Abstract
AIM: To conduct a meta-analysis comparing laparoscopic (LGD2) and open D2 gastrectomies (OGD2) for the treatment of advanced gastric cancer (AGC).
METHODS: Randomized controlled trials (RCTs) and non-RCTs comparing LGD2 with OGD2 for AGC treatment, published between 1 January 2000 and 12 January 2013, were identified in the PubMed, Embase, and Cochrane Library databases. Primary endpoints included operative outcomes (operative time, intraoperative blood loss, and conversion rate), postoperative outcomes (postoperative analgesic consumption, time to first ambulation, time to first flatus, time to first oral intake, postoperative hospital stay length, postoperative morbidity, incidence of reoperation, and postoperative mortality), and oncologic outcomes (the number of lymph nodes harvested, tumor recurrence and metastasis, disease-free rates, and overall survival rates). The Cochrane Collaboration tools and the modified Newcastle-Ottawa scale were used to assess the quality and risk of bias of RCTs and non-RCTs in the study. Subgroup analyses were conducted to explore the incidence rate of various postoperative morbidities as well as recurrence and metastasis patterns. A Begg’s test was used to evaluate the publication bias.
RESULTS: One RCT and 13 non-RCTs totaling 2596 patients were included in the meta-analysis. LGD2 in comparison to OGD2 showed lower intraoperative blood loss [weighted mean difference (WMD) = -137.87 mL, 95%CI: -164.41--111.33; P < 0.01], lower analgesic consumption (WMD = -1.94, 95%CI: -2.50--1.38; P < 0.01), shorter times to first ambulation (WMD = -1.03 d, 95%CI: -1.90--0.16; P < 0.05), flatus (WMD = -0.98 d, 95%CI: -1.30--0.66; P < 0.01), and oral intake (WMD = -0.85 d, 95%CI: -1.67--0.03; P < 0.05), shorter hospitalization (WMD = -3.08 d, 95%CI: -4.38--1.78; P < 0.01), and lower postoperative morbidity (odds ratio = 0.78, 95%CI: 0.61-0.99; P < 0.05). No significant differences were observed between LGD2 and OGD2 for the following criteria: reoperation incidence, postoperative mortality, number of harvested lymph nodes, tumor recurrence/metastasis, or three- or five-year disease-free and overall survival rates. However, LGD2 had longer operative times (WMD = 57.06 min, 95%CI: 41.87-72.25; P < 0.01).
CONCLUSION: Although a technically demanding and time-consuming procedure, LGD2 may be safe and effective, and offer some advantages over OGD2 for treatment of locally AGC.
Keywords: D2 lymph node dissection, Gastrectomy, Gastric cancer, Laparoscopy, Meta-analysis
Core tip: The Japanese Gastric Cancer Association guidelines stipulate that D2 gastrectomy is required for the treatment of advanced gastric cancer. Due to its technical difficulty and the lack of long-term results, the application of laparoscopic D2 gastrectomy (LGD2) remains questionable. Based on the results of this study, LGD2 had similar reoperation incidence, mortality, and oncologic outcomes compared with the open D2 gastrectomy for locally advanced gastric cancer treatment. Furthermore, LGD2 was associated with lower intraoperative blood loss, lower analgesic consumption, quicker recovery, shorter hospitalization, and lower morbidity, albeit with longer operative time.
INTRODUCTION
Gastric cancer is the third most common cancer and the second leading cause of cancer-related deaths in the world[1]. Radical gastrectomy, with lymph node dissection, is essential to cure this type of cancer[2]. The first reported usage of laparoscopic gastrectomy (LG) for early gastric cancer (EGC) came from Kitano et al[3]. Currently, LG is the accepted treatment of choice for EGC due to low postoperative pain, faster recovery, shorter hospital stay, and a better cosmetic outcome compared with open gastrectomy (OG)[4-7]. Three non-randomized clinical trials (non-RCTs) reported comparable five-year long-term oncologic outcomes using this type of treatment[8-10].
Uyama et al[11] were the first to report the use of LG with D2-extended lymph node dissection (LGD2) for the treatment of advanced gastric cancer (AGC) in 2000. The Japanese Gastric Cancer Association (JGCA) guidelines stipulate that D2 gastrectomy is required for treating AGC[12,13]. In the last decade, only a few surgeons worldwide, particularly in East Asia, have performed LGD2 to treat AGC[14-32]. However, the application of this treatment remains dubious due to its technical difficulty and the lack of long-term results[19,23,27,29,31,32].
According to the JGCA guidelines, D2 dissection of stations 12a or 10 can be technically demanding due to the serious risks of organ injury, bleeding, and/or bile and pancreatic leakage from a major vessel[29,32]. Nodal dissection can increase morbidity and mortality rates similar to those of open resections[33-35]. The laparoscopic approach for treatment of tumors with serosal invasion also risks the peritoneal seeding of malignant cells during the procedure. Several theories regarding the etiology of port-site recurrence, associated with pneumoperitoneum and visceral manipulation, have been proposed[36]. Another concern is the lack of long-term oncologic outcomes[31,32]. A meta-analysis of seven case-control studies comparing laparoscopy-assisted distal gastrectomy with OG for AGC revealed that LG was associated with better short-term outcomes and comparable three-year overall survival rates. However, these studies were comprised of only 1271 cases, as well as D1, D1+, and D2 lymph node dissections[37]. Consequently, we performed meta-analyses to evaluate whether LGD2 is an acceptable alternative to OGD2 for AGC treatment.
MATERIALS AND METHODS
Literature search
All RCTs and non-RCTs comparing LGD2 with OGD2 for AGC were identified by searching the PubMed, EMBASE, and Cochrane Library databases for studies published between 1 January 2000 and 12 January 2013. Only articles published in English or Chinese were included in this study. The following medical subject headings and free-text terms were used: stomach neoplasms; stomach cancer; gastric carcinoma; gastric cancer; laparoscopy; laparoscopic; minimally invasive; laparotomy; conventional gastrectomy; OG; D2 lymph node dissection; extended; radical. Additional relevant articles were identified using references of relevant articles and previous meta-analyses. The PubMed database was used to search for additional studies and trials using authors’ names and the “related articles” function. The World Health Organization International Clinical Trials Registry Platform, Clinical Trials, Cochrane Central Register of Controlled Trials, and Chinese Clinical Trial Register were used to identify any ongoing RCTs.
Definitions
Based on the preoperative clinical assessment or postoperative pathologic examination, AGC was defined as cancerous growth invading beyond the submucosal layer of the stomach. Locally AGC is the subgroup of AGC excluding stage IV. LG was defined as total LG or laparoscopy-assisted gastrectomy. In all included studies, D2 lymph node dissection was performed according to the JGCA lymph node classification[38]. The evaluated endpoints were classified as operative outcomes (operative time, intraoperative blood loss, and conversion rate), postoperative outcomes (postoperative analgesic consumption, time to first ambulation, time to first flatus, time to first oral intake, length of postoperative hospital stay, postoperative morbidity, incidence of reoperation, and postoperative mortality), and oncologic outcomes (number of lymph nodes harvested, tumor recurrence and metastasis, and disease-free and overall survival rates). The primary endpoints were postoperative morbidity and mortality as well as disease-free and overall survival rates. Other variables were considered as secondary endpoints.
Inclusion and exclusion criteria
The analyses included studies comparing LGD2 with OGD2 in patients with AGC. In cases when more than one publication reported on a single trial, only the most recent data were included, unless relevant outcomes were reported only in earlier publications. The following criteria were applied to exclude a study: < 40 cases; combined examination of AGC and EGC cases and/or D1-D3 lymphadenectomy, which prevented extraction of relevant or the authors’ provision of such data by email; malignant stromal tumors, benign disease, or emergency operations; use of hand-assisted LG, gasless laparoscopic surgery, or robotic surgery.
Method of review
The meta-analyses were performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement[39]. Two reviewers (Zou ZH and Zhao LY) independently evaluated all retrieved studies to determine if they met the criteria, to assess study quality, and extract data. The study team resolved all of their disagreements through discussion to reach a consensus.
Methodological quality assessment
The Cochrane Collaboration Handbook 5.1.0 was used to independently determine the quality and risk of bias of RCTs[40]. Following domains were assessed: sequence generation; allocation concealment; completeness of outcome data; selective outcome reporting; baseline comparability of groups; dropout rates. The risk of bias in each domain was assessed and classified as low, high, or unclear. Blinding methods were not examined in this review because both LGD2 and OGD2 are invasive, and the patients were informed preoperatively about the planned procedures.
The methodological quality of non-RCTs was assessed using the modified Newcastle-Ottawa scale[41]. Patient selection, comparability of LGD2 and OGD2 groups, and assessment of measured outcomes were examined. In assessing comparability between groups, focus was on the variables that might affect primary endpoints such as, patient age and sex, pathologic tumor-node-metastasis stage, type of gastrectomy, resection margin, tumor size, histologic type, reconstruction, and adjuvant treatment.
Studies were scored using an ordinal star scale, with higher scores representing higher quality. A maximum of one star was awarded to a study for each numbered item within the selection and outcome assessment. A maximum of two stars was awarded for the comparability of the two groups. The quality of each study was graded as level 1 (0-5 stars) or level 2 (6-9 stars).
Statistical analysis
Review Manager (RevMan, version 5.0; The Cochrane Collaboration, Oxford, United Kingdom) and STATA (version 11.2; STATA Corporation, College Station, TX, United States) software were used for statistical analyses. Weighted mean differences (WMDs) with 95%CIs were calculated for continuous variables, including operative time, intraoperative blood loss, postoperative analgesic consumption, time to first ambulation, time to first flatus, time to first oral intake; length of postoperative hospital stay, and number of harvested lymph nodes. The odds ratios (ORs) with 95%CIs were calculated for dichotomous variables, including postoperative morbidity and mortality rates, incidence of reoperation, tumor recurrence, and metastasis. The hazard ratios (HRs) with 95%CIs extracted from Kaplan-Meier curves were used for disease-free and overall survival rates[42,43]. A random effects model was used to pool studies with significant heterogeneity, as determined by the χ2 test (P ≤ 0.10) and the inconsistency index (I2 ≥ 50%)[44,45].
An alternative statistical effect model was used to reanalyze the data for the sensitivity analysis (e.g., a random effects model instead of a fixed effects model or vice versa). The incidences of various postoperative morbidities and recurrence, and metastasis patterns were determined using subgroup analyses. The Begg’s test was used to assess the presence of publication bias. Publication bias was present when the continuity-corrected Pr >|z| value was ≤ 0.1[46].
RESULTS
Descriptive assessment and study characteristics
Of the 493 publications identified in the initial literature search, 14 trials (1 RCT, 13 non-RCTs) were included in the analyses[19-32]. A total of 2596 participants (1328 in the LGD2 group and 1268 in the OGD2 group) were included in the study (Figure 1, Table 1).
Figure 1.
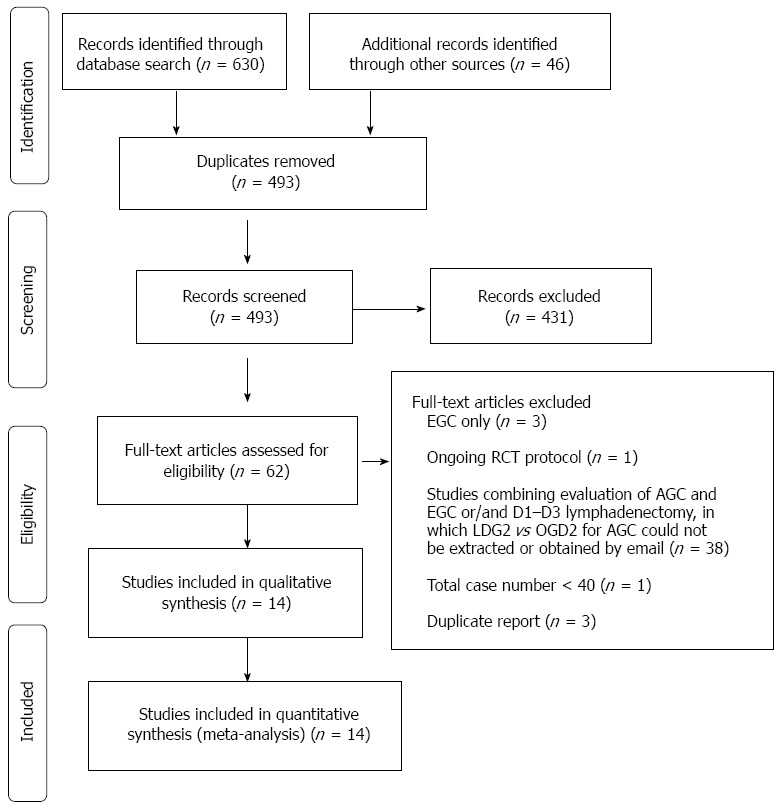
Flow chart of the identification and inclusion of studies. Studies evaluating laparoscopic gastrectomy with D2 (LGD2) were identified to evaluate the procedure as an acceptable alternative to open gastrectomy with D2 (OGD2) for locally advanced gastric cancer (AGC); EGC: Early gastric cancer; RCT: Randomized controlled trial.
Table 1.
Characteristics and quality of studies included in the meta-analysis
| Publication | Study design | Cases (L/O) | Type of gastrectomy | Type of laparoscopy | Mean follow-up (mo) | Matching criteria1 | Quality score |
| Shinohara et al[32] | Non-RCT | 186/123 | DG, TG, PG | TLG | 48.8 | 1, 2, 3, 4, 7, 8, 9 | 8 |
| Kim et al[31] | Non-RCT | 88/88 | DG, TG, PG | LAG | L: 53.7; O: 58.1 | 1, 2, 3, 4, 5, 7 | 8 |
| Wang et al[30] | Non-RCT | 210/180 | DG, TG, PG | LAG | L: median 24; O: median 26 | 1, 2, 3, 4, 5 | 7 |
| Sato et al[29] | Non-RCT | 32/118 | DG, TG, PG | LAG | 43 | 1 | 7 |
| Hamabe et al[28] | Non-RCT | 66/101 | DG, TG | LAG | L: 30.4; O: 53.5 | 1, 2, 3, 4, 7, 9 | 6 |
| Chen et al[27] | Non-RCT | 224/112 | DG, TG | LAG | NS | 1, 2, 3, 4, 6, 7, 8 | 7 |
| Zang et al[26] | Non-RCT | 156/156 | TG | LAG | NS | 1, 2, 3, 4, 6, 8 | 6 |
| Shuang et al[25] | Non-RCT | 35/35 | DG | LAG | L: 36.5; O: 38.5 | 5, 8 | 6 |
| Scatizzi et al[24] | Non-RCT | 30/30 | DG | TLG | 18 | 1, 2, 3, 4, 6 | 7 |
| Cai et al[23] | RCT | 49/47 | DG, TG, PG | LAG | 22.1 | 1, 2, 3, 4, 5, 6, 7 | RCT |
| Huang et al[22] | Non-RCT | 66/69 | DG | LAG | Range: 1-19 | 1, 2, 3, 4, 6, 7, 8 | 7 |
| Du et al[21] | Non-RCT | 82/94 | TG | LAG | 2.5 | 1, 2, 3, 4, 5, 7 | 7 |
| Du et al[20] | Non-RCT | 78/90 | DG | LAG | 25.2 | 1, 2, 3, 4, 5, 6, 7 | 7 |
| Hur et al[19] | Non-RCT | 26/25 | DG | LAG | 29.0 | 1, 2, 3, 4, 5, 7, 8, 9 | 7 |
Matching criteria: 1 = age; 2 = sex; 3 = pathologic tumor-node-metastasis stage; 4 = type of gastrectomy; 5 = resection margin; 6 = tumor size; 7 = histologic type; 8 = reconstruction; 9 = adjuvant treatment. DG: Distal gastrectomy; L: Laparoscopic gastrectomy; LAG: Laparoscopy-assisted gastrectomy; NS: Not stated; O: Open gastrectomy; PG: Proximal gastrectomy; RCT: Randomized controlled trial; TG: Total gastrectomy; TLG: Total laparoscopic gastrectomy.
Study quality
A methodological quality assessment revealed that the RCT had unclear random sequence generation, satisfactory allocation concealment, adequately addressed incomplete outcome data, and had no selective outcome reporting[23]. The quality of all 13 non-RCTs was level 2 (6-9 stars) on the Newcastle-Ottawa scale (Table 1).
Meta-analyses of operative outcomes
Thirteen studies provided operative time data[19-25,27-32]. The LGD2 group’s weighted mean operative time was 57.06 min longer than in the OGD2 group (95%CI: 41.87-72.25; P < 0.01), with significant heterogeneity among studies (I2 = 90%; P < 0.01) (Table 2, Figure 2A).
Table 2.
Meta-analysis results of endpoints from all available studies
| Measured outcome | Studies (n) | Patients (n) | OR, WMD, or HR | 95%CI | P |
Heterogeneity test |
Pr >|z| | ||
| I2 | P | ||||||||
| Operative outcomes | |||||||||
| Operative time | 13 | 2300 | 57.06 | 41.87 | 72.25 | < 0.00001 | 90% | < 0.00001 | 0.502 |
| Intraoperative blood loss | 11 | 2064 | -137.87 | -164.41 | -111.33 | < 0.00001 | 90% | < 0.00001 | 0.533 |
| PO outcomes | |||||||||
| PO analgesic consumption | 4 | 441 | -1.94 | -2.50 | -1.38 | < 0.00001 | 77% | 0.005 | 0.308 |
| Time to first ambulation | 5 | 977 | -1.03 | -1.90 | -0.16 | 0.02 | 97% | < 0.00001 | 1.000 |
| Time to first flatus | 9 | 1588 | -0.98 | -1.30 | -0.66 | < 0.00001 | 89% | < 0.00001 | 0.536 |
| Time to first oral intake | 6 | 987 | -0.85 | -1.67 | -0.03 | 0.04 | 86% | < 0.00001 | 1.000 |
| Length of PO hospital day | 10 | 1782 | -3.08 | -4.38 | -1.78 | < 0.00001 | 88% | < 0.00001 | 0.721 |
| Overall morbidity | 13 | 2284 | 0.78 | 0.61 | 0.99 | 0.04 | 14% | 0.30 | 0.161 |
| Anastomotic stenosis | 12 | 2108 | 0.89 | 0.36 | 2.16 | 0.79 | 0% | 0.74 | 0.308 |
| Anastomotic leakage | 13 | 2284 | 0.74 | 0.36 | 1.50 | 0.40 | 0% | 0.80 | 1.000 |
| Duodenal stump leakage | 13 | 2284 | 1.12 | 0.42 | 3.01 | 0.82 | 0% | 0.83 | 1.000 |
| Pancreatic fistula/ pancreatitis | 13 | 2284 | 0.75 | 0.37 | 1.52 | 0.42 | 0% | 0.91 | 0.308 |
| Intra-abdominal bleeding | 13 | 2284 | 0.99 | 0.41 | 2.38 | 0.98 | 0% | 0.83 | 1.000 |
| Ileus | 12 | 2108 | 0.56 | 0.21 | 1.46 | 0.23 | 0% | 0.73 | 1.000 |
| Wound problems | 13 | 2284 | 0.56 | 0.34 | 0.93 | 0.03 | 0% | 0.66 | 0.152 |
| Pneumonia | 13 | 2284 | 0.38 | 0.21 | 0.71 | 0.002 | 17% | 0.29 | 1.000 |
| Reoperation | 7 | 1289 | 1.58 | 0.58 | 4.31 | 0.37 | 0% | 0.63 | 1.000 |
| Mortality | 13 | 2284 | 0.69 | 0.21 | 2.26 | 0.54 | 0% | 0.64 | - |
| Oncologic outcomes | |||||||||
| Lymph nodes harvested (n) | 13 | 2526 | -0.11 | -2.72 | 2.50 | 0.94 | 95% | < 0.00001 | 0.537 |
| Tumor recurrence/metastasis | 8 | 1587 | 0.79 | 0.60 | 1.04 | 0.09 | 20% | 0.27 | 0.035 |
| Local/lymphatic recurrence | 5 | 853 | 0.79 | 0.46 | 1.34 | 0.38 | 0% | 0.41 | 0.296 |
| Peritoneal recurrence | 5 | 853 | 1.20 | 0.70 | 2.07 | 0.50 | 0% | 0.50 | 0.296 |
| Distant metastasis | 5 | 853 | 0.67 | 0.42 | 1.07 | 0.09 | 45% | 0.12 | 0.089 |
| Three-year DFS | 4 | 703 | 1.02 | 0.64 | 1.61 | 0.94 | 0% | 0.88 | - |
| Three-year overall survival | 8 | 1363 | 0.87 | 0.59 | 1.27 | 0.46 | 0% | 0.99 | - |
| Five-year DFS | 3 | 652 | 1.02 | 0.66 | 1.57 | 0.92 | 0% | 0.67 | - |
| Five-year overall survival | 3 | 652 | 0.79 | 0.46 | 1.34 | 0.38 | 0% | 0.90 | - |
DFS: Disease-free survival; HR: Hazard ratio; OR: Odds ratio; PO: Postoperative; WMD: Weighted mean difference.
Figure 2.
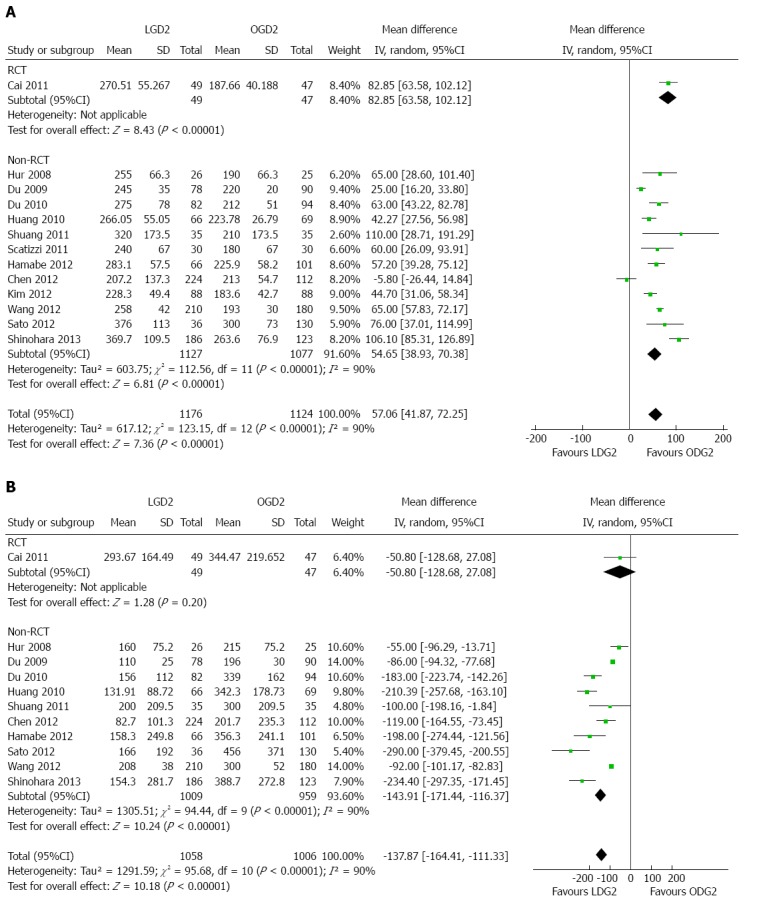
Meta-analyses of procedure characteristics. A: Weighted mean operative time; B: Intraoperative blood loss. LGD2: Laparoscopic gastrectomy with D2 extended lymph node dissection; OGD2: Open gastrectomy with D2 extended lymph node dissection; RCT: Randomized controlled trial.
Blood loss data was found in 11 studies[19-23,25,27-30,32], revealing a significantly lower blood loss in the LGD2 compared to the OGD2 groups (WMD = -137.87 mL, 95%CI: -164.41--111.33; P < 0.01), with significant heterogeneity among studies (I2 = 90%; P < 0.01) (Figure 2B).
Laparoscopic procedure conversion rates were documented in eight studies, ranging from 0.00 to 6.67%, with a weighted average of 1.68%[19,21-24,28,30,32]. Four articles reported the following reasons for converting to open procedures: hemorrhage (n = 2); overlarge tumor (n = 2); common bile duct injury (n = 1); obesity (n = 1); technical difficulty (n = 1); lack of pneumoperitoneum (n = 1); failure of the linear stapler (n = 1); dense adhesion after open sigmoidectomy (n = 1); relatively fixed tumor (n = 1); small incision metastasis (n = 1).
Meta-analyses of postoperative outcomes
Analgesic administration was reported by only four articles included in this study[21,22,24,25]. Meta-analysis revealed a significantly lower frequency of analgesic administration in the LGD2 group than in the OGD2 group (WMD = -1.94, 95%CI: -2.50--1.38; P < 0.01), with significant heterogeneity among studies (I2 = 77%; P < 0.01) (Table 2, Figure 3A).
Figure 3.
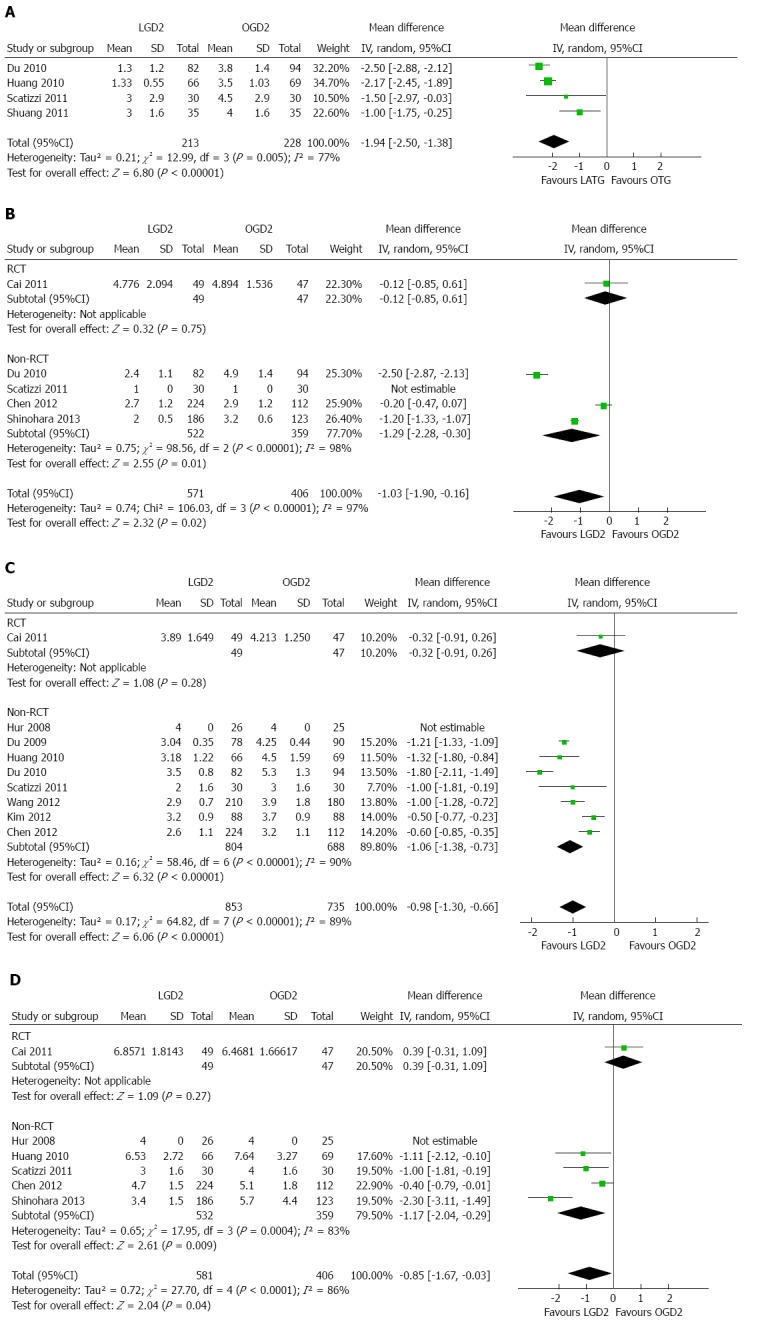
Meta-analyses of patient characteristics. A: Analgesic consumption; B: Time to first ambulation; C: Time to first flatus; D: Time to first oral intake. LGD2: Laparoscopic gastrectomy with D2 extended lymph node dissection; OGD2: Open gastrectomy with D2 extended lymph node dissection; RCT: Randomized controlled trial.
The time to first ambulation was reported in five papers[21,23,24,27,32]. This time was significantly shorter in the LGD2 group than in the OGD2 group (WMD = -1.03 d, 95%CI: -1.90--0.16; P < 0.05), with significant heterogeneity among studies (I2 = 97%; P < 0.01) (Figure 3B).
The time to first flatus was reported in nine articles[19-24,27,30,31]. The time was significantly shorter in the LGD2 group than in the OGD2 group (WMD = -0.98 d, 95%CI: -1.30--0.66; P < 0.01), with significant heterogeneity among studies (I2 = 89%; P < 0.01) (Figure 3C).
The time to first oral intake was reported in six papers[19,22-24,27,32]. Meta-analysis demonstrated this time was significantly shorter in the LGD2 group than in the OGD2 group (WMD = -0.85 d, 95%CI: -1.67--0.03; P < 0.05), with significant heterogeneity among studies (I2 = 86%; P < 0.01) (Figure 3D).
The length of postoperative hospitalization was reported in 10 articles[19,20,22-25,27,28,30,32]. The LGD2 group had significantly shorter postoperative hospitalization than the OGD2 group (WMD = -3.08 d, 95%CI: -4.38--1.78; P < 0.01). There was a significant heterogeneity among studies (I2 = 88%; P < 0.01) (Figure 4A).
Figure 4.
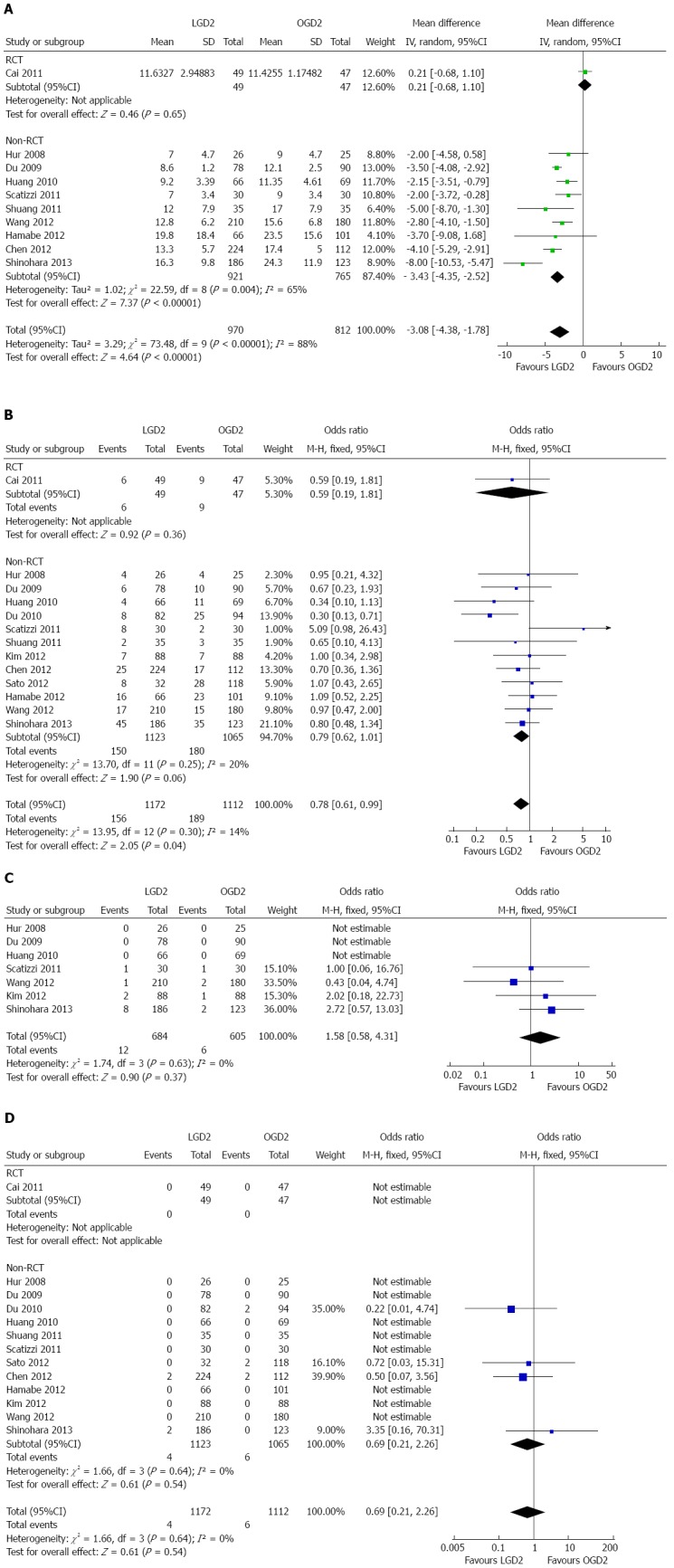
Meta-analyses of postoperative events. A: Postoperative hospitalization; B: Morbidity; C: Reoperation; D: Mortality. LGD2: Laparoscopic gastrectomy with D2 extended lymph node dissection; OGD2: Open gastrectomy with D2 extended lymph node dissection; RCT: Randomized controlled trial.
The postoperative morbidity rates were reported in 13 studies[19-25,27-32]. Meta-analysis demonstrated a significantly lower overall postoperative morbidity after LGD2 than after OGD2 (OR = 0.78, 95%CI: 0.61-0.99; P < 0.05), with no significant heterogeneity among studies (I2 = 14%) (Figure 4B).
The subgroup analyses showed significantly lower incidence rates of wound problems (wound infection and dehiscence) and pneumonia in the LGD2 group. No difference in the incidence rate of major surgical site complications, such as anastomotic stenosis, anastomotic leakage, duodenal stump leakage, pancreatic fistula or pancreatitis, and intra-abdominal bleeding, was found between the two groups (Table 2). Subgroup analyses demonstrated no significant differences between groups in major surgical site complications with regard to surgical extensions (distal gastrectomy/proximal gastrectomy/total gastrectomy). This includes anastomotic stenosis, anastomotic leakage, duodenal stump leakage, pancreatic fistula or pancreatitis, and intra-abdominal bleeding.
The reoperation incidence rate was reported in seven articles[19,20,22,24,30-32]. No significant difference in this parameter was found between the LGD2 and OGD2 groups (OR = 1.58, 95%CI: 0.58-4.31) with no significant heterogeneity among studies (I2 = 0%) (Figure 4C).
The postoperative mortality rates were reported in 13 studies[19-25,27-32] with no significant difference in the rate between the LGD2 and OGD2 groups (OR = 0.69, 95%CI: 0.21-2.26) with no significant heterogeneity among studies (I2 = 0%) (Figure 4D).
Meta-analyses of oncologic outcomes
The number of lymph nodes harvested was reported in 13 studies[19-24,26-32]. Although meta-analysis showed no significant difference in this parameter between the two groups (WMD = -0.11, 95%CI: -2.72-2.50), there was significant heterogeneity among the studies (I2 = 95%; P < 0.01) (Table 2, Figure 5A).
Figure 5.
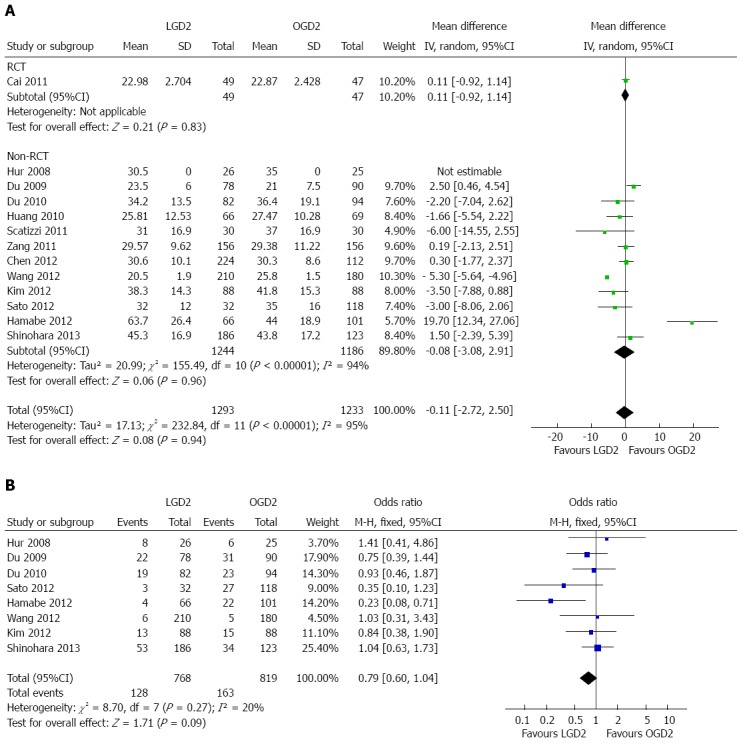
Meta-analyses of lymph node harvest and tumor recurrence. A: Lymph nodes harvested; B: Tumor recurrence and metastasis. LGD2: Laparoscopic gastrectomy with D2 extended lymph node dissection; OGD2: Open gastrectomy with D2 extended lymph node dissection; RCT: Randomized controlled trial.
Tumor recurrence and metastasis were recorded in eight studies[19-21,28-32]. The meta-analysis showed no statistical difference between the LGD2 and OGD2 groups (OR = 0.79, 95%CI: 0.60-1.04), as well as no significant heterogeneity among studies (I2 = 20%) (Figure 5B). Subgroup analyses showed no significant difference in recurrence and metastasis patterns between the groups (Table 2).
Four trials involving 703 patients provided three-year disease-free survival rates[19,28,31,32]. Three trials involving 652 patients provided five-year disease-free survival rates[28,31,32]. The two groups showed no significant difference in three-year (HR = 1.02, 95%CI: 0.64-1.61) (Figure 6A) or five-year (HR = 1.02, 95%CI: 0.66-1.57) (Figure 6B) disease-free survival rates (Figure 6). There was no significant heterogeneity among studies (I2 = 0% for both rates) (Table 2).
Figure 6.
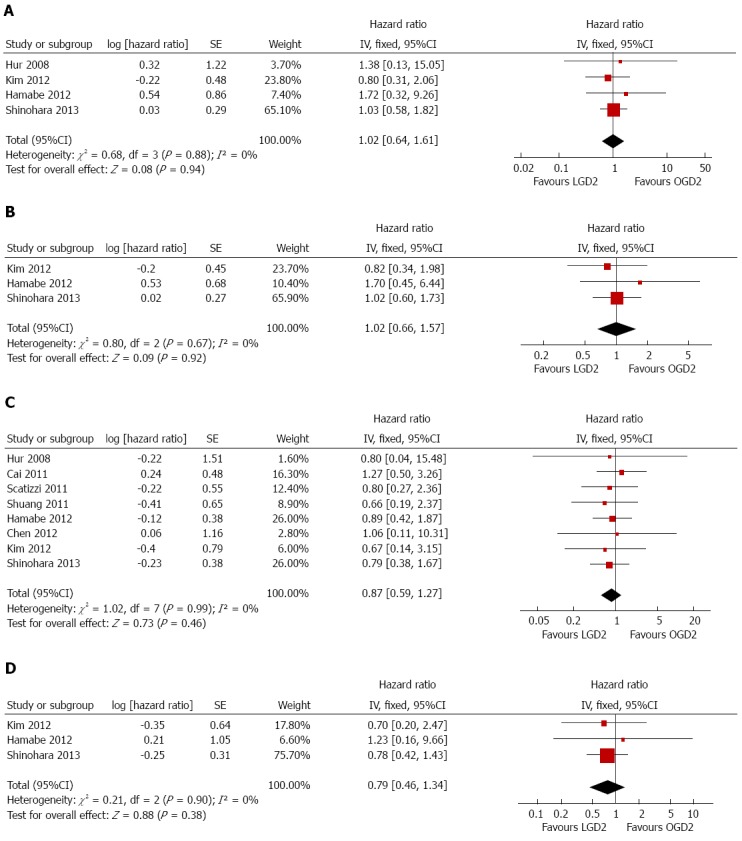
Meta-analyses of treatment outcomes between laparoscopic and open D2 gastrectomy. A: Three-year disease-free survival; B: Five-year disease-free survival; C: Three-year overall survival; D: Five-year overall survival. LGD2: Laparoscopic gastrectomy with D2 extended lymph node dissection; OGD2: Open gastrectomy with D2 extended lymph node dissection.
Eight trials involving 1363 patients provided three-year overall survival rates[19,23-25,27,28,31,32], and three trials involving 652 patients provided five-year overall survival rates[28,31,32]. The two groups showed no significant differences in three-year (HR = 0.87, 95%CI: 0.59-1.27) (Figure 6C) or five-year (HR = 0.79, 95%CI: 0.46-1.34) (Figure 6D) overall survival rates, accompanied with no significant heterogeneity among studies (I2 = 0% for both). Among the studies included, only Shinohara et al[32] presented calculated disease-free and overall survival rates after LGD2 and OGD2 with regard to tumor stage, with no significant differences observed between the two groups.
Sensitivity analysis and publication bias
Study results were reanalyzed using alternative (random or fixed effects) models showing no significant difference in pooled effects, except comparable incidences of overall morbidity in the two groups (OR = 0.78, 95%CI: 0.59-1.02). Furthermore, the studies showed no significant heterogeneity (I2 = 14%). Endpoint analysis revealed no strong evidence of bias (Begg’s rank correlation test, continuity-corrected Pr >|z| > 0.1), except for tumor recurrence/metastasis (Pr >|z| = 0.035) and distant metastasis (Pr >|z| = 0.089).
DISCUSSION
This meta-analysis examined whether LGD2 is an acceptable alternative to OGD2 for AGC from a clinical perspective. The results suggest that despite LGD2 being a technically demanding and time-consuming procedure with longer operative times and acceptable conversion rates, it can be used to achieve long-term prognoses. Comparison between LGD2 and OGD2 showed similar numbers of harvested lymph nodes, tumor recurrence and metastasis rates, and disease-free and overall survival rates. Furthermore, LGD2 provides better short-term prognoses with lower postoperative pain, faster recovery, and shorter hospital stays. There was a lower postoperative morbidity associated with LGD2, which may have been due to the minimal invasiveness, reduced postoperative pain, earlier ambulation, and fewer pulmonary complications associated with the LGD2 procedure, though some comparable major surgical-site complications and postoperative mortality remained. Hence, LGD2 may provide better short-term prognoses than OGD2.
The results of the present study suggest equivalent long-term oncologic results can be obtained with LGD2 as with an open radical surgery. This finding mainly reflects similar pathologic tumor-node-metastasis stages in the two groups and the prioritization of and adherence to oncologic principles, such as en bloc resection, the no-touch technique, and systemic lymphadenectomy[22]. However, there are still challenges associated with the LGD2 procedure, including a learning curve for training and the mastery of essential techniques of distal LG with systemic lymphadenectomy for treating major EGC, which requires experience from 60-90 cases[47]. Thus, LGD2 is not recommended in small-volume centers.
The present meta-analysis has several limitations. First, all but one of the included studies were observational. Second, most of the included studies were conducted at tertiary centers and major institutions in East Asia (eight in China, three in Japan, two in South Korea, and one in Italy). Hence, the included patients might not reflect general patient populations. Furthermore, any application of the conclusions to Western patients should be performed cautiously. Third, because > 95% of patients had locally AGC with stages ranging from IB to III, the conclusions should be applied only to similar cases. Fourth, the studies showed significant heterogeneity in operative time, intraoperative blood loss, postoperative analgesic consumption, time to first ambulation, time to first flatus, time to first oral intake, length of postoperative hospital stay, and number of lymph nodes harvested. Differences in study design, sample size, adjuvant treatment, and other factors might explain this heterogeneity. Additionally, calculations using the random effects model yielded more conservative estimates of statistical significance. Finally, this meta-analysis was performed at the study level and did not address or incorporate individual factors at the patient level.
In conclusion, although LGD2 is a technically demanding and time-consuming procedure, the results of this meta-analysis suggest it may be an acceptable alternative to OGD2 for locally AGC. The procedure may yield comparable oncologic results and better short-term prognoses than OGD2. However, additional clinical trials are needed for further evaluation of this procedure. We identified seven ongoing RCTs comparing the use of LGD2 and OGD2 to treat AGC in East Asia (three in China, three in South Korea, and one in Japan) (Table 3)[48-54]. The results of these trials will help researchers address this question in the future.
Table 3.
Ongoing randomized controlled trials comparing laparoscopic and open D2 gastrectomy for advanced gastric cancer
| Contact | Country | Sample size | Type of cancer | Start date | Completion date |
| Li et al48] | China | 1056 | Locally AGC | 2012/9/1 | 2018/6/1 |
| Shi et al49] | China | 328 | Locally AGC | 2010/2/1 | 2015/2/1 |
| Huang et al50] | China | 111 | AGC | 2011/11/1 | Not stated |
| Han et al51] | South Korea | 1050 | Locally AGC | 2011/10/1 | 2016/9/1 |
| Kim et al52] | South Korea | 204 | Locally AGC | 2010/6/1 | 2016/12/1 |
| Kim et al53] | South Korea | 124 | Locally AGC | 2008/8/1 | 2013/7/1 |
| Tsuyoshi et al54] | Japan | 500 | Locally AGC | 2009/11/1 | Not stated |
COMMENTS
Background
Laparoscopic gastrectomy is gaining popularity worldwide as a minimally invasive alternative treatment to traditional open surgery in treating gastric cancer. The Japanese Gastric Cancer Association guidelines stipulate that D2 gastrectomy is required to cure advanced gastric cancer. However, the application of laparoscopic D2 gastrectomy (LGD2) remains questionable due to its technical difficulty and the lack of long-term results.
Research frontiers
The authors performed a meta-analysis comparing LGD2 with open D2 gastrectomy (OGD2) in patients with advanced gastric cancer, evaluating endpoints of operative, postoperative, and oncological outcomes.
Innovations and breakthroughs
Compared with OGD2, LGD2 is a safer and more effective method, with lower overall morbidity, enhanced postoperative recovery, and comparable oncologic outcomes.
Applications
LGD2 is safe and effective, and offers some advantages over OGD2 in treatment of locally advanced gastric cancer. However, well-designed, prospective, multicenter, randomized controlled trials comparing LGD2 with OGD2 for treatment of advanced gastric cancer are warranted before recommending LGD2 for wider use in surgical practice.
Peer review
The paper is a well-organized and structured meta-analysis of currently available data on the benefits of using LGD2 over OGD2 for the treatment of advanced gastric cancer. The authors concluded that LGD2 is safe and effective in treating locally advanced gastric cancer. This meta-analysis is well written and an important addition of knowledge to successful treatment of locally advanced gastric cancer.
Footnotes
P- Reviewer: Basso N, Kim HH, Park KU, Wang DR S- Editor: Ma N L- Editor: AmEditor E- Editor: Zhang DN
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s–275s. doi: 10.1200/JCO.2003.09.172. [DOI] [PubMed] [Google Scholar]
- 3.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 4.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 6.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 7.Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg. 2013;257:640–646. doi: 10.1097/SLA.0b013e31826fd541. [DOI] [PubMed] [Google Scholar]
- 8.Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Laparoscopic assisted distal gastrectomy for early gastric cancer: Five years’ experience. Surgery. 2005;137:317–322. doi: 10.1016/j.surg.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee SE, Kim YW, Lee JH, Ryu KW, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Nam BH, et al. Developing an institutional protocol guideline for laparoscopy-assisted distal gastrectomy. Ann Surg Oncol. 2009;16:2231–2236. doi: 10.1245/s10434-009-0490-9. [DOI] [PubMed] [Google Scholar]
- 10.An JY, Heo GU, Cheong JH, Hyung WJ, Choi SH, Noh SH. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol. 2010;102:77–81. doi: 10.1002/jso.21554. [DOI] [PubMed] [Google Scholar]
- 11.Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hasumi A. Laparoscopic D2 lymph node dissection for advanced gastric cancer located in the middle or lower third portion of the stomach. Gastric Cancer. 2000;3:50–55. doi: 10.1007/pl00011690. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y, Luo H, Zhang Y. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011;25:2960–2966. doi: 10.1007/s00464-011-1652-y. [DOI] [PubMed] [Google Scholar]
- 15.Cui M, Xing JD, Yang W, Ma YY, Yao ZD, Zhang N, Su XQ. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18:833–839. doi: 10.3748/wjg.v18.i8.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT, Jeong CY, Ha WS. Risk of recurrence after laparoscopy-assisted radical gastrectomy for gastric cancer performed by a single surgeon. Surg Endosc. 2011;25:872–878. doi: 10.1007/s00464-010-1286-5. [DOI] [PubMed] [Google Scholar]
- 17.Orsenigo E, Di Palo S, Tamburini A, Staudacher C. Laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: a monoinstitutional Western center experience. Surg Endosc. 2011;25:140–145. doi: 10.1007/s00464-010-1147-2. [DOI] [PubMed] [Google Scholar]
- 18.Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008;22:1161–1164. doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 19.Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol. 2008;98:515–519. doi: 10.1002/jso.21155. [DOI] [PubMed] [Google Scholar]
- 20.DU XH, Li R, Chen L, Shen D, Li SY, Guo Q. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer: initial experience. Chin Med J (Engl) 2009;122:1404–1407. [PubMed] [Google Scholar]
- 21.Du J, Zheng J, Li Y, Li J, Ji G, Dong G, Yang Z, Wang W, Gao Z. Laparoscopy-assisted total gastrectomy with extended lymph node resection for advanced gastric cancer--reports of 82 cases. Hepatogastroenterology. 2010;57:1589–1594. [PubMed] [Google Scholar]
- 22.Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B. Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg. 2010;27:291–296. doi: 10.1159/000281818. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 24.Scatizzi M, Kröning KC, Lenzi E, Moraldi L, Cantafio S, Feroci F. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a case-control study. Updates Surg. 2011;63:17–23. doi: 10.1007/s13304-011-0043-1. [DOI] [PubMed] [Google Scholar]
- 25.Shuang J, Qi S, Zheng J, Zhao Q, Li J, Kang Z, Hua J, Du J. A case-control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg. 2011;15:57–62. doi: 10.1007/s11605-010-1361-1. [DOI] [PubMed] [Google Scholar]
- 26.Zang WD, Zhang H, Chen LC, Zhuo CH, Ying MG. [Advantage of perisplenic hilar lymph node dissection by laparoscopy-assisted total gastrectomy (D2) over conventional open total gastrectomy for advanced gastric cancer] Zhonghua Zhong Liu Zazhi. 2011;33:864–867. [PubMed] [Google Scholar]
- 27.Chen QY, Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Lu J. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: a case control study. World J Surg Oncol. 2012;10:248. doi: 10.1186/1477-7819-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamabe A, Omori T, Tanaka K, Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012;26:1702–1709. doi: 10.1007/s00464-011-2096-0. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Shimada M, Kurita N, Iwata T, Nishioka M, Morimoto S, Yoshikawa K, Miyatani T, Goto M, Kashihara H, et al. Comparison of long-term prognosis of laparoscopy-assisted gastrectomy and conventional open gastrectomy with special reference to D2 lymph node dissection. Surg Endosc. 2012;26:2240–2246. doi: 10.1007/s00464-012-2167-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang DR, Zhao JG, Yu HF, Wang LH, Jiang GQ, Li YK, Chen J. [Laparoscopic versus open surgery for D2 gastrectomy in advanced gastric cancer] Zhonghua Wei Chang Wai Ke Zazhi. 2012;15:964–966. [PubMed] [Google Scholar]
- 31.Kim KH, Kim MC, Jung GJ, Choi HJ, Jang JS, Kwon HC. Comparative analysis of five-year survival results of laparoscopy-assisted gastrectomy versus open gastrectomy for advanced gastric cancer: a case-control study using a propensity score method. Dig Surg. 2012;29:165–171. doi: 10.1159/000338088. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286–294. doi: 10.1007/s00464-012-2442-x. [DOI] [PubMed] [Google Scholar]
- 33.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 34.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 35.Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643–649. doi: 10.1002/bjs.6936. [DOI] [PubMed] [Google Scholar]
- 36.Azagra JS, Goergen M, De Simone P, Ibañez-Aguirre J. Minimally invasive surgery for gastric cancer. Surg Endosc. 1999;13:351–357. doi: 10.1007/s004649900988. [DOI] [PubMed] [Google Scholar]
- 37.Qiu J, Pankaj P, Jiang H, Zeng Y, Wu H. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:1–7. doi: 10.1097/SLE.0b013e3182747af7. [DOI] [PubMed] [Google Scholar]
- 38.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0.) Hoboken: John Wiley & Sons Inc; 2011. [Google Scholar]
- 41.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis, 2013. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 42.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–1537. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 46.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 47.Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc. 2009;23:1259–1264. doi: 10.1007/s00464-008-0142-3. [DOI] [PubMed] [Google Scholar]
- 48.Li G. Multicenter study on laparoscopic distal subtotal gastrectomy for advanced gastric cancer (CLASS-01). NLM identifier NCT01609309. Available from: http://clinicaltrials.gov/ct2/show/NCT01609309. Accessed Mar 20, 2013.
- 49.Shi Y. Comparison of laparoscopic vs open gastrectomy for advanced gastric cancer: a prospective randomized trial. NLM identifier NCT01043835. Available from: http://clinicaltrials.gov/ct2/show/NCT01043835. Accessed Mar 20, 2013.
- 50.Huang CM. Prospective randomized controlled trial of laparoscopic D2 radical gastrectomy and open surgery for advanced gastric cancer. ChiCTR identifier ChiCTR-TRC-11001340. Available from: http://www.chictr.org/en/proj/show.aspx?proj=66. Accessed Mar 20, 2013.
- 51.Han SU. Efficacy of laparoscopic subtotal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (KLASS-02-RCT). NLM identifier NCT01456598. Available from: http://clinicaltrials.gov/ct2/show/NCT01456598. Accessed Mar 20, 2013.
- 52.Kim YW. Feasibility study of laparoscopy-assisted d2 distal gastrectomy to treat advanced gastric cancer (COACT_1001). NLM identifier NCT01088204. Available from: http://clinicaltrials.gov/ct2/show/NCT01088204. Accessed Mar 20, 2013.
- 53.Kim W. Comparison of the laparoscopy-assisted distal gastrectomy and open distal gastrectomy for advanced gastric cancer. NLM identifier NCT00741676. Available from: http://clinicaltrials.gov/ct2/show/NCT00741676. Accessed Mar 20, 2013.
- 54.Tsuyoshi E. Randomized Controlled Trial to Evaluate Laparoscopic vs Open Surgery for Advanced Gastric Cancer (JLSSG0901: Adv.GC-LAP/OPEN, PII/III). JPRN identifier JPRN-UMIN000003420. Available from: http://apps.who.int/trialsearch/trial.aspx?TrialID=JPRN-UMIN000003420. Accessed Mar 20, 2013.


