biliopancreatic diversion with or without duodenal switch; RYGB: Roux-en-Y gastric bypass; SG: Sleeve gastrectomy.
Ghrelin
Ghrelin is a 28-amino acid peptide produced in different sites of the endothelial system. It was identified in 1999 as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a) in the pituitary gland[1,18]. Subsequently, in rodents it was observed that ghrelin determine an increase in feeding and weight gain and develop a orexigenic role in energy balance[10].
The ghrelin is principally produced from cells located in the oxyntic glands of the fundus and body of the stomach (also known as P/D1 cells). Ghrelin levels support an ultradian rhythm-rising encode a corresponding pattern of NPY discharge for daily meal patterning[1,19]. It has been shown that ghrelin cells express the clock genes PER1 and PER2, and the ghrelin release is entrained to peak just before regular feeding times[1,20]. A rising serum ghrelin level induces an increase in food seeking behavior and the initiation of feeding. After food intake, ghrelin levels are quickly suppressed[19]. Ghrelin effects on the brain are mediated in part by the vagus nerves and in part by a humoral action.
Numerous sites of the CNS, that include the ARC, the nucleus accumbens, the parabrachial nucleus, dorsomedial and lateral hypothalamic area and the subfornical part of the circumventricular organs, contain ghrelin receptors or ghrelin-stimulated activity that play a significant role in energy homeostasis. In the ARC, after stimulation of NPY/AgRP neurons it observe ghrelin orexigenic action[10].
Obese subjects show low ghrelin levels while lean subjects show high ghrelin levels. Ghrelin fasting levels appear rise in diet-induced or exercise-induced weight loss, and this may result in augmented sensation of hunger with failure to lose excess weight[21]. Thus, maintaining a weight set point might depend from ghrelin in, particularly in the condition of weight loss resistance.
Ghrelin levels, before and after RYGB, are reported in twenty-three studies about (Tables 2 and 3). A significant early decrease in ghrelin was found in five out of the seven studies that reported ghrelin levels within the first 14 d after surgery. Some of these studies compared patients undergoing RYGB with a control group of patients undergoing other non resective gastric operations such as AGB or Nissen fundoplication[1,22,23]. Only RYGB resulted in a significant reduction in postoperative ghrelin levels.
Table 2.
Short term (< 3 mo) fasting serum hormonal changes after bariatric surgery
| RYGB | AGB | SG | BPD/DS | |
| Ghrelin | ↓ [22,26,72,74,75, 87-91]= [23-25,36,45,88,89]↑ (abs. .inf.) | ↓ (abs. .inf.)= [24,25,27]↑[27] | ↓ [26,27,45]= (abs. .inf.)↑ (abs. .inf.) | ↓ [108]= [108,111,112]↑ [109-111] |
| GLP-1 | ↓ (abs. .inf.)= [23,24,26,36,37,89,115-117] ↑ (abs. .inf.) | ↓ (abs. .inf.)= [24]↑ (abs. .inf.) | ↓ (abs. .inf.)= [26]↑ (abs. .inf.) | ↓ (abs. .inf.)= [112,122-125]↑(125,126) |
| PYY | ↓ [23]= [24,25,36,46,76,89,91] ↑ (abs. .inf.) | ↓ (abs. .inf.)= [24,25]↑ (abs. .inf.) | ↓ (abs. .inf.)= [46]↑ [26,45] | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) |
| Insulin | ↓ [22,23,25,26,87,89,90,115,117,118,127-129]= [25, 46,89,91,116]↑ [88] | ↓ [21,22]= [25]↑ (abs. .inf.) | ↓ [26,48]= (abs. .inf.)↑ (abs. .inf.) | ↓ [122,123]= (abs. .inf.)↑ (abs. .inf.) |
| Leptin | ↓ [23-25,89,90,117,127-129]= (abs. .inf.)↑ (abs. .inf.) | ↓ [25]= [24]↑ [21] | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ [111,112,122]= (abs. .inf.)↑ (abs. .inf.) |
| Adiponectin | ↓ [72, 87]= [87]↑ (abs. .inf.) | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ (abs. .inf.)= [122]↑ (abs. .inf.) |
↓: Decreased; =: Not significant; ↑: Increased; abs. inf.: Absence of information. The number inside the Table indicate the related references. RYGB: Roux-en-Y gastric bypass; AGB: Adjustable gastric band; SG: Sleeve gastrectomy; BPD/DS: Biliopancreatic diversion with or without duodenal switch; GLP-1: Glucagon-like peptide-1; PYY: Peptide tyrosine-tyrosine.
Table 3.
Long term (> 3 mo) fasting serum hormonal changes after bariatric surgery
| RYGB | AGB | SG | BPD/DS | |
| Ghrelin | ↓ [77,85,92,97,100]= [24,25,45,72, 87-89,91,95,99,101]↑ [45, 73,78-83,86,88,93,94,96,99] | ↓(abs. .inf.)= [24,25,103,104,106]↑[28,27,92,101,102,104,105,107] | ↓ [28,27,45,107,114]= (abs. .inf.)↑ (abs. .inf.) | ↓ [54,97]= [96,113]↑ [111,112] |
| GLP-1 | ↓ [120]= [24,25,26,37,80,89,95,116,119,121]↑ (abs. .inf.) | ↓ [120]= [24,25,106,120]↑ [102] | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) |
| PYY | ↓ (abs. .inf.)= [24,25,89,91]↑ [25,45,81,96,119,120] | ↓ (abs. .inf.)= [24,25]↑ [120] | ↓ (abs. .inf.)= (abs. .inf.)↑[45] | ↓ (abs. .inf.)= (abs. .inf.)↑ (96) |
| Insulin | ↓[25,78,78,87,89,74,94,95,97,98,99,100,102,120,121,127,129-135]= [24,25,80,89,91]↑ [82,84,95,99,130] | ↓ [25,92,102,104,120,131]= [106]↑ (abs. .inf.) | ↓[48]= (abs. .inf.)↑ (abs. .inf.) | ↓ (97,113,126)= (abs. .inf.)↑ (abs. .inf.) |
| Leptin | ↓ [24,25,78,79,89,92-94,96-99,101,127,129,132,135]= (abs. .inf.)↑ (abs. .inf.) | ↓ [25,92,101-104,106,132,136]= [24]↑ (abs. .inf.) | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ [97,111-113]= [54,96]↑ (abs. .inf.) |
| Adiponectin | ↓ (abs. .inf.)↑ [78,79,84,87,90,93,94,97,99,131,133,134] | ↓ (abs. .inf.) | ↓ (abs. .inf.)= (abs. .inf.)↑ (abs. .inf.) | ↓ (abs. .inf.)= [54]↑ [97] |
↓: Decreased; =: Not significant; ↑: Increased; abs. inf.: Absence of information. The number inside the Table indicate the related references. RYGB: Roux-en-Y gastric bypass; AGB: Adjustable gastric band; SG: Sleeve gastrectomy; BPD/DS: Biliopancreatic diversion with or without duodenal switch; GLP-1: Glucagon-like peptide-1; PYY: Peptide tyrosine-tyrosine.
There are not consistent researches of longer-term changes in ghrelin levels after RYGB (Tables 2 and 3). Several studies reported preoperative and postoperative circulating ghrelin levels between 1 mo and 2 years after surgery.
In some of these researches the evaluation of a single serum ghrelin concentration in the morning after an overnight fast was performed; in others the meal-associated suppression of ghrelin was assessed. Some researchers evaluated ‘‘active’’ ghrelin, the metabolically active isoform including a unique post-translational octanoylation of the serine-3 hydroxyl group[1,18], while other group also detected the inactive des-acyl isoform[1].
This variability in the methods as well as the differences among assays restricts the studies comparisons.
Although considerable physiologic variations in serum ghrelin levels may contribute to conflicting findings, two observations emphasize the expected decreased ghrelin levels in patients after RYGB. Cummings et al[24] determined the 24-h plasma ghrelin profiles, body composition, insulin levels, leptin levels, and insulin sensitivity in 13 obese subjects before and after a six-month dietary program for weight loss. The 24-h ghrelin profiles were also determined in 5 subjects who lost weight after RYGB and 10 normal-weight controls; 5 of the 13 obese subjects who participated in the dietary program were matched with the subjects in the gastric-bypass group as obese controls[24]. The increase in the plasma ghrelin level with the sbsequent diet-induced weight loss is consistent with the hypothesis that ghrelin plays a role in the long-term regulation of body weight[24]. RYGB is correlated with considerably suppressed ghrelin levels, probably contributing to the weight-reducing effect of the procedure[24]. Nevertheless, the findings reported by Cummings et al[24] is not in line with other investigators: some studies have found an unchanged ghrelin spike before a meal and normal suppression following a meal after RYGB[25,26]. These inconsistencies may be also due to different techniques in performing RYGB.
Korner et al[26] showed that the differences in levels of gut hormones may play a role in promoting greater weight loss and insulin sensitivity after RYGB compared with AGB.
The changes in ghrelin after SG were measured in different studies: fasting ghrelin levels are decreased at all time points up to 5 years of follow-up. Moreover, some studies tried to evaluate and compare the effects of RYGB[27] or AGB[28,29] with the SG on fasting ghrelin levels, which showed to be decreased.
These studies lend credence that the ghrelin suppression after both SG and RYGB may be part of the mechanism that contributes to T2DM remission.
Glucagon-like peptide-1
Glucagon-like peptide-1 (7-36) amide (GLP-1) is a 30-residue peptide hormone released from intestinal L cells following nutrient consumption, as reported by Donnelly[30]. It potentiates the glucose-induced secretion of insulin from pancreatic beta cells, increases insulin expression, but also inhibits beta-cell apoptosis. Donnelly[30] has reported that GLP-1 promotes beta-cell neogenesis and satiety, reduces glucagon secretion, delays gastric emptying, and increases peripheral glucose disposal. Intact peptide is inactivated during passage across the hepatic bed by the enzyme dipeptidyl peptidase-4 (DPP-IV) associated with the hepatocytes, and further degraded by the peripheral tissues, while the kidney is important for the final elimination of the metabolites. GLP-1 is also a neurotransmitter locates in numerous areas of the brain (paraventricular nucleus and dorsomedial hypothalamus, dorsovagal complex, thalamus, and pituitary). The GLP-1 receptor is G-protein-coupled and is expressed in the pancreatic islets, lung, hypothalamus, stomach, heart and kidney[31].
Peripheral infusion of GLP-1 or PYY3-36 reduces food intake in healthy, obese, and diabetic subjects[32]. These two hormones, in combination with other anorectic gut hormones, act as peripheral sensory inputs, integrated in the CNS, modulating appetite and energy expenditure.
GLP-1 and PYY3-36 directly act on the stomach to slow gastric emptying and acid secretion functioning as an ‘‘ileal brake’’mechanism to intestinal motility, preventing an exceedingly rapid transit and allowing time for digestion and absorption of nutrients in the proximal intestine[1,33].
GLP-1, one of the gut hormones called incretins, has an important role in enhancing insulin secretion by the pancreas[1]. The increased insulin response to enteral as opposed to parenteral glucose is defined the‘‘incretin effect’’[34]. Both in vivo and in vitro researches have showed that GLP-1 increase insulin secretion in the beta cell. Moreover, glucagon secretion is inhibited by GLP-1 while insulin sensitivity is increased.
Apoptosis is contrasted by GLP-1 while beta cell regeneration is promoted[35]. Diabetic patients show a blunted first-phase insulin response following a meal, partly due to a decreased incretin response[1,36].
Exenatide, a GLP-1 receptor agonist, is currently used for the treatment of T2DM. A strong GLP-1 response was reported 10 years after RYGB, suggesting a long-lasting effect. This phenomenon may play a key role in maintaining T2DM remission and weight loss after RYGB. Following RYGB, fasting GLP-1 levels are usually unchanged (Tables 2 and 3). Nevertheless, the GLP-1 response to a glucose or mixed meal is consistently enhanced (Tables 2 and 3). This effect occurs within two days following surgery[37] and remains stable for one year[25,26,38].
Jiménez et al[39] showed that an enhanced GLP-1 response to meal intake in T2DM patients is not sufficient to maintain normal glucose tolerance in the long term after RYGBP.
Similar to RYGB, a large number of studies have shown unchanged fasting GLP-1 and a significant increase in response to a glycemic challenge.
Peterli et al[27] prospectively evaluated GLP-1 changes following SG in randomized patients to receive either SG or RYGB. Curiously, following a test meal, GLP-1 results augmented in the SG patients and PYY response similar to RYGB patients. Another, there was a rapid resolution of T2DM in both groups. Their results do not confirm the foregut theory that hypothesizes that bypassing the duodenum and proximal small intestine is important to achieve the diabetes remission following bariatric surgery. Another hypothesis suggests that the rapid gastric transit arises as consequence of both early exposure of the distal intestine to nutrients and stimulation of the ‘‘ileal brake’’ mechanism[1,33]. This combination of effects influences digestive process and feeding behavior.
Peptide tyrosine tyrosine
PYY1-36 is co-secreted with GLP-1 and OXM by L-cells of the distal small bowel and colon in proportion to calorie content of the meal[1]. The main circulating and active form, PYY3-36, is formed by cleavage of PYY1-36 by the enzyme DPP-IV[40]. Serum levels rise within 15 min after initiating a meal and peak 1-2 h later, remaining elevated for various hours[41]. An anorectic effect of PYY3-36 has also been reported[42]. Similar to GLP-1, an anorectic CNS action was reported and it acts by reducing gastric emptying and acid production, by inhibiting pancreatic exocrine function, and by slowing intestinal transit time. Obese subjects show a reduced PYY3-36 response to feeding that might represent a cause of excessive appetite[43].
PYY3-36 has the anorectic action on the ARC through the Y2 receptors expressed at NPY/AgRP neuronal level, beyond in the nucleus of the solitary tract and the nodose ganglion of the vagus. Most of PYY3-36 anorectic effect is likely mediated by the vagus nerve[44].
Following RYGB, similar to GLP-1, there is a forward and particular increase in PYY3-36 response to a glycemic challenge (Tables 2 and 3). Along with GLP-1, this contributes to decreased appetite and rapid weight loss[40,45]. Three studies also reported a significantly increased fasting level of PYY3-36 following RYGB. Following the AGB, two studies[25,26] failing to demonstrate a variation in either fasting PYY or meal-stimulated response. This aspect showed no agreement with the enhanced response following RYGB.
Three researches[27,46,47] have showed the changes in meal-stimulated PYY3-36 response before and after SG reinforcing Peterli’s conclusions regarding an increased GLP-1 response to SG; the reserches showed an elevated meal-stimulated PYY3-36 response, that was similar to the change in PYY3-36 after RYGB.
Insulin
Insulin develops numerous roles in energy metabolism and is hormone more widely. Elevated serum glucose levels following a meal stimulate, particularly, insulin release by pancreatic beta cells. Insulin release is also induced by several amino acids (alanine, glycine, and arginine), acetylcholine from vagal nerve endings, and some incretins, including GLP-1 and glucose-dependent insulinotropic polypeptide (GIP)[1].
Insulin, among its various functions, is central to energy balance. Postprandial secretion of insulin lowers spikes of glucose levels following a meal by storing glucose as glycogen in muscle and liver and as triglycerides in adipose tissue. Vice versa, low serum insulin levels in fasting situations end up in mobilization of glucose from the liver and fatty acids from muscle. Consequently a major ATP amount is produced by β-oxidation, from adipose tissue.
Insulin has both long- and short-term effects on energy homeostasis. Insulin shows a negative feedback role on feeding by direct effects on the CNS[1]. In animal studies, decreased food intake and weight loss is caused by insulin after injected at central level. NPY/AgRP neurons in the ARC are inhibited by insulin. Its levels have also been suggested as long-term indicators of overall energy stores. Overweight compared with lean subjects showed higher basal and meal-stimulated insulin levels[48].
Fasting insulin levels are decreased due to RYGB and BPD/DS (Tables 2 and 3). Obese patients with T2DM shows a rapid improvement in glycemic control or impaired glucose tolerance. This is the result of: (1) a restored first-phase insulin response to a glycemic challenge due to an enhanced incretin effect; and (2) an improved insulin sensitivity related to weight loss.
Fasting insulin levels are also reduced after AGB. Nevertheless, the enhanced incretin effect in response to a meal is not observed. In diabetic patients, insulin sensitivity improves with weight loss.
SG also results in rapid resolution of diabetes in T2DM patients with decreased fasting insulin levels and a rapid improvement of the first phase insulin response and insulin sensitivity[27,49].
Leptin
Leptin is a 167-amino acid protein produced in several sites in the body, in particular by adipocytes - and it acts as a signal of overall body energy stores rising in proportion to total body fat mass[50].
Leptin is produced mainly in white adipose tissue, although it is also produced in brown adipose tissue, placenta, ovaries, skeletal muscle, stomach, breast, bone marrow, pituitary, and liver[1].
Several researches have showed that leptin acts on the hypothalamus, where it inhibits NPY/AgRP receptor neurons in the ARC while stimulates α-MSH neurons[1,9,11]. The OB-Rb receptor woold seems to be binded to leptin. The JAK-STAT and MAPK signal transduction pathways woold seems to be activated by leptin[1].
Leptin acts on appetite and energy balance oppositely to the action of grelin. Although in mice leptin appears to act as a significant physiological brake on appetite, its role in humans requires further studies, because of minimal weight loss obtained in obese patients taking leptin analogues, as reported by Harvey et al[1].
Harvey et al[1] has reported that leptin resistance in obesity has been proposed as one possible interpretation of this finding. Another hypothesis is that, in humans, relative leptin deficiency is essentially the more potent stimulus at times of negative energy balance, such as starvation and diet-induced weight loss, as reported by Harvey et al[1]. In this way the weight loss recovery is facilitated.
In different studies the serum leptin measurement after metabolic surgery was regularly performed; the results demonstrated its decrease with weight[45]. At the moment, there are no data demonstrating strong differences among leptin levels after equivalent weight loss due to RYGB or diet/exercise. Therefore, it should be excluded a direct bariatric surgery effect on serum leptin concentration.
Adiponectin
High-molecular-weight adiponectin is a 244-amino acid protein produced in white adipose tissue[1]. Serum adiponectin levels are inversely proportional to body fat mass, BMI, waist-to-hip ratio, serum insulin, and glucose levels[1]. In some studies, obese subjects compared with lean controls show a reduction of fasting adiponectin levels[51]. Nevertheless, the response to a meal appear to be exaggerated in obese subjects[51].
In the pathogenesis of insulin resistance in T2DM woold seems to have a role adiponectin. In rodents, hepatic gluconeogenesis is improved by adiponectin administration while fatty acid utilization in muscle cells appears to be increased[52]. Moreover, in a study comparing Pima Indians - a population with a high propensity for obesity and T2DM - and Caucasians, hypoadiponectinemia is more closely related to the degree of insulin resistance[53], high levels being protective against developing T2DM[54].
About the adiponectin levels before and after RYGB, just one study reported an increase in fasting adiponectin. No significant difference in adiponectin levels after BPD/DS were found in three out of four studies[51-54], despite 44% decrease of total body weight reported by Kotidis et al[55]. The differences could be related, among other things, to the presence of adiponectin gene mutations that alter its physiological functional role and affect its levels in the body. For this reason, we suggest to perform molecular investigations in order to clarify the mutational state of adiponectin gene and the effect of possible mutations on level outcomes.
Following AGB, adiponectin levels also rise as the weight reduction occurs. To our knowledge, no study has investigated changes in adiponectin following SG.
Tables 2 and 3 summarize short and long term effect of bariatric procedures on serum fasting gut hormones considered in this review.
ADAPTED BARIATRIC SURGERY
Actually our knowledges on the mechanisms of energy homeostasis are increased, newer bariatric procedures have been developed to achieve specific metabolic goals. Surgical procedures designed to treat T2DM in non-morbidly obese patients, such as the ileal interposition[56] and duodenal-jejunal bypass[57], are being evaluated in Institutional Review Board-approved researches. Bariatric surgery improve strongly diabetic as showed in most of the studies. Discontinuation of antidiabetic medications and remission of T2DM after bariatric surgery were put in evidence in 86.8% and 64.7% of the patients, respectively, with fasting plasma glucose and glycated hemoglobin (HbA1c) slightly above normal range[57]. Furthermore, bariatric surgery provided adequate glycemic control for 30.1% of the patients using insulin prior to surgery[57]. It has been showed, in some studies, that malabsorptive bariatric techniques have higher diabetes remission rates than restrictive ones[57-59]. T2DM usually finds the solution within few days to some weeks following malabsorptive procedures such as RYGB and BPD; in both cases a significant weight loss is previously achieved[57]. Although the exact mechanism is not yet completely understood, some studies support the idea that malabsorptive procedures involving rerouting of food might control high glucose levels in T2DM by bursting insulin sensitivity and/or by improving β-cell function[57]. Obviously, both mechanisms favor losing weight and reducing caloric intake[59-62]. Some studies have described that acute insulin response to intravenous glucose and early phase insulin response to oral glucose load significantly improve within a month following gastrointestinal bypass surgery[57,60,63]. Mechanisms for these mutations could be due to the important decrease in insulin resistance and the increase in GLP-1 postprandial plasma levels immediatly after surgery[57]. Actually, two hypotheses, named hindgut and foregut theory, have been proposed to explain T2DM improvment after metabolic surgery and surgical-induced weight loss. The former theory claims that surgical nutrient rerouting to the small intestine distal part ends up in elevated GLP-1 secretion and concomitant GLP-1 glucose-lowering effects; the latter hypothesis emphasizes that surgical bypass of the foregut prevents the release of a not yet well-defined nutrient, that functions as a pro-diabetes stimulus in predisposed subjects[64]. The weight loss effect of metabolic surgery on T2DM in not severely obese patients (BMI ≥ 35 kg/m2) might be lower than that on T2DM with a higher BMI (≥ 40 kg/m2)[57]. The understanding of the above mechanism is the key to success in metabolic surgery. There is no strong evidence describing the effectiveness of metabolic surgery on long-term follow-up in obese diabetic individuals[57].
LINKING PHARMACOTHERAPY AND BARIATRIC SURGERY
The therapeutic effect of metabolic surgery are not observed in all patients. For example, up to 15% of patients fail to achieve a weight loss over 30%[1,65] and a minority of diabetic patients will still have inadequate glycemic control following after bariatric surgery[1,66]. In others, with weight regain the presurgical hormonal conditions comes back. A hormonal panel may point to a particular feedback system that is limiting the overall success. Targeted pharmacotherapy might be necessary and sufficient to suppress the system and achieve the metabolic goal. As clearly depicted in their work, Le Roux et al[45] enforced jejunoileal bypass on rats and compared these to sham-operated rats. Increased PYY3-36 caused weight loss in jejunoileal bypass rats. Antagonism of the PYY with a specific neutralizing antibody led the rats to re-gain weight, whereas administration of PYY3-36 resulted in additional weight.
Outcome of bariatric surgery on insulin sensitivity and secretion is different in relation to the type of surgery methods that was performed[67]. In fact, while RYGB enhances insulin secretion after a meal, thus improving glucose metabolism, BPD/DS acts through the improvement in insulin sensitivity allowing the subsequent reduction of insulin hypersecretion, a typical feature of the insulin resistance state[67]. Gastric banding action is expected to be mediated only through weight loss, and the effect of sleeve gastrectomy remains still to be clarified. Incretin secretion is mostly elevated under nutrient stimulation after gastric bypass, likely leading to an overstimulation of pancreatic β-cells, and resulting in the elevated insulin secretion.
RYGB intervention affects fasting GLP-1, PYY or ghrelin levels and produces greater improvement in insulin sensitivity compared with diet at equivalent weight loss in T2DM patients[68]. No beneficial effect was observed in nondiabetic subjects at this early time-point.
No change in fasting GLP-1 concentrations after massive weight loss achieved with bariatric surgery was reported. In particular, after biliopancreatic diversion in morbidly obese patients without diabetes mellitus[69].
All hormones showed changes from baseline: some variations have been identified soon after surgery (ghrelin, leptin, adiponectin), whereas others were preserved in the long term (GLP-1, PYY, ghrelin, leptin adiponectin), as summarized in Tables 2 and 3[70-136]. At present no clinical studies are available combining bariatric procedures with specific pharmacotherapy for metabolic complications. However this topic appears to be of increasing interest, especially if it is implemented by pharmacogenomic data that could illustrate the specific pattern of drug susceptibly belonging to each patient.
NAFLD AND BARIATRIC SURGERY
Bariatric surgery was historically related with reduced prevalence and severity of liver disease. However, in some studies, the progression of liver disease in patients postoperatively was reported. In the early age of bariatric surgery, jejunoileal bypass was the most usually enforced procedure. A considerable portion of patients developed advanced liver disease, probably due to bacterial overgrowth and endotoxemia in the bypassed intestine, resulting in bacterial translocation and liver disease. Nevertheless, the jejunoileal bypass surgery has rarely been enforced in recent years due to the multiple complications arising from this procedure.
In a recent review the association between bariatric surgery (RYGB) and ghrelin reduction was underlined; ghrelin is known to stimulate insulin counter regulatory hormones, reduce adiponectin, and block hepatic insulin signalling[137]. Moreover, RGYB is also associated with GLP-1 high levels; consequently it enhances glucose tolerance by enhancing insulin secretion, suppressing glucagon production, inhibiting gastric emptying, and increasing B cell mass[137]. For this reason, bariatric surgery is probably to have potential benefit in ameliorating this gut hormones that strongly contribute to NAFLD pathogenesis[137]. Nevertheless, Hafeez et al[137] concluded that further investigations are needed to determine (1) the benefit of bariatric surgery in NAFLD patients at high risk of developing liver cirrhosis; and (2) the role of bariatric surgery in modulation of NAFLD complications such as diabetes and cardiovascular disease. The outcomes of the future researches will evaluate whether bariatric surgery will be one of the prescribed choice for treatment of the most progressive NAFLD type[137].
GUT MICROBIOTA AND BARIATRIC SURGERY
Bariatric surgery is generally the only available treatment for severe obesity that regularly develops and sustains considerable weight loss[138]. This surgery leads to some mutations in acid exposure to the gastric remnant and proximal small bowel. It also acts by restricting the amount and types of food that can be easly ingested, by promoting a modest degree of nutrient malabsorption by shortening the length of the small bowel. In addition, the RYGB may result in intestinal dysmotility. Actually, very little is known about the mutations in the gut microbiome that occur after RYGB, and, Kong et al[139] showed an increase in gut microbiome richness and in the number of associations between gut microbiome and white adipose tissue genes. Variations of gut microbiome were correlated with mutations in white adipose tissue gene expression[139]. These findings stimulate deeper explorations of the mechanisms linking gut micro-biome and white adipose tissue pathological alterations in human obesity and its variations after weight loss[139]. In this field, genomic, transcriptomic and bioinformatics analysis could offer a potent integrated tool to clarify the majority of unclear aspects.
Osto et al[140] showed that RYGB surgery might differently modify the gut microbiome composition in the three distinct anatomical sections of the small intestine compared to sham surgery. RYGB induced variations in the microbiota of the alimentary limb and the common channel resembling those seen after prebiotic treatment or weight loss by dieting, as reported by Osto et al[140]. These variations may be associated with altered production of intestinal hormones known to control energy balance. Postsurgical modulation of gut microbiome may significantly contribute to the beneficial metabolic effects of RYGB surgery[140], not excluding those on NAFLD.
Ashrafian et al[141] reported that, in rats, surgically induced metabolic shifts identify some of the potential mechanisms that contribute toward bariatric cardio-protection through gut microbiota ecological fluxes and an entero-cardiac axis to shield against metabolic syndrome of cardiac dysfunction.
Recently, Sweeney and Morton[142], in their review, have been provided the practicing surgeon with (1) an update on the state of a quickly innovating branch of clinical bioinformatics, specifically, the micro-biome; (2) a new understanding of the micro-biome changes after RYGB and weight loss; and (3) a basis for understanding further clinical applications of studies of the distal gut micro-biome, such as in Crohn’s disease, ulcerative colitis, and infectious colitis.
We suggest that the application of bioinformatics tools for better understanding the most significant micro-biome features could be cross-linked with the results of the expression hormone gene analysis conducted in the patients after weight loss. In this way it is possible to interpret the actual contribute of micro-biome in treatment outcomes.
FUTURE DIRECTIONS
Bariatric surgery leads to certain variations in the hormonal milieu that develop weight loss in obese subjects acting on energy balance regulation, particularly on food intake. Bariatric surgery is also related to some potential beneficial effects on co-morbid diseases correlated with obesity such as T2DM, metabolic syndrome[71,143,144] and NAFLD. Translational researches about the relative contribution of abounding signaling pathways in energy homeostasis will probably appear in combined treatment strategies containing both pharmacotherapy and surgery. Future research in these areas is warranted.
Surgery aimed principally at diseases such as diabetes and not weight loss are referred to as “metabolic surgery”, as reported by Cohen et al[145]. Cohen et al[145] showed that metabolic surgery has been proven to be safe and effective, and although more data are needed, it is unquestionable that a new discipline has been founded. Metabolic surgery can effectively treat T2DM in individuals with any BMI, including < 35 kg/m2, who do no respond to standard medical therapy[145].
Bariatric surgery can be enforced safely with acceptable low complication rate and mortality by specialized teams.
Changes in gut hormones, containing increases in GLP-1, PYY, and oxyntomodulin, decrease in GIP and ghrelin, or the combined action of all these hormones, might have a role in induction and long-term maintenance of weight loss[146]. In particular, it has been recently demonstrated by Holst[147] that GLP-1 and PYY appear to contribute tightly to the reduction in food intake after bypass and, thus, to the weight loss.
Actually, there are no data indicating that a reduced secretion of the hormones is associated with the pathogenesis of obesity and/or T2DM, but impaired secretion usually observed in obesity (and hence also in diabetes) may contribute to the development, as reported by Holst[147]. These hormones have subsequently become attractive novel targets for the development of obesity and T2DM therapies[148].
The previous study of the hormones mutational state with molecular tools could relate the pathogenesis aspects to the therapy aspects: if the presence of pathogenic mutations is highlighted by genomic analysis, its effects could affect or impair the hormone physiological role. In this case also the therapy outcomes could be influenced by the genes variations; consequently therapy strategy must be design to overcome the problem.
In fact, by recapitulating the gut hormone secretion changes after bariatric surgery, drugs based on gut hormones represent an exciting possibility for the treatment of obesity and T2DM[149]. Nevertheless, the timing, the exact variations of gut hormones and the relative importance of these ones in the metabolic improvement post-bariatric surgery remain to be further clarified[150].
Dixon et al[151] has reported that bariatric surgery, in addition to its profound weight-reduction effects, leads to a durable resolution of T2DM. Therefore, Dixon et al[151] has hypothesized that a cure for this disease may be obtainable, both by surgery as well as with drugs or devices that mimic surgery effects.
All these studies are safely interesting and will probably improve our knowledge on the pathogenesis of several metabolic diseases. This aspect is positively related to the possibility to identify new and exciting therapeutic opportunities. However, high-level controlled trials evaluating in particular long term benefits of bariatric surgery in obesity and its co-morbid diseases such as T2DM and the metabolic syndrome are required[152].
Recently, Lee et al[153] suggested that the novel anorexic hormone nesfatin-1 and another new hormone, the obestatin, might contribute to the marked improvement in glycemic homeostasis and weight loss in diabetics after RYGB and SG.
Nesfatin-1 is a recently identified 82-amino-acid peptide derived from the precursor protein, nucleobindin2 (NUCB2)[154]. The brain distribution of NUCB2/nesfatin-1 at the mRNA and protein level along with functional studies in rodents support a role for NUCB2/nesfatin-1 as a novel satiety molecule acting through leptin-independent mechanisms[154].
Consequently may be very significant the quantification of nesfatin levels in obese patients by using the appropriate methods transcriptomic-based.
Obestatin is a recently discovered 23-amino acid peptide encoded by the ghrelin gene[155,156]. Although in the original Zhang’s works obestatin appeared to suppress food intake and decrease gastric emptying[155-157], consequently antagonizing the orexigenic effect of ghrelin, subsequent researches in rodents showed controversial results[155-161]. Obestatin is present not only in the gastrointestinal tract, but also in the spleen, mammary gland, breast milk, and plasma[155,156,160]. Obestatin seems to function as part of a complex gut-brain network whereby hormones and substances from the stomach, intestine and the brain about satiety or hunger[155,156,160].
Lee et al[153] concluded that RYGB and SG produce differential influences with regards to circulating nesfatin-1 and obestatin levels in non-morbidly obese, T2DM patients. Circulating nesfatin-1 may modulate glucose homeostasis in two surgical procedures, and participate in regulating body weight in SG. However, many questions are still to be answered, in particular the receptor involved in the peptide’s actions, and the processing of NUCB2 to nesfatin-1 in hypotalamic or gut tissues still remain elusive. Furthermore, signaling mechanisms directly associated with the action of nesfatin-1 have been little explored[153].
CONCLUSION
It is important to emphasize the role of the major peptides released by the enteroendocrine system, which promote satiety and modulate energy homeostasis and utilization, as well as those that control fat absorption and intestinal permeability, as reported by Mells et al[162]. Clarifying new functions for enteroendocrine system-related peptides and developing pharmacologic peptide analogues offer future pharmacologic chances for obesity-related human disease, of which NAFLD is such important a part[4].
Bariatric surgery could be the most effective treatment for obesity and co-morbidities, often within days after surgery, independently of weight loss[163] and it is actually the only therapy available for obesity which results in long-term, sustained weight loss[148].
We hypothesize that gut hormones might induce and mainten the weight loss to long-term, could determine the improvement of obesity-related co-morbidities and could help to detect new drug targets and improved surgical procedures.
Footnotes
P- Reviewer: Demonacos C, M’Koma AE S- Editor: Ding Y L- Editor: A E- Editor: Zhang DN
References
- 1.Harvey EJ, Arroyo K, Korner J, Inabnet WB. Hormone changes affecting energy homeostasis after metabolic surgery. Mt Sinai J Med. 2010;77:446–465. doi: 10.1002/msj.20203. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375–3384. doi: 10.3748/wjg.v19.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 2010;140:648–652. doi: 10.3945/jn.109.111179. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140:578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 9.Obici S. Minireview: Molecular targets for obesity therapy in the brain. Endocrinology. 2009;150:2512–2517. doi: 10.1210/en.2009-0409. [DOI] [PubMed] [Google Scholar]
- 10.Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology. 2013;154:709–717. doi: 10.1210/en.2012-1421. [DOI] [PubMed] [Google Scholar]
- 11.Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept. 2012;2012:287457. doi: 10.1155/2012/287457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollywood A, Ogden J, Pring C. The impact of a bariatric rehabilitation service on weight loss and psychological adjustment--study protocol. BMC Public Health. 2012;12:275. doi: 10.1186/1471-2458-12-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field BC, Chaudhri OB, Bloom SR. Obesity treatment: novel peripheral targets. Br J Clin Pharmacol. 2009;68:830–843. doi: 10.1111/j.1365-2125.2009.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsesmeli N, Coumaros D. The future of bariatrics: endoscopy, endoluminal surgery, and natural orifice transluminal endoscopic surgery. Endoscopy. 2010;42:155–162. doi: 10.1055/s-0029-1243876. [DOI] [PubMed] [Google Scholar]
- 15.Emerging Technologies and Clinical Issues Committees of the ASMBS. American Society for Metabolic and Bariatric Surgery Position Statement on emerging endosurgical interventions for treatment of obesity. Surg Obes Relat Dis. 2009;5:297–298. doi: 10.1016/j.soard.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Burton PR, Brown WA. The mechanism of weight loss with laparoscopic adjustable gastric banding: induction of satiety not restriction. Int J Obes (Lond) 2011;35 Suppl 3:S26–S30. doi: 10.1038/ijo.2011.144. [DOI] [PubMed] [Google Scholar]
- 17.Botella Romero F, Milla Tobarra M, Alfaro Martínez JJ, García Arce L, García Gómez A, Salas Sáiz MÁ, Soler Marín A. [Bariatric surgery in duodenal switch procedure: weight changes and associated nutritional deficiencies] Endocrinol Nutr. 2011;58:214–218. doi: 10.1016/j.endonu.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 19.Kalra SP, Kalra PS. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides. 2004;38:201–211. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Frühbeck G, Diez-Caballero A, Gómez-Ambrosi J, Gil MJ, Monreal I, Salvador J, Cienfuegos JA. Disruption of the leptin-insulin relationship in obese men 24 hours after laparoscopic adjustable silicone gastric banding. Obes Surg. 2002;12:366–371. doi: 10.1381/096089202321088174. [DOI] [PubMed] [Google Scholar]
- 23.Frühbeck G, Diez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–309. doi: 10.1056/NEJM200401153500323. [DOI] [PubMed] [Google Scholar]
- 24.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 25.Bose M, Machineni S, Oliván B, Teixeira J, McGinty JJ, Bawa B, Koshy N, Colarusso A, Laferrère B. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18:1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 28.Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–1029. doi: 10.1381/0960892054621125. [DOI] [PubMed] [Google Scholar]
- 29.Catheline JM, Fysekidis M, Dbouk R, Boschetto A, Bihan H, Reach G, Cohen R. Weight loss after sleeve gastrectomy in super superobesity. J Obes. 2012;2012:959260. doi: 10.1155/2012/959260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2012;166:27–41. doi: 10.1111/j.1476-5381.2011.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–399. doi: 10.1210/rp.56.1.377. [DOI] [PubMed] [Google Scholar]
- 32.Steinert RE, Poller B, Castelli MC, Drewe J, Beglinger C. Oral administration of glucagon-like peptide 1 or peptide YY 3-36 affects food intake in healthy male subjects. Am J Clin Nutr. 2010;92:810–817. doi: 10.3945/ajcn.2010.29663. [DOI] [PubMed] [Google Scholar]
- 33.Young AA, Gedulin BR, Rink TJ. Dose-responses for the slowing of gastric emptying in a rodent model by glucagon-like peptide (7-36) NH2, amylin, cholecystokinin, and other possible regulators of nutrient uptake. Metabolism. 1996;45:1–3. doi: 10.1016/s0026-0495(96)90192-4. [DOI] [PubMed] [Google Scholar]
- 34.Cobble M. Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:8. doi: 10.1186/1758-5996-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowzee AM, Cawley NX, Chiorini JA, Di Pasquale G. Glucagon-like peptide-1 gene therapy. Exp Diabetes Res. 2011;2011:601047. doi: 10.1155/2011/601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willard FS, Sloop KW. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Exp Diabetes Res. 2012;2012:470851. doi: 10.1155/2012/470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 38.Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg. 2013;257:894–899. doi: 10.1097/SLA.0b013e31826b8603. [DOI] [PubMed] [Google Scholar]
- 40.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60:810–818. doi: 10.2337/db10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizrahi M, Ben Ya’acov A, Ilan Y. Gastric stimulation for weight loss. World J Gastroenterol. 2012;18:2309–2319. doi: 10.3748/wjg.v18.i19.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145:12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Small CJ, Bloom SR. The therapeutic potential of gut hormone peptide YY3-36 in the treatment of obesity. Expert Opin Investig Drugs. 2005;14:647–653. doi: 10.1517/13543784.14.5.647. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Jayasena CN, Bloom SR. The gut hormones in appetite regulation. J Obes. 2011;2011:528401. doi: 10.1155/2011/528401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 47.Valderas JP, Irribarra V, Boza C, de la Cruz R, Liberona Y, Acosta AM, Yolito M, Maiz A. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab. 2010;95:1069–1075. doi: 10.1210/jc.2009-0983. [DOI] [PubMed] [Google Scholar]
- 48.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664–669. doi: 10.1016/j.surg.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 51.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 53.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 54.Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne) 2011;2:62. doi: 10.3389/fendo.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotidis EV, Koliakos G, Papavramidis TS, Papavramidis ST. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006;16:554–559. doi: 10.1381/096089206776944940. [DOI] [PubMed] [Google Scholar]
- 56.Gill RS, Karmali S, Sharma AM. Treating type 2 diabetes mellitus with sleeve gastrectomy in obese patients. Obesity (Silver Spring) 2011;19:701–702. doi: 10.1038/oby.2010.261. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu H, Timratana P, Schauer PR, Rogula T. Review of Metabolic Surgery for Type 2 Diabetes in Patients with a BMI & lt; 35 kg/m(2) J Obes. 2012;2012:147256. doi: 10.1155/2012/147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, Nguyen NT. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254:410–20; discussion 420-2. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee WJ, Chong K, Chen CY, Chen SC, Lee YC, Ser KH, Chuang LM. Diabetes remission and insulin secretion after gastric bypass in patients with body mass index & lt; 35 kg/m2. Obes Surg. 2011;21:889–895. doi: 10.1007/s11695-011-0401-6. [DOI] [PubMed] [Google Scholar]
- 61.Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, Ferrannini E. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–2143. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 62.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 63.Scopinaro N, Adami GF, Papadia FS, Camerini G, Carlini F, Fried M, Briatore L, D’Alessandro G, Andraghetti G, Cordera R. Effects of biliopanceratic diversion on type 2 diabetes in patients with BMI 25 to 35. Ann Surg. 2011;253:699–703. doi: 10.1097/SLA.0b013e318203ae44. [DOI] [PubMed] [Google Scholar]
- 64.Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270–2276. doi: 10.1007/s00125-009-1511-8. [DOI] [PubMed] [Google Scholar]
- 65.Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc. 2009;23:2302–2306. doi: 10.1007/s00464-008-0322-1. [DOI] [PubMed] [Google Scholar]
- 66.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu JL, Ramshaw BJ, Papanicolaou DA, Ziegler TR, Smith CD. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–784. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 67.Castagneto-Gissey L, Mingrone G. Insulin sensitivity and secretion modifications after bariatric surgery. J Endocrinol Invest. 2012;35:692–698. doi: 10.3275/8470. [DOI] [PubMed] [Google Scholar]
- 68.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, McMahon DJ, Korner J. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring) 2011;19:2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Luis D, Pacheco D, Conde R, Primo D, Aller R, Izaola O. Basal GLP-1 levels in morbidly obese patients following biliopancreatic diversion surgery. Ann Nutr Metab. 2012;61:70–73. doi: 10.1159/000339378. [DOI] [PubMed] [Google Scholar]
- 70.Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, Ghatei MA, Bloom SR, le Roux CW, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr. 2011;35:169–180. doi: 10.1177/0148607110381403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 72.Couce ME, Cottam D, Esplen J, Schauer P, Burguera B. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- 73.Sundbom M, Holdstock C, Engström BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 74.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 75.Morínigo R, Casamitjana R, Moizé V, Lacy AM, Delgado S, Gomis R, Vidal J. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–1116. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 76.Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 77.Frühbeck G, Rotellar F, Hernández-Lizoain JL, Gil MJ, Gómez-Ambrosi J, Salvador J, Cienfuegos JA. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–1215. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 78.Holdstock C, Engström BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 79.Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, Gutiérrez C, Simón I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 80.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–39. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Arnes J, Gallego-Perales JL, Rivas-Marin J, Morcillo S, Cardona I, Soriguer F. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18:1424–1429. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- 82.García de la Torre N, Rubio MA, Bordiú E, Cabrerizo L, Aparicio E, Hernández C, Sánchez-Pernaute A, Díez-Valladares L, Torres AJ, Puente M, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 83.Pardina E, López-Tejero MD, Llamas R, Catalán R, Galard R, Allende H, Vargas V, Lecube A, Fort JM, Baena-Fustegueras JA, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19:1414–1423. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 84.Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 85.Roth CL, Reinehr T, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:29–35. doi: 10.1007/s11695-008-9568-x. [DOI] [PubMed] [Google Scholar]
- 86.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–350. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 87.Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47–55. doi: 10.1007/s11695-008-9642-4. [DOI] [PubMed] [Google Scholar]
- 88.Hanusch-Enserer U, Brabant G, Roden M. Ghrelin concentrations in morbidly obese patients after adjustable gastric banding. N Engl J Med. 2003;348:2159–2160. doi: 10.1056/NEJM200305223482125. [DOI] [PubMed] [Google Scholar]
- 89.Hanusch-Enserer U, Cauza E, Brabant G, Dunky A, Rosen H, Pacini G, Tüchler H, Prager R, Roden M. Plasma ghrelin in obesity before and after weight loss after laparoscopical adjustable gastric banding. J Clin Endocrinol Metab. 2004;89:3352–3358. doi: 10.1210/jc.2003-031438. [DOI] [PubMed] [Google Scholar]
- 90.Schindler K, Prager G, Ballaban T, Kretschmer S, Riener R, Buranyi B, Maier C, Luger A, Ludvik B. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Invest. 2004;34:549–554. doi: 10.1111/j.1365-2362.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 91.Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, Patterson C, Weinshel E, Fielding GA, Ren C, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089–1096. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg. 2009;19:357–362. doi: 10.1007/s11695-008-9688-3. [DOI] [PubMed] [Google Scholar]
- 93.Adami GF, Cordera R, Marinari G, Lamerini G, Andraghetti G, Scopinaro N. Plasma ghrelin concentratin in the short-term following biliopancreatic diversion. Obes Surg. 2003;13:889–892. doi: 10.1381/096089203322618713. [DOI] [PubMed] [Google Scholar]
- 94.Mingrone G, Granato L, Valera-Mora E, Iaconelli A, Calvani MF, Bracaglia R, Manco M, Nanni G, Castagneto M. Ultradian ghrelin pulsatility is disrupted in morbidly obese subjects after weight loss induced by malabsorptive bariatric surgery. Am J Clin Nutr. 2006;83:1017–1024. doi: 10.1093/ajcn/83.5.1017. [DOI] [PubMed] [Google Scholar]
- 95.Mingrone G, Nolfe G, Gissey GC, Iaconelli A, Leccesi L, Guidone C, Nanni G, Holst JJ. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52:873–881. doi: 10.1007/s00125-009-1288-9. [DOI] [PubMed] [Google Scholar]
- 96.García-Unzueta MT, Fernández-Santiago R, Domínguez-Díez A, Vazquez-Salví L, Fernández-Escalante JC, Amado JA. Fasting plasma ghrelin levels increase progressively after biliopancreatic diversion: one-year follow-up. Obes Surg. 2005;15:187–190. doi: 10.1381/0960892053268453. [DOI] [PubMed] [Google Scholar]
- 97.Adami GF, Cordera R, Andraghetti G, Camerini GB, Marinari GM, Scopinaro N. Changes in serum ghrelin concentration following biliopancreatic diversion for obesity. Obes Res. 2004;12:684–687. doi: 10.1038/oby.2004.79. [DOI] [PubMed] [Google Scholar]
- 98.Valera Mora ME, Manco M, Capristo E, Guidone C, Iaconelli A, Gniuli D, Rosa G, Calvani M, Mingrone G. Growth hormone and ghrelin secretion in severely obese women before and after bariatric surgery. Obesity (Silver Spring) 2007;15:2012–2018. doi: 10.1038/oby.2007.240. [DOI] [PubMed] [Google Scholar]
- 99.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, Prager G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 100.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4; discussion 4-5. [PubMed] [Google Scholar]
- 102.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 105.Reinehr T, Roth CL, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–1577. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 106.de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros Mazon S, da Silva CA, Geloneze B, Muscelli E, Alegre SM. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–320. doi: 10.1007/s11695-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 107.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 108.Marfella R, Barbieri M, Ruggiero R, Rizzo MR, Grella R, Mozzillo AL, Docimo L, Paolisso G. Bariatric surgery reduces oxidative stress by blunting 24-h acute glucose fluctuations in type 2 diabetic obese patients. Diabetes Care. 2010;33:287–289. doi: 10.2337/dc09-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valverde I, Puente J, Martín-Duce A, Molina L, Lozano O, Sancho V, Malaisse WJ, Villanueva-Peñacarrillo ML. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–397. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 111.Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC, Luciani A, Salerni B, Mittenperger F, Nodari S, et al. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res. 2004;36:111–115. doi: 10.1055/s-2004-814222. [DOI] [PubMed] [Google Scholar]
- 112.Molina A, Vendrell J, Gutiérrez C, Simón I, Masdevall C, Soler J, Gómez JM. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–621. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 113.Ramos AP, de Abreu MR, Vendramini RC, Brunetti IL, Pepato MT. Decrease in circulating glucose, insulin and leptin levels and improvement in insulin resistance at 1 and 3 months after gastric bypass. Obes Surg. 2006;16:1359–1364. doi: 10.1381/096089206778663706. [DOI] [PubMed] [Google Scholar]
- 114.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trakhtenbroit MA, Leichman JG, Algahim MF, Miller CC, Moody FG, Lux TR, Taegtmeyer H. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med. 2009;122:435–442. doi: 10.1016/j.amjmed.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coughlin CC, Finck BN, Eagon JC, Halpin VJ, Magkos F, Mohammed BS, Klein S. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity (Silver Spring) 2007;15:640–645. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 117.Riedl M, Vila G, Maier C, Handisurya A, Shakeri-Manesch S, Prager G, Wagner O, Kautzky-Willer A, Ludvik B, Clodi M, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–2312. doi: 10.1210/jc.2007-2383. [DOI] [PubMed] [Google Scholar]
- 118.Serra A, Granada ML, Romero R, Bayés B, Cantón A, Bonet J, Rull M, Alastrue A, Formiguera X. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin Nutr. 2006;25:400–408. doi: 10.1016/j.clnu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 119.Swarbrick MM, Austrheim-Smith IT, Stanhope KL, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 2006;49:2552–2558. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 120.Vila G, Riedl M, Maier C, Struck J, Morgenthaler NG, Handisurya A, Prager G, Ludvik B, Clodi M, Luger A. Plasma MR-proADM correlates to BMI and decreases in relation to leptin after gastric bypass surgery. Obesity (Silver Spring) 2009;17:1184–1188. doi: 10.1038/oby.2009.22. [DOI] [PubMed] [Google Scholar]
- 121.Vilarrasa N, Vendrell J, Sánchez-Santos R, Broch M, Megia A, Masdevall C, Gomez N, Soler J, Pujol J, Bettónica C, et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol (Oxf) 2007;67:679–686. doi: 10.1111/j.1365-2265.2007.02945.x. [DOI] [PubMed] [Google Scholar]
- 122.Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes Surg. 2004;14:659–665. doi: 10.1381/096089204323093453. [DOI] [PubMed] [Google Scholar]
- 123.Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, Ebenbichler CF, Stadlmann S, Moser PL, Tilg H. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–777. doi: 10.1016/j.jhep.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 124.Ram E, Vishne T, Maayan R, Lerner I, Weizman A, Dreznik Z, Konstantin B, Seror D, Pnina V. The relationship between BMI, plasma leptin, insulin and proinsulin before and after laparoscopic adjustable gastric banding. Obes Surg. 2005;15:1456–1462. doi: 10.1381/096089205774859146. [DOI] [PubMed] [Google Scholar]
- 125.Diker D, Vishne T, Maayan R, Weizman A, Vardi P, Dreznik Z, Seror D, Ram E. Impact of gastric banding on plasma adiponectin levels. Obes Surg. 2006;16:1057–1061. doi: 10.1381/096089206778026244. [DOI] [PubMed] [Google Scholar]
- 126.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–1581. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 127.Engl J, Bobbert T, Ciardi C, Laimer M, Tatarczyk T, Kaser S, Weiss H, Molnar C, Tilg H, Patsch JR, et al. Effects of pronounced weight loss on adiponectin oligomer composition and metabolic parameters. Obesity (Silver Spring) 2007;15:1172–1178. doi: 10.1038/oby.2007.627. [DOI] [PubMed] [Google Scholar]
- 128.Poitou C, Lacorte JM, Coupaye M, Bertrais S, Bedel JF, Lafon N, Bouillot JL, Galan P, Borson-Chazot F, Basdevant A, et al. Relationship between single nucleotide polymorphisms in leptin, IL6 and adiponectin genes and their circulating product in morbidly obese subjects before and after gastric banding surgery. Obes Surg. 2005;15:11–23. doi: 10.1381/0960892052993431. [DOI] [PubMed] [Google Scholar]
- 129.Salinari S, Bertuzzi A, Iaconelli A, Manco M, Mingrone G. Twenty-four hour insulin secretion and beta cell NEFA oxidation in type 2 diabetic, morbidly obese patients before and after bariatric surgery. Diabetologia. 2008;51:1276–1284. doi: 10.1007/s00125-008-1007-y. [DOI] [PubMed] [Google Scholar]
- 130.Camastra S, Manco M, Frascerra S, Iaconelli A, Mingrone G, Ferrannini E. Daylong pituitary hormones in morbid obesity: effects of bariatric surgery. Int J Obes (Lond) 2009;33:166–172. doi: 10.1038/ijo.2008.226. [DOI] [PubMed] [Google Scholar]
- 131.Rosa G, Mingrone G, Manco M, Euthine V, Gniuli D, Calvani R, Calvani M, Favuzzi AM, Castagneto M, Vidal H. Molecular mechanisms of diabetes reversibility after bariatric surgery. Int J Obes (Lond) 2007;31:1429–1436. doi: 10.1038/sj.ijo.0803630. [DOI] [PubMed] [Google Scholar]
- 132.Bruno C, Fulford AD, Potts JR, McClintock R, Jones R, Cacucci BM, Gupta CE, Peacock M, Considine RV. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95:159–166. doi: 10.1210/jc.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 134.Czupryniak L, Pawlowski M, Kumor A, Szymanski D, Loba J, Strzelczyk J. Predicting maximum Roux-en-Y gastric bypass-induced weight reduction--preoperative plasma leptin or body weight? Obes Surg. 2007;17:162–167. doi: 10.1007/s11695-007-9042-1. [DOI] [PubMed] [Google Scholar]
- 135.Maruna P, Gürlich R, Fried M, Frasko R, Chachkhiani I, Haluzik M. Leptin as an acute phase reactant after non-adjustable laparoscopic gastric banding. Obes Surg. 2001;11:609–614. doi: 10.1381/09608920160556814. [DOI] [PubMed] [Google Scholar]
- 136.Heinonen MV, Purhonen AK, Miettinen P, Pääkkönen M, Pirinen E, Alhava E, Akerman K, Herzig KH. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130:7–13. doi: 10.1016/j.regpep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 137.Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes. 2013;2013:839275. doi: 10.1155/2013/839275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 139.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 140.Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav. 2013;119:92–96. doi: 10.1016/j.physbeh.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 141.Ashrafian H, Li JV, Spagou K, Harling L, Masson P, Darzi A, Nicholson JK, Holmes E, Athanasiou T. Bariatric surgery modulates circulating and cardiac metabolites. J Proteome Res. 2014;13:570–580. doi: 10.1021/pr400748f. [DOI] [PubMed] [Google Scholar]
- 142.Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg. 2013;148:563–569. doi: 10.1001/jamasurg.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.del Genio F, Alfonsi L, Marra M, Finelli C, del Genio G, Rossetti G, del Genio A, Contaldo F, Pasanisi F. Metabolic and nutritional status changes after 10% weight loss in severely obese patients treated with laparoscopic surgery vs integrated medical treatment. Obes Surg. 2007;17:1592–1598. doi: 10.1007/s11695-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 144.Del Genio F, Del Genio G, De Sio I, Marra M, Alfonsi L, Finelli C, Contaldo F, Pasanisi F. Noninvasive evaluation of abdominal fat and liver changes following progressive weight loss in severely obese patients treated with laparoscopic gastric bypass. Obes Surg. 2009;19:1664–1671. doi: 10.1007/s11695-009-9891-x. [DOI] [PubMed] [Google Scholar]
- 145.Cohen R, Caravatto PP, Petry T. Metabolic Surgery for Type 2 Diabetes in Patients with a BMI of & lt; 35 kg/m(2): A Surgeon’s Perspective. Obes Surg. 2013;23:809–818. doi: 10.1007/s11695-013-0930-2. [DOI] [PubMed] [Google Scholar]
- 146.Ionut V, Burch M, Youdim A, Bergman RN. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity (Silver Spring) 2013;21:1093–1103. doi: 10.1002/oby.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol. 2013;13:983–988. doi: 10.1016/j.coph.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 148.Troke RC, Tan TM, Bloom SR. The future role of gut hormones in the treatment of obesity. Ther Adv Chronic Dis. 2014;5:4–14. doi: 10.1177/2040622313506730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan T, Bloom S. Gut hormones as therapeutic agents in treatment of diabetes and obesity. Curr Opin Pharmacol. 2013;13:996–1001. doi: 10.1016/j.coph.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Hage MP, Safadi B, Salti I, Nasrallah M. Role of Gut-Related Peptides and Other Hormones in the Amelioration of Type 2 Diabetes after Roux-en-Y Gastric Bypass Surgery. ISRN Endocrinol. 2012;2012:504756. doi: 10.5402/2012/504756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- 152.Busetto L, Sbraccia P, Frittitta L, Pontiroli AE. The growing role of bariatric surgery in the management of type 2 diabetes: evidences and open questions. Obes Surg. 2011;21:1451–1457. doi: 10.1007/s11695-011-0471-5. [DOI] [PubMed] [Google Scholar]
- 153.Lee WJ, Chen CY, Ser KH, Chong K, Chen SC, Lee PC, Liao YD, Lee SD. Differential influences of gastric bypass and sleeve gastrectomy on plasma nesfatin-1 and obestatin levels in patients with type 2 diabetes mellitus. Curr Pharm Des. 2013;19:5830–5835. doi: 10.2174/13816128113198880010. [DOI] [PubMed] [Google Scholar]
- 154.Stengel A, Taché Y. Minireview: nesfatin-1--an emerging new player in the brain-gut, endocrine, and metabolic axis. Endocrinology. 2011;152:4033–4038. doi: 10.1210/en.2011-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang JV, Li L, Huang Q, Ren PG. Obestatin receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:89–107. doi: 10.1016/B978-0-12-386933-3.00003-0. [DOI] [PubMed] [Google Scholar]
- 156.Martins C, Kjelstrup L, Mostad IL, Kulseng B. Impact of sustained weight loss achieved through Roux-en-Y gastric bypass or a lifestyle intervention on ghrelin, obestatin, and ghrelin/obestatin ratio in morbidly obese patients. Obes Surg. 2011;21:751–758. doi: 10.1007/s11695-011-0399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 158.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148:1648–1653. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carlini VP, Perez MF, Salde E, Schiöth HB, Ramirez OA, de Barioglio SR. Ghrelin induced memory facilitation implicates nitric oxide synthase activation and decrease in the threshold to promote LTP in hippocampal dentate gyrus. Physiol Behav. 2010;101:117–123. doi: 10.1016/j.physbeh.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 160.Carlini VP, Schiöth HB, Debarioglio SR. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem Biophys Res Commun. 2007;352:907–912. doi: 10.1016/j.bbrc.2006.11.112. [DOI] [PubMed] [Google Scholar]
- 161.Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vázquez MJ, Wiedmer P, Castañeda TR, et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148:21–26. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- 162.Mells JE, Anania FA. The role of gastrointestinal hormones in hepatic lipid metabolism. Semin Liver Dis. 2013;33:343–357. doi: 10.1055/s-0033-1358527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]


