SUMMARY
Angiogenesis inhibitors cause increased hypoxia in tumors and this results in the induction of cytoprotective autophagy. Targeting this adaptation using autophagy inhibitors can overcome resistance to anti-angiogenic therapy and enhance the antitumor effects.
In this issue of Clinical Cancer Research, Selvakumaran and colleagues use a preclinical model of colorectal cancer to demonstrate that tumors treated with a combination regimen containing anti-angiogenic and cytotoxic agents depend on autophagy for survival 1. Inhibition of autophagy synergistically enhanced the efficacy of this regimen. This type of synthetic lethal interaction is termed ‘induced essentiality’ 2.
The vascular endothelial growth factor (VEGF)-blocking antibody, bevacizumab (Avastin®, Genentech), is approved for treatment of metastatic colorectal, renal, non-small cell lung cancer and for glioblastoma. In these settings treatment with bevacizumab results in improved progression-free survival (PFS) but rarely overall survival (OS). Bevacizumab acts by preventing tumor neoangiogenesis and destabilizing existing immature vasculature, and these effects starve the tumor of oxygen and nutrients resulting in reduced growth of primary and metastatic tumors. In some cases the beneficial effects of bevacizumab may be due to vascular normalization resulting in improved drug delivery and radiation sensitivity 3. To better understand the observed differences in response it is necessary to identify key determinants of sensitivity and resistance.
Antiangiogenic agents produce a number of adverse biological changes in the tumor that may be responsible for development of resistance to angiogenesis inhibition and subsequent disease progression 4. These changes include enhanced invasion through derepression of MET signaling and increased intratumoral hypoxia 5, 6. One approach to add potency or overcome resistance to antiangiogenic agents is to target these prosurvival adaptations.
Targeting the increased hypoxic fraction that occurs following antiangiogenic therapy using low dose topoisomerase I inhibitors that block the accumulation HIF-1α is particularly effective in enhancing the efficacy of angiogenesis inhibitors 7. An alternative approach to targeting HIF-1 itself is to target one or several of the phenotypic changes that occur during hypoxia including altered metabolism, enhanced motility and invasion or increased autophagic flux. Several reports have validated this approach. For example, inhibition of hypoxic pH regulation using the carbonic anhydrase inhibitor, acetazolamide, or knockdown of CA9 sensitized tumors to bevacizumab 8.
Autophagy is a process whereby cytoplasmic macromolecules, protein aggregates and mitochondria are degraded by the lysosome and recycled to promote cell survival during starvation or energy stress 9. Autophagy is induced during hypoxic exposures by several independent transcriptional and post-translational pathways, highlighting the importance of this process in these conditions. The suppression of oxidative phosphorylation that occurs in hypoxia causes an increase in AMP/ATP ratio and subsequent activation of AMPK. AMPK acts to reinstate energy homeostasis by phosphorylation and activation of ULK1, a key initiator of autophagy. In parallel, AMPK inhibits mTORC1 activity and this derepresses ULK1 also resulting in increased induction of autophagy. HIF-1α also contributes to the induction of autophagy during hypoxia by promoting targeted mitochondrial autophagy via transcription of BNIP3 and BNIP3L. In addition, ATF4-dependent transcriptional upregulation of MAP1LC3B replenishes LC3 pools to maintain high levels of autophagic flux during periods of hypoxia and amino acid deprivation 10. ATF4 also transcriptionally upregulates ULK1 and this contributes to the induction of autophagy in some contexts 11.
The prosurvival role of autophagy in hypoxia suggests that inhibiting this adaptive process represents an appealing strategy to sensitize tumors to angiogenesis inhibitors and slow the onset of acquired resistance. Indeed, preclinical studies support the value of this approach. Inhibition of autophagy using chloroquine (CQ) or by genetic depletion of ATG7 enhanced the efficacy of bevacizumab in preclinical glioblastoma models 6. However, as angiogenesis inhibitors are used in combination with chemotherapeutic agents it is necessary to determine the effects of autophagy inhibition in this setting. Selvakumaran et al., have addressed this shortcoming in the literature by combining CQ with bevacizumab and oxaliplatin in a preclinical model of colorectal cancer 1.
Hypoxic cells are often resistant to cytotoxic agents. Indeed, hypoxia caused a modest resistance to oxaliplatin in all eight colorectal cell lines investigated (median 1.5-fold higher IC50). Inhibition of autophagy using pharmacological agents that act at diverse steps of the autophagy process or genetic depletion of essential autophagy genes reversed the resistance to oxaliplatin in hypoxia, suggesting that autophagy plays a prosurvival role in these circumstances.
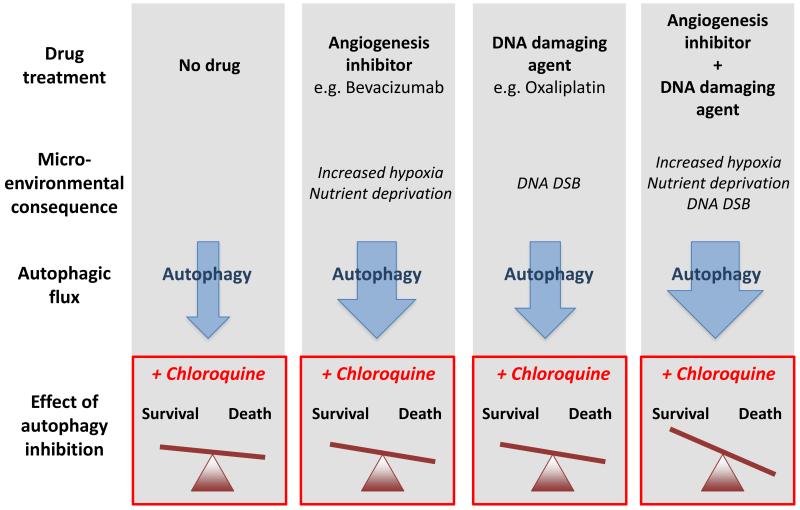
In HT29 xenografts, treatment with CQ resulted in a modest tumor growth delay suggesting that autophagy inhibition alone has some efficacy in this model. Treatment with either bevacizumab or oxaliplatin also modestly inhibited tumor growth and resulted in induction of autophagy, recapitulating their in vitro observations. Addition of CQ to either oxaliplatin or bevacizumab inhibited the induction of autophagy observed with each agent and provided an additional increase in tumor growth delay. The combination of oxaliplatin and bevacizumab provided greater efficacy than either agent alone and also led to the induction of autophagy. Tumor growth inhibition was further enhanced when CQ was added to the combination of oxaliplatin and bevacizumab and autophagy inhibition was demonstrated. Notably, CQ was able to increase the efficacy of oxaliplatin or bevacizumab in an additive manner, yet when the three agents were combined the effect was synergistic. These findings suggest that following treatment with the combination of bevacizumab and oxaliplation cells rely on increased autophagic flux for survival. Exploiting this induced essentiality using inhibitors of autophagy leads to synergistic antitumor effects (Figure 1).
Figure 1.
Autophagic flux in tumor cells is increased by treatment with angiogenesis inhibitors (e.g bevacizumab), DNA damaging drugs (e.g. oxaliplatin) and the combination. The approximate increase in flux is indicated by arrow width. Autophagy inhibition using chloroquine results in some cell killing on its own (no drug), more killing when combined with either bevacizumab or oxaliplatin and greatly enhanced killing when the three agents are combined. However, the baseline parameters to predict these effects are unknown. DSB, double strand breaks.
A large number of clinical trials investigating CQ or hydroxychloroquine (HCQ) are underway. HCQ is considered less toxic and is, therefore, likely to be better tolerated when incorporated into existing chemotherapeutic regimens. Several Phase 1/2 studies combine HCQ with regimens containing angiogenesis inhibitors including XELOX + bevacizumab, FOLFOX + bevacizumab and carboplatin-taxol + bevacizumab (clinicaltrials.gov; NCT01006369, NCT01206530 and NCT01649947).
Both CQ and HCQ have prolonged plasma half-lives (40 to 60 days), low blood clearance (e.g. 96 mL/min for HCQ) and high variability of blood concentrations with an eleven-fold range of drug concentrations following similar doses, so pharmacokinetic analysis and dosage guidance is critical to evaluate these agents. The steady-state blood concentrations of HCQ are approximately 1 mg/L (3 μM) following daily administration of 300 mg, suggesting that concentrations required to inhibit autophagy in tumors may be achievable, although this has not been formally demonstrated. Confirmation of autophagy inhibition using imaging and serial biopsies to stain for pharmacodynamic (PD) biomarkers is necessary to confirm that any observed effects of CQ or HCQ are indeed due to inhibition of autophagy. Current PD markers for autophagy inhibition are limited relying on either direct visualization of autophagy in cells using electron microscopy or immunohistochemical staining for autophagy markers including p62, Beclin1 and LC3. Further development and validation of these markers is necessary. Assays to predict which patients will benefit from autophagy inhibitors are also needed.
Notably, autophagy inhibition also synergizes with radiotherapy 10. These observations suggest that the main role for CQ/HCQ in anticancer therapy will be in potentiating the effects of multimodal combination regimens that contain DNA damaging drugs, angiogenesis inhibitors, molecular targeted agents and radiotherapy. Bevacizumab causes a rapid reduction in tumor perfusion and consequently reduced drug delivery and this feature needs to be considered during optimization of rationally designed chemotherapy schedules to maintain adequate drug delivery 12.
Acknowledgements
Cancer Research UK, the European Commission (Metoxia Grant No. 222741), Breast Cancer Reseacrh Foundation and Oxford NHS Biomedical Research Centre.
Footnotes
No conflicts of interest
References
- 1.Selvakumaran M, Amaravadi R, Vasilevskaya IA, O’Dwyer PJ. Autophagy Inhibition Sensitizes Colon Cancer Cells to Anti-angiogenic and Cytotoxic Therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-12-1542. [DOI] [PubMed] [Google Scholar]
- 2.Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145:30–8. doi: 10.1016/j.cell.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harbor perspectives in medicine. 2012;2:a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nature reviews Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer research. 2012;72:1773–83. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Molecular cancer therapeutics. 2009;8:1867–77. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre A, Patiar S, Wigfield S, Li JL, Ledaki I, Turley H, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:3100–11. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Molecular cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. The Journal of clinical investigation. 2010;120:127–41. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pike LR, Singleton DC, Buffa F, Abramczyk O, Phadwal K, Li JL, et al. Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. The Biochemical journal. 2013;449:389–400. doi: 10.1042/BJ20120972. [DOI] [PubMed] [Google Scholar]
- 12.Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]