Abstract
The CDK inhibitors, p18INK4c and p16INK4a, both have the credentials of tumor suppressors in human cancers and mouse models. For p16INK4a, the underlying rationale is its role in senescence but the selective force for inactivation of p18INK4c in incipient cancer cells is less clear. Here we show that in human fibroblasts undergoing replicative or oncogene-induced senescence, there is a marked decline in the levels of p18INK4c protein and RNA, which mirrors the accumulation of p16INK4a. Down-regulation of INK4c is not dependent on p16INK4a and RAS can promote the loss of INK4c without cell cycle arrest. Down-regulation of p18INK4c correlates with reduced expression of menin and E2F1 but is unaffected by acute cell cycle arrest or inactivation of the retinoblastoma protein (pRb). Collectively, our data question the idea that p18INK4c acts as a backup for loss of p16INK4a and suggest that the apparent activation of p18INK4c in some settings represents delayed senescence rather than increased expression. We propose that the contrasting behavior of the two very similar INK4 proteins could reflect their respective roles in senescence versus differentiation.
Keywords: INK4, CDK inhibitors, senescence, E2F1, menin
Introduction
The INK4 family of cyclin-dependent kinase (CDK) inhibitors, p16INK4a, p15INK4b, p18INK4c and p19INK4d, are structurally and functionally similar (1). They comprise between 3.5 and 5 ankyrin-type repeats and bind directly to CDK4 and CDK6, thereby preventing or destabilizing the interaction of these kinases with regulatory D-type cyclins. Although the INK4 proteins demonstrate equivalent affinity for CDK4 and CDK6 in vitro and when over-expressed (2), the prevailing impression is that they associate preferentially with CDK6 in physiological settings (3-6).
The prototypic member of the family, p16INK4a, is an accredited tumor suppressor that sustains inactivating germline mutations in familial melanoma and is incapacitated by diverse mechanisms in a variety of sporadic cancers (1). Its tumor suppressor functions are best explained by its role in senescence, either in response to an activated oncogene or in cells that reach the end of their proliferative lifespan (7). The INK4b gene, which is tandemly linked to INK4a on human chromosome 9p21, is also implicated in senescence (7) and has credentials as a tumor suppressor in some contexts, but inactivating mutations of p15INK4b are very rare (1).
The same applies to p18INK4c and p19INK4d but there has recently been renewed interest in the potential role of INK4c in tumor suppression. The most compelling evidence comes from mice carrying null alleles of Ink4c. These animals develop spontaneous pituitary adenomas late in life (8) but when Ink4c deficiency is combined with other gene knockouts, the range and frequency of tumors becomes more extensive. For example, mice lacking both p18Ink4c and p27Kip1 develop a tumor spectrum reminiscent of human multiple endocrine neoplasia type 1 (MEN1) (9). Critically, menin, the product of the MEN1 gene, has been shown to activate p27KIP1 and p18INK4c by enhancing the binding of trithorax group (TrxG) complexes to the respective promoters (10, 11). Crossing of the knockout mice implicates Ink4c as the critical target of menin in this system (12). Another important example is the combination of Ink4c and p53 deficiency that results in early onset hemangiosarcoma and medullablastoma and nicely models the genetic background of the human disease (13, 14).
In addition to these mouse models, loss or silencing of human p18INK4c has been recorded in a number of human malignancies (14-23). Glioblastoma multiforme (GBM) is an intriguing example because homozygous deletions and inactivating mutations of INK4c are observed in a tumor that is frequently associated with defects in the p16INK4a-CDK4-pRb pathway (20-23). As a number of the samples showed concordant loss of both INK4a and INK4c, implying that the selection against these components is not mutually exclusive, some have proposed that p18INK4c acts as a backup for p16INK4a (21, 23).
In considering what drives the inactivation of p18INK4c in human cancers, we investigated whether it contributes to replicative or oncogene-induced senescence. Remarkably, the accumulation of p16INK4a in senescent human fibroblasts is accompanied by the virtual elimination of p18INK4c. Loss of p18INK4c correlates with a reduction in the total levels of menin and E2F1, which positively regulate INK4c, but we saw no inverse correlation between INK4c and pRb status in human cancer cells, or between p16INK4a and p18INK4c levels as previously suggested. Rather, depletion of p16INK4a delays the onset of senescence and concomitant down-regulation of p18INK4c.
Materials and Methods
Cell culture and retroviral infection
Conditions for the serial propagation and retroviral infection of human diploid fibroblasts (Hs68, TIG3, BF, IMR90, Leiden and Q34) and mouse embryo fibroblasts (MEF) were as previously described (24-27). As these are primary cells with a finite lifespan, they are considered to be genetically stable and have not been authenticated. The panel of human tumor cells lines, described previously (28) were authenticated by DNA fingerprinting by the in-house Cell Services facility. Retroviral vectors encoding HA-tagged p16INK4a, p18INK4c, p21CIP1, and SV40 large T-antigen were as described (24, 29) and the E2F1:ER. ER:RAS, ΔRAF:ER and Bmi1 vectors were kindly provided by Kristian Helin, Paul Khavari, Martin McMahon and Maarten van Lohuizen, respectively. The various ER fusions proteins were induced by addition of either 100 or 500nM 4-hydroxytamoxifen (OHT). For the cell cycle arrest experiments, cells were treated with hydroxyurea (1mM), nocodazole (0.3μM), or RO 3306 (9 μM) for 24 h or with the CDK4/6 inhibitor PD-033299 (500ng/ml) for up to 20 days. In some experiments, cells were treated with MG132 (20μM) for 24h or with cycloheximide (20μM). Staining for SA-β-galactosidase activity and DAPI foci followed standard protocols (24).
Immunoprecipitation and immunoblotting
Cell lysates were prepared in TNN buffer (50mM Tris.HCl, pH 7.5, 120 mM NaCl, 5mM EDTA, 0.5% NP40) with complete EDTA free protease inhibitor (Roche) plus 1mM PMSF, and analyzed essentially as described previously (24). Primary antibodies were as follows: CDK4 (sc-601), CDK6 (sc-177), E2F1 (sc-22820), p18INK4c (sc-1208), mouse p16Ink4a (sc-1207), β-tubulin (sc-9104), p53 (DO-1, sc-126), cyclin E (sc-247), cyclin A (sc-601) and p21CIP1 (sc-397) were from Santa Cruz; MEK1/2 (#9122) was from Cell Signalling; menin (AB2605), HRP conjugated anti-GAPDH (AB9482) and a monoclonal antibody (mAb) against CDK6 (AB77674) were from Abcam; CDK4 (DCS31) and p18INK4c (DCS118) mAbs were from Novus; the cyclin D1 mAb (DCS6) was from BD Pharmingen; a pan-Ras mAb (OP41) was from Oncogene Research; the mAb against p16INK4a (JC8) was provided by LRI Cell Services using cells originally obtained from J. Koh and E. Harlow. We also generated two rabbit antisera raised against bacterially expressed p18INK4c (FST1 and FST4) and have previously described in-house reagents against cyclin D1 (287.3), CDK6 (LBO-1) and p16INK4a (DPAR12) (28).
Quantitative reverse transcription and PCR
Total RNA was extracted using the Ultra Pure RNA extraction Kit (Roche) (Roche). Reverse transcription was carried out with 0.5–1 μg of RNA using MultiScribe reverse transcriptase and random hexamer primers (Applied Biosystems). One fiftieth of the cDNA was used as a template for quantitative real-time PCR (qRT-PCR) on ABI 7900HT (Applied Biosystems) using Power SYBR Green Master Mix (Applied Biosystems) or TaqMan Universal PCR Master Mix (Applied Biosystems). Expression was normalized to GAPDH. For detection of the INK4a transcript, annealing and extension was done for 1 min at 66°C, while for other transcripts this step was performed at 60°C. Sequences of the primers can be found in Supplementary Table 1
RNA interference
The validated shRNA against p16INK4a in the pRetroSuper vector was as described (30). Three different shRNAs targeting human p18INK4c were generated in pRetroSuper using the following 19-nucleotide sequences: A- 5′-CTGGTTTCGCTGTCATTCA-3′, B- 5′-GGGAACCTGCCCTTGCACT-3 and C- 5′-GGGGGACACCGCCTGTGAT-3′. The C sequence was the most effective.
Chromatin immunoprecipitation
Cells were harvested and processed for chromatin immunoprecipitation as described (31). After sonication, the chromatin was centrifuged to pellet the debris and pre-cleared at 4°C for 2h with protein A-agarose and protein G-sepharose. The samples were immunoprecipitated with 4μg of relevant antibody or a control IgG at 4°C overnight, followed by a 3h incubation with blocked protein A- or protein G-sepharose. The antibody-chromatin complexes were extensively washed with ice-cold IP buffer (150mM NaCl, 50mM Tris-HCl (pH 7.5), 5mM EDTA. 0.5% NP40 and 1% Triton X-100) and eluted with 1% SDS, 100mM NaHCO3, 10mM DTT at 65°C for 5min and RT for 15min. Crosslinking was reversed by incubation at 65°C overnight following the addition of NaCl to 150mM. The samples were treated with 100 μg/ml RNase (Roche) for 30 min at 37 °C and DNA was purified using a Qiagen PCR cleanup kit. Antibodies against H3K4me3 (clone 07-473 or MC315) and H3K27me3 (clone 07-449) were from Millipore, and Menin (AB2605) from Abcam.
Gel filtration
The procedures for gel filtration and associated data were as described in ref (32).
Results
Down-regulation of p18INK4c in replicative and oncogene-induced senescence
When primary human diploid fibroblasts (HDFs) reach the end of their proliferative lifespan in culture, there is a substantial accumulation of p16INK4a, preceded by a peak in p21CIP1 expression which subsequently declines (25). However, to our knowledge, there have been no reports on the expression of p18INK4c in this classical model of senescence. We found that whereas p18INK4c levels remained relatively constant with cumulative population doublings, they declined dramatically when the cells became senescent, essentially the mirror image of p16INK4a expression levels (Fig. 1A). This pattern of expression was recapitulated in different HDFs irrespective of their maximum lifespan and basal levels of p16INK4a and p18INK4c (data not shown).
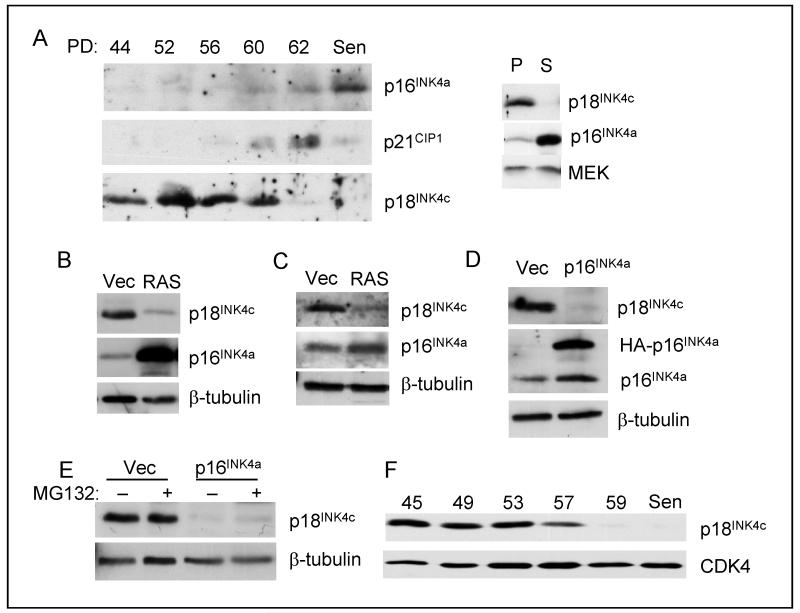
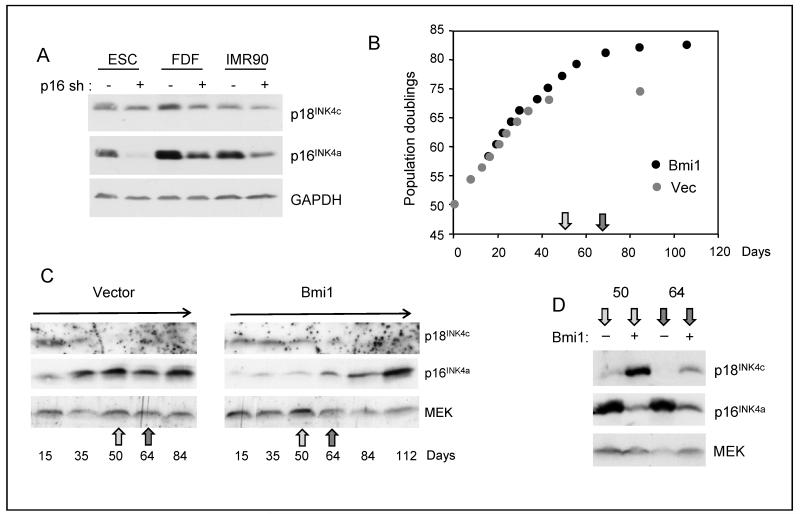
Figure 1. Downregulation of p18INK4c at senescence.
A. HDFs (TIG3) were passaged continuously until they reached replicative senescence (Sen). Cell lysates prepared at the indicated population doublings (PD) were fractionated by SDS-PAGE and immunoblotted for p16INK4a, p21CIP1 and p18INK4c. Right panel shows a direct comparison between proliferating (P) and senescent (S) cells. B. HDFs (TIG3) were infected with a retrovirus encoding H-RASG12V or the empty vector control (Vec). Following drug selection, the levels of p18INK4c and p16INK4a were assessed by immunoblotting. C. Primary MEFs were infected with a retrovirus encoding H-RASG12V or the empty vector control (Vec). The levels of p18Ink4c and p16Ink4a were assessed by immunoblotting. D. HDFs (Hs68) were infected with a retrovirus encoding HA-tagged p16INK4a. The levels of p18INK4c and p16INK4a were assessed by immunoblotting. E. MG132 had no effect on p18INK4c levels in cells transduced with HA-p16INK4a or empty vector. F. The Leiden strain of p16INK4a-deficient fibroblasts were passaged until they reached senescence. Samples taken at the indicated PDs were immunoblotted for p18INK4c, with CDK4 as a loading control.
As replicative senescence reflects both telomere-dependent and independent events, we asked whether a similar phenomenon occurs during oncogene-induced senescence. Expression of H-RASG12V in HDFs caused a marked down-regulation of p18INK4c, contrasting with the anticipated up-regulation of p16INK4a (Fig. 1B). Analogous effects were observed in mouse embryo fibroblasts (MEFs) (Fig. 1C) and with other oncogenes (data not shown).
Down-regulation of p18INK4c at senescence is independent of p16INK4a
One simple explanation for these observations would be that the accumulation of p16INK4a at senescence might displace p18INK4c from CDK complexes, resulting in its turnover. Indeed, ectopic expression of HA-tagged p16INK4a in early passage HDFs caused a marked reduction of p18INK4c (Fig. 1D). However, there are several reasons why this explanation is untenable. First, both p18INK4c and p16INK4a are stable proteins with half-lives in excess of 15 hours as judged by blocking protein synthesis with cycloheximide or by pulse-chase analyses (Suppl. Fig. S1) and (5, 28, 33, 34). Second, the down-regulation of p18INK4c was not reversed by treatment with the proteasome inhibitor MG132 (Fig. 1E). Third, extinction of p18INK4a at senescence was observed in the Leiden strain of human fibroblasts (24), which lack functional p16INK4a (Fig. 1F).
Down-regulation of p18INK4c RNA at senescence
As these data imply that the loss of p18INK4c at senescence is not caused by protein turnover, we asked whether the down-regulation of INK4c occurs at the RNA level. When different strains of HDF, including p16INK4a-deficient strains, were passaged to senescence, there was a marked reduction in the levels of INK4c RNA relative to early passage cells, as assessed by qRT-PCR (Fig. 2A and 2B). INK4c RNA levels also declined in cells infected with an H-RASG12V retrovirus (Fig. 2C) or following addition of OHT to HDFs transduced with a RAS:ER fusion protein (Fig. 2D). As expected, INK4a RNA levels were substantially increased under the same conditions. Unlike other members of the INK4 family, the INK4c gene specifies two distinct transcripts that originate from alternative promoters but encode the same protein (35). Using primers that discriminate between the two transcripts, we confirmed that both are down-regulated by RAS (Suppl. Fig. S2).
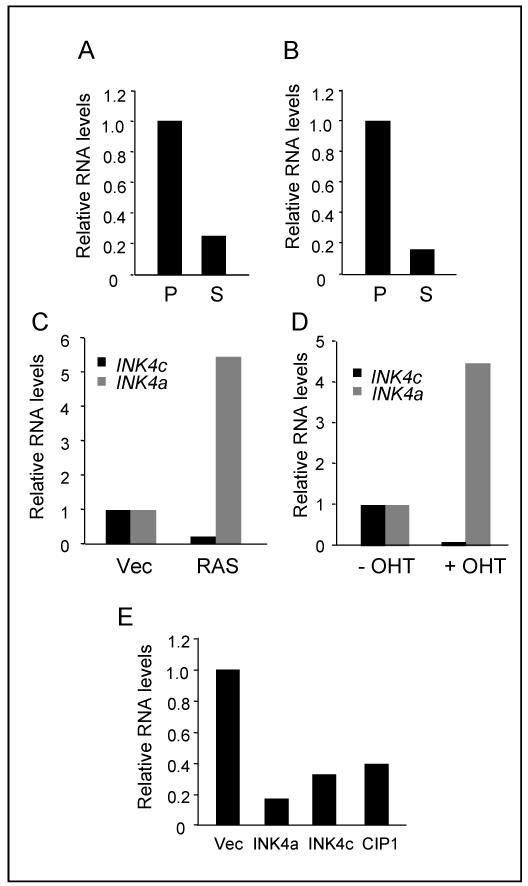
Figure 2. Downregulation of INK4c RNA at senescence.
A and B. The Hs68 (A) and Leiden (B) strains of HDF were passaged to senescence and the relative levels of INK4c RNA in proliferating (P) and senescent (S) cells were assessed by reverse transcription and quantitative real-time PCR (qRT-PCR). C. Relative levels of INK4c and INK4a RNAs in HDFs (Hs68) infected with a retrovirus encoding H-RASG12V or empty vector control (Vec). D. Relative levels of INK4c and INK4a RNAs following addition of tamoxifen (OHT) to HDFs (BF) expressing ER:H-RASG12V. E. Relative levels of INK4c RNA in HDFs (Hs68) transduced with retroviral vectors encoding HA-tagged versions of p16INK4a, p18INK4c or p21CIP1. Note that endogenous INK4c RNA was measured using primer sets that do not detect the ectopically expressed INKc cDNA.
HDFs arrested by over-expression of p16INK4a or other members of INK4 and CIP/KIP families acquire many features of senescence although they do not display the senescence-associated secretory phenotype associated with chronic DNA damage (36, 37). We therefore investigated the fate of p18INK4c in these circumstances. Interestingly, ectopic expression of p21CIP1, p16INK4a or p18INK4c itself resulted in down-regulation of the endogenous INK4c gene (Fig. 2E). These findings suggest that transcriptional silencing of INK4c is a consistent feature of senescent cells but make it unlikely that the loss of p18INK4c has a causal role in the phenotype.
Regulation of p18INK4c by menin
The INK4a locus is subject to regulation by the Polycomb group (PcG) of transcriptional repressors (7) via trimethylation of histone H3 on lysine 27 (H3K27me3). Although published datasets indicate that INK4c is also occupied by PcG complexes in embryonic stem cells (38), we found no evidence for the H3K27me3 mark at INK4c in human fibroblasts under conditions in which it is readily detectable at INK4a (Suppl. Fig. S3). As previously shown (26), H-RASG12V caused a loss of H3K27me3 at INK4a.
The repressive effects of PcG complexes can be countered by the TrxG proteins and a number of studies have shown that INK4c is positively regulated by the tumor suppressor menin as part of a TrxG complex (10, 11). In line with these reports, we found that in human fibroblasts undergoing H-RASG12V-induced senescence, there was a measurable decrease in the levels of menin and H3K4me3 at the INK4c locus (Figs. 3A and 3B). In essentially all of the situations in which we documented a senescence-associated decline in INK4c expression, there was a concomitant but less pronounced decline in the levels of menin RNA (Fig. 3C -G).
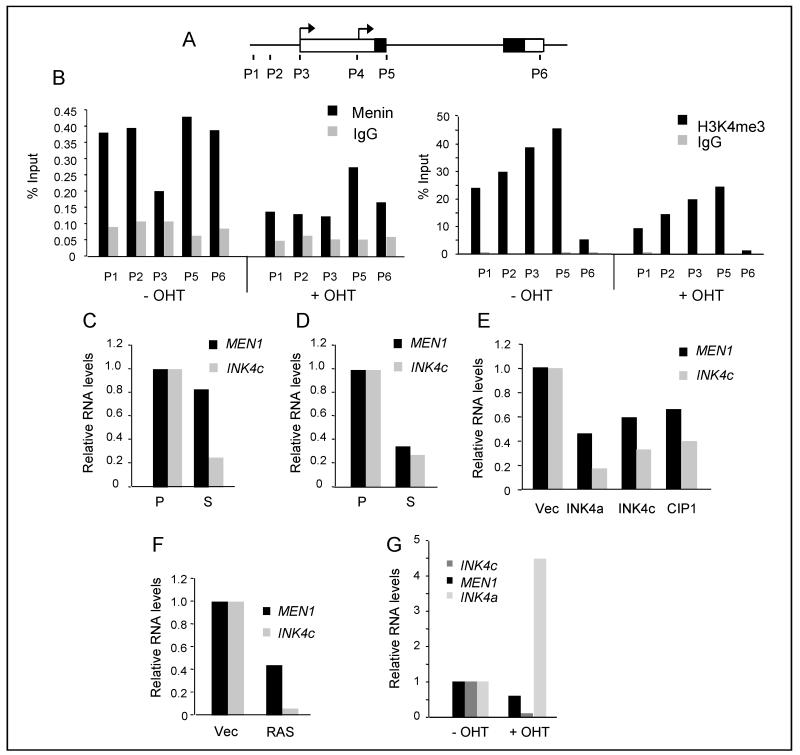
Figure 3. Role of menin in the down-regulation of INK4c.
A. Diagram of the human INK4c locus with exons depicted as boxes and coding domains shaded. The location of the transcriptional start sites (arrows) and primer sets P1-P6 used for ChIP analyses are indicated. B. ChIP of menin and H3K4me3 at the INK4c locus before and after treatment of IMR90 ER:RAS cells with OHT. Enrichment for each primer set was assessed by qPCR relative to a non-specific IgG and expressed as percentage of input. C and D. Comparison of MEN1 and INK4c RNA levels in proliferating (P) and senescent (S) populations of Hs68 (C) and Leiden (D) fibroblasts (same samples as in Fig. 2A and B). E. Relative levels of MEN1 and INK4c RNA in cells transduced with retroviral vectors encoding HA-tagged versions of p16INK4a, p18INK4c or p21CIP1, as in Fig. 2C. F. Relative levels of INK4c, MEN1 and INK4a RNAs in Hs68 cells infected with a retrovirus encoding H-RASG12V or empty vector control (Vec), as in Fig. 2D. G. Relative levels of INK4c, MEN1 and INK4a RNAs following addition of tamoxifen (OHT) to BF cells expressing ER:H-RASG12V (as in Fig. 2E).
Regulation of p18INK4c by E2F
As it seemed unlikely that the changes in menin levels could account for the dramatic down-regulation of INK4c, we considered the possibility that as an E2F-regulated gene (21, 39, 40), INK4c was simply reacting to cell cycle arrest. However, agents that cause acute arrest in different cell cycle phases had no effect on the levels of p18INK4c RNA or protein, and we saw no substantive changes in p18INK4c levels following serum stimulation of quiescent cells (Suppl. Fig. S4A and S4B, and data not shown). Moreover, p18INK4c levels were relatively unaffected by introduction of SV40 large T-antigen whereas p16INK4a expression was clearly elevated in this setting (Fig 4A). We also surveyed p18INK4c expression in a panel of human tumor cell lines that we had previously used to demonstrate the inverse correlation between pRb status and p16INK4a expression (28). There was no consistent relationship between p16INK4a and p18INK4c expression or between p18INK4c and pRb status (Fig 4B), in line with an earlier report (41).
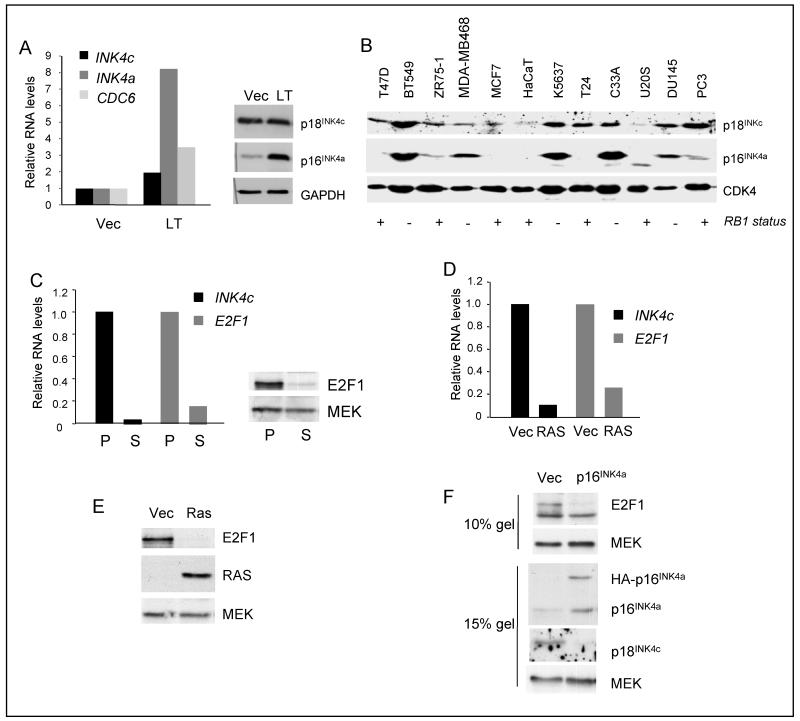
Figure 4. Regulation of p18INK4c by E2F1.
A. Relative levels of INK4c, INK4a and CDC6 RNAs in Hs68 cells infected with a retrovirus encoding SV40 large T-Ag (LT) or empty vector control (Vec) (left panel). Lysates from the same cells were fractionated by SDS-PAGE and immunoblotted for p18INK4c and p16INK4a as indicated (right). B. Survey of p18INK4c and p16INK4a expression in a series of human cancer cell lines from different anatomical sites: breast (T47D, BT5499, ZR75-1 MDA-MB468 and MCF7), keratinocyte (HaCaT), bladder (K5637 and T24), cervix (C33A), bone (U2OS) and prostate (DU145 and PC3). The +/− below each lane refers to the functional status of the RB1 gene in these cell lines, based on published literature. CDK4 was used as a loading control. C. Comparison of E2F1 RNA (left) and protein (right) levels in proliferating (P) and senescent (S) HDFs (TIG3). D. Comparison of INK4c and E2F1 RNA expression in HDFs (IMR90) infected with a retrovirus encoding H-RASG12V (Ras) or empty vector (Vec). E. Down-regulation of E2F1 protein in HDFs (TIG3) cells transduced with H-RASG12V. F. Down-regulation of E2F1 in HDFs infected with a retrovirus encoding HA-p16INK4a.
Taken together, our data imply that p18INK4c expression is largely unaffected by the fluctuations of E2F activity that occur during the cell cycle or as a consequence of pRb inactivation. However, it was quite clearly induced by over-expression of E2F1, as confirmed using HDFs expressing a tamoxifen-regulated ER-E2F1 fusion protein (Suppl. Fig. S4C and S4D). We therefore asked whether the down-regulation of INK4c at senescence correlated with reduced expression of E2F1. As shown in Figure 4C-F, the levels of E2F1 RNA and protein are consistently and dramatically reduced in fibroblasts undergoing either replicative, oncogene- or p16-induced senescence.
Distinction between activation and delayed repression of INK4c
It was previously reported that shRNA-mediated knockdown of p16INK4a in normal astrocytes caused an increase in the levels of p18INK4c (21). As this appeared to be at odds with our findings, we performed analogous experiments in human fibroblasts. In general, we found that shRNA-mediated knockdown of p16INK4a had no discernible impact on the levels of p18INK4c despite robust depletion of p16INK4a (Fig. 5A). This negative result was confirmed in multiple experiments with several different strains of logarithmically dividing cells (not shown). However, we occasionally noted higher levels of p18INK4c in p16INK4a -knockdown cells that had been in culture for some time. We reasoned that agents that suppress or disable the pRb-p16INK4a pathway can extend the replicative lifespan of cells and potentially delay the down-regulation of p18INK4c that occurs at senescence. Depending on when the cells are analyzed, this could give the impression that p18INK4c levels are elevated relative to the control cells.
Figure 5. Distinction between activation and delayed repression.
A. A validated shRNA was used to knockdown p16INK4a in several strains of logarithmically growing HDFs and its effects on p16INK4a and p18INK4c were assessed by immunoblotting. B. Lifespan extension in TIG3 cells infected with a retrovirus encoding mouse Bmi1 compared to empty vector (Vec). C. The cells described in panel B were harvested at the indicated days post-infection and the levels of p16INK4a and p18INK4c were assessed by immunoblotting. D. Direct comparison of lysates prepared at days 50 and 64 (indicated by the arrows in panel B) from cells infected with Bmi1 virus (+) or empty vector (−).
To explore this possibility, we monitored the changes in p16INK4a and p18INK4c expression in human fibroblasts infected with a retrovirus encoding the PcG protein Bmi1. As expected (25), Bmi1 enabled the TIG3 strain of fibroblasts to grow for ~10 additional population doublings relative to the cells infected with the empty vector (Fig. 5B). It also reduced the basal levels of p16INK4a but did not prevent the eventual accumulation of p16INK4a at senescence (Fig. 5C). Importantly, the p18INK4c levels declined much later in the Bmi1-transduced cells than in the controls. To illustrate this point more clearly, Figure 5D shows a direct comparison of cell lysates prepared on days 50 and 64 post-infection. At these time points, cells expressing Bmi1 appear to have substantially more p18INK4a than the control, giving the false impression that p18INK4c is being activated by Bmi1. Similar effects were observed in cells whose lifespan was extended by over-expression of CDK4 or p16-shRNA (data not shown).
Senescence-dependent and -independent effects on p18INK4c
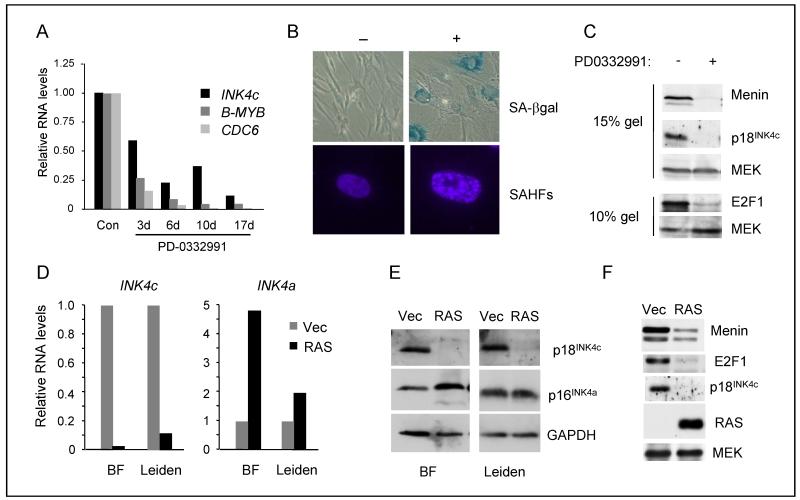
One of the characteristic features of senescent fibroblasts is the appearance of senescence-associated heterochromatin foci (SAHF) which cause irreversible silencing of a subset of E2F target genes (42). We wondered whether the silencing of INK4c at senescence was related to the formation of SAHFs. To avoid altering the levels of key components, we treated HDFs with the CDK4/6 inhibitor PD-0332991, which causes a pRb-dependent G1 arrest (43) and was recently shown to induce senescence in GBM cells that have co-deletion of INK4a and INK4c (22, 23). The stable arrest caused by PD-0332991 was accompanied by a decline in the expression of p18INK4c RNA, as well as other E2F target genes (Fig. 6A). Importantly, the cells developed characteristic features of senescence, and stained positively for SA-β-galactosidase and SAHFs (Fig. 6B). Of note, the loss of p18INK4c expression was accompanied by down-regulation of both menin and E2F1 (Fig. 6C). However, for technical reasons, we were unable to draw a direct correlation between the appearance of SAHFs and elimination of p18INK4c at the single cell level.
Figure 6. Senescence-dependent and -independent downregulation of p18INK4c.
A. HDFs (Hs68) treated with the CDK4/6 inhibitor PD0332991 were harvested at the indicated intervals and levels of INK4c, B-MYB and CDC6 RNA were assessed by qRT-PCR and normalized to the untreated control. B. HDFs (TIG3) treated with and without PD0332991 for 14d were stained for SA-β-galactosidase activity and with DAPI to visualize SAHFs. C. Lysates from the cells described in panel B were immunoblotted for p18INK4c, p16INK4a, menin, and E2F1 with MEK as a loading control. D. HDFs (BF and Leiden) were infected with a retrovirus encoding H-RASG12V or empty vector and the relative levels of INK4c and INK4a RNA were assessed by qRT-PCR and normalized to the vector only control. E. Lysates from the cells described in panel D were immunoblotted for p18INK4c and p16INK4a. F. Lysates from Leiden cells infected with a retrovirus encoding H-RASG12V or empty vector were immunoblotted for menin, E2F1, p18INK4c and RAS.
Reversing the logic, we asked whether H-RASG12V would cause down-regulation of INK4c in the absence of SAHFs. The Leiden strain of p16INK4a-deficient HDFs are resistant to oncogene-induced senescence under conditions that efficiently arrest other HDF strains (24). H-RASG12V caused down-regulation of INK4c and up-regulation of the mutant INK4a in Leiden cells, although the effects were less dramatic than in the control fibroblasts (Fig. 6D and 6E). As H-RASG12V caused down-regulation of p18INK4c in MEFs (Fig. 1D) where SAHFs are not observed (44), our data suggest that SAHFs are not required for the down-regulation of INK4c by H-RASG12V. Importantly, the correlation between p18INK4c, E2F1 and menin expression still applied in Leiden cells despite the avoidance of senescence (Fig. 6F). Taken together, our data suggest that there could be two distinct pathways leading to down-regulation of INK4c, one instigated by CDK inhibition and the other instigated by RAS that appears to be independent of CDK inhibition.
Consequences of p18INK4c downregulation in senescent cells
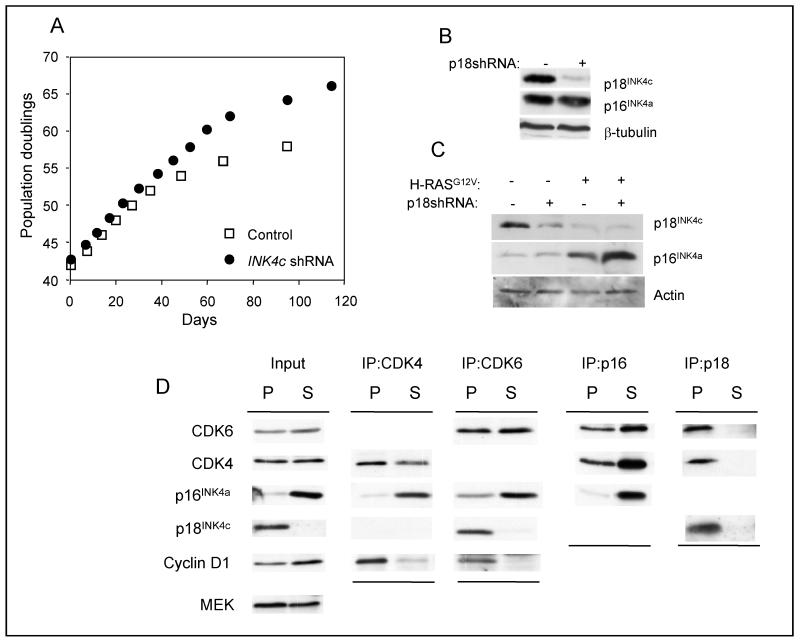
At face value, the behavior of p18INK4c at senescence is at odds with its role as a tumor suppressor. Loss or down regulation of p18INK4c would in principle make it more difficult for cells to mount a senescence-like arrest in response to oncogenic signals by depleting the total INK4 pool. To investigate this possibility, we used shRNA to knock down the expression of p18INK4c in primary HDFs. As exemplified in Figure 7, cells transduced with p18INKc shRNA had a proliferative advantage and extended lifespan relative to control cells. Similar effects were observed in p16INK4a-deficient strains of HDF (not shown). However, the cells eventually underwent replicative senescence. Moreover, cells expressing p18INK4c shRNA remained susceptible to H-RASG12V-induced senescence. Interestingly, these cells had higher levels of p16INK4a at senescence, as compared to controls, suggesting an increased dependence on p16INK4a to enforce the arrest (Fig. 7C).
Figure 7. p18INK4c is not required for replicative or oncogene induced senescence.
A. Hs68 cells infected with a retrovirus encoding either shRNA against p18INK4c or a non-specific shRNA (Con) were passaged until they reached M1 senescence. B. Immunoblotting for p18INK4c and p16INK4a in cells infected with p18INK4c shRNA. C. Expression of H-RASG12V in cells transduced with p18INK4c shRNA caused oncogene-induced senescence accompanied by a further reduction of p18INK4c levels and enhanced expression of p16INK4a. D. Lysates from proliferating (P) and senescent (S) fibroblasts were immunoprecipitated with rabbit antibodies against CDK6, CDK4, p18INK4c and p16INK4a. The proteins were fractionated by SDS-PAGE and immunoblotted with mouse monoclonal antibodies against the indicated proteins.
This result would be consistent with the idea that the total levels of INK4 proteins in the cell might determine the availability of CDK4 and CDK6 to associate with the D-type cyclins. Human fibroblasts contain substantially more CDK4 than CDK6 RNA, as judged by deep sequencing (unpublished results of Helen Pemberton and the authors), and we and others have previously demonstrated that only a small fraction of the total CDK6 is associated with D-type cyclins (5, 32). Excluding the high-molecular weight complexes with heat shock chaperone proteins, most of the CDK6 is bound by INK4 proteins in 1:1 complexes of around 50-60 kDa whereas the bulk of the CDK4 is in 150-170 kDa complexes with D-cyclins and CIP/KIP proteins (Supp. Fig. S5A). In proliferating cells, CDK6 is primarily associated with p18INK4c as judged by co-immunoprecipitation, but in senescent cells, virtually all of the CDK6 becomes bound to p16INK4a (Fig. 7D and Suppl. Fig. S5B). Although CDK4 can be found associated with p16INK4a and p18INK4c in early passage cells, its association with cyclin D1 is preserved at senescence despite the excess of p16INK4a (Fig 7D and (32)). In summary, the down-regulation of p18INK4c at senescence results in re-assortment of the D-cyclins, CDKs and CDK inhibitors to retain CDK6 in an apparently inactive state.
Discussion
A motivation for this study was the evidence that p18INK4c acts as a tumor suppressor not only in mouse models but also in a number of human cancers. Remarkably, deletions of INK4c have been found in tumors that also have defects in INK4a (17, 21-23, 45) prompting questions about the selective rationale for concomitant inactivation of two members of the same family. As mutation or silencing of p16INK4a can enable incipient cancer cells to escape from oncogene-induced senescence, it was suggested that p18INK4c serves as a backup in these circumstances, causing pressure to escape from p18INK4c-mediated arrest (21, 23). The data we describe make this scenario unlikely.
In primary human fibroblasts undergoing senescence, whether in response to an activated oncogene, continuous passaging or CDK inhibitors, there is a marked decline in the expression of p18INK4c which is effectively a mirror image of the dramatic accumulation of p16INK4a in most of these settings. The down-regulation of INK4c occurs at the RNA level and has been previously documented in gene expression profiles of RAS-induced senescence (46-48), although these studies did not discuss the implications. We find that the effects on p18INK4c are not dependent on the p16INK4a status of the cell or on cell cycle arrest per se.
In seeking potential mechanisms, we considered known activators of INK4c such as menin (10-12) and E2F1 (21, 39, 40). Although H-RASG12V caused reduced binding of menin at the INK4c locus and the levels of menin declined in all of the situations in which we documented down-regulation of p18INK4c, the effects were relatively modest and unlikely to account for the almost complete silencing of INK4c.
A more cogent case can be made for E2F1. In situations where we observed loss of p18INK4c expression, we invariably saw a substantial decline in the total levels of E2F1. Again, our findings agree with published evidence that E2F1 is down-regulated in replicative and H-RASG12V-induced senescence (46, 49). However, the interpretation is complicated by the fact that agents that modulate E2F1 activity rather than its total levels, such as cell cycle arrest or inactivation of pRb, had little effect on p18INK4c. While it is clear that the response of E2F target genes can be context dependent, our data do not support the notion of a linear pathway through which loss of p16INK4a induces E2F-dependent expression of p18INK4c (21).
The concept of a linear pathway was based on evidence that shRNA-mediated knockdown of p16INK4a in primary astrocytes resulted in up-regulation of p18INK4c (21). However, this inverse relationship was not apparent in the logarithmically growing human fibroblasts studied here and, while there could be differences between cell types, our data suggest that the passage history of the cells can have an important bearing on the interpretation of such experiments. Agents that neutralize p16INK4a, such as PcG proteins, INK4a shRNAs, or ectopic expression of CDK4, have the potential to extend the replicative lifespan of cells and thereby delay the down-regulation of p18INK4c. Depending on how close the control cells are to senescence, this can give the false impression that p18INK4c is being up-regulated.
Although they are virtually indistinguishable biochemically, a substantial body of evidence implies that the INK4 family of proteins and their target kinases, CDK4 and CDK6, have distinct roles. Whereas p16INK4a is primarily involved in the implementation of senescence in response to diverse forms of stress, most studies link p18INK4c with differentiation and suggest that p18INK4c and CDK6 are partners in crime (3, 4, 6, 50-53). Thus, the over-expression of CDK6 or loss of p18INK4c, as observed in a variety of human cancers, would be expected to impair differentiation.
Interestingly, in terminally differentiated myoblasts, virtually all of the CDK6 is bound to p18INK4c (3) whereas in senescent fibroblasts almost all of the CDK6 is bound by p16INK4a. This suggests a fundamental difference between these states of cell cycle arrest. But what purpose is served by expressing p18INK4c and CDK6 if the major fraction of both proteins is locked in a futile embrace and why would the substitution of p18INK4c by p16INK4a at senescence make a difference? One possibility would be that senescence requires an INK4 protein that is more able to inhibit CDK4. Another is that while differentiation is reversible, as evident from the reprogramming of pluripotency in somatic cells, senescence is generally considered to be irreversible. Interestingly, INK4c levels decline when human fibroblasts are transduced with the OSKM reprogramming factors, consistent with reports that senescence acts as a barrier to reprogramming (A. Banito and J. Gil, personal communication). It will be interesting to explore the dynamics of p16INK4a and p18INK4c expression and their regulation by PcG complexes during this process.
Supplementary Material
Acknowledgements
We are grateful to past and present members of the Molecular Oncology Laboratory who have contributed to various stages of this study, particularly Maria Starborg, Francesca Stott, Margarida Ruas, Fiona Gregory, Hollie Chandler and Marc Rodriguez-Niedenführ, and to Jesus Gil for comments on the manuscript. We also thank Pfizer for providing PD-0332991 and the many named researchers who have provided reagents.
References
- 1.Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:F115–77. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 2.Thullberg M, Bartkova J, Khan S, Hansen K, Ronnstrand L, Lukas J, et al. Distinct versus redundant properties among members of the INK4 family of cyclin-dependent kinase inhibitors. FEBS Lett. 2000;470:161–6. doi: 10.1016/s0014-5793(00)01307-7. [DOI] [PubMed] [Google Scholar]
- 3.Franklin DS, Xiong Y. Induction of p18INK4c and its predominant association with CDK4 and CDK6 during myogenic differentiation. Mol Biol Cell. 1996;7:1587–99. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Induction of cell cycle arrest and B cell terminal differentiation by CDK inhibitor p18(INK4c) and IL-6. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 5.Parry D, Mahony D, Wills K, Lees E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol. 1999;19:1775–83. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovalev GI, Franklin DS, Coffield VM, Xiong Y, Su L. An important role of CDK inhibitor p18(INK4c) in modulating antigen receptor-mediated T cell proliferation. J Immunol. 2001;167:3285–92. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–77. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 8.Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin DS, Godfrey VL, O’Brien DA, Deng C, Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000;20:6147–58. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–54. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659–64. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai F, Pei XH, Nishikawa T, Smith MD, Xiong Y. p18Ink4c, but not p27Kip1, collaborates with Men1 to suppress neuroendocrine organ tumors. Mol Cell Biol. 2007;27:1495–504. doi: 10.1128/MCB.01764-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zindy F, Nilsson LM, Nguyen L, Meunier C, Smeyne RJ, Rehg JE, et al. Hemangiosarcomas, medulloblastomas, and other tumors in Ink4c/p53-null mice. Cancer Res. 2003;63:5420–7. [PubMed] [Google Scholar]
- 14.Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–67. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Aguilera A, Delgado J, Camacho FI, Sanchez-Beato M, Sanchez L, Montalban C, et al. Silencing of the p18INK4c gene by promoter hypermethylation in Reed-Sternberg cells in Hodgkin lymphomas. Blood. 2004;103:2351–7. doi: 10.1182/blood-2003-07-2356. [DOI] [PubMed] [Google Scholar]
- 16.Morishita A, Masaki T, Yoshiji H, Nakai S, Ogi T, Miyauchi Y, et al. Reduced expression of cell cycle regulator p18(INK4C) in human hepatocellular carcinoma. Hepatology. 2004;40:677–86. doi: 10.1002/hep.20337. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni MS, Daggett JL, Bender TP, Kuehl WM, Bergsagel PL, Williams ME. Frequent inactivation of the cyclin-dependent kinase inhibitor p18 by homozygous deletion in multiple myeloma cell lines: ectopic p18 expression inhibits growth and induces apoptosis. Leukemia. 2002;16:127–34. doi: 10.1038/sj.leu.2402328. [DOI] [PubMed] [Google Scholar]
- 18.Bartkova J, Thullberg M, Rajpert-De Meyts E, Skakkebaek NE, Bartek J. Cell cycle regulators in testicular cancer: loss of p18INK4C marks progression from carcinoma in situ to invasive germ cell tumours. Int J Cancer. 2000;85:370–5. doi: 10.1002/(sici)1097-0215(20000201)85:3<370::aid-ijc13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch M, Morz M, Pinzer T, Schackert HK, Schackert G. Frequent loss of the CDKN2C (p18INK4c) gene product in pituitary adenomas. Genes Chromosomes Cancer. 2009;48:143–54. doi: 10.1002/gcc.20621. [DOI] [PubMed] [Google Scholar]
- 20.Solomon DA, Kim JS, Jenkins S, Ressom H, Huang M, Coppa N, et al. Identification of p18 INK4c as a tumor suppressor gene in glioblastoma multiforme. Cancer Res. 2008;68:2564–9. doi: 10.1158/0008-5472.CAN-07-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiedemeyer R, Brennan C, Heffernan TP, Xiao Y, Mahoney J, Protopopov A, et al. Feedback circuit among INK4 tumor suppressors constrains human glioblastoma development. Cancer Cell. 2008;13:355–64. doi: 10.1016/j.ccr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–38. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A. 2010;107:11501–6. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookes S, Rowe J, Ruas M, Llanos S, Clark PA, Lomax M, et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. Embo J. 2002;21:2936–45. doi: 10.1093/emboj/cdf289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookes S, Rowe J, Gutierrez Del Arroyo A, Bond J, Peters G. Contribution of p16(INK4a) to replicative senescence of human fibroblasts. Exp Cell Res. 2004;298:549–59. doi: 10.1016/j.yexcr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Barradas M, Anderton E, Acosta JC, Li S, Banito A, Rodriguez-Niedenfuhr M, et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev. 2009;23:1177–82. doi: 10.1101/gad.511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maertens GN, El Messaoudi-Aubert S, Racek T, Stock JK, Nicholls J, Rodriguez-Niedenfuhr M, et al. Several distinct polycomb complexes regulate and co-localize on the INK4a tumor suppressor locus. PLoS ONE. 2009;4:e6380. doi: 10.1371/journal.pone.0006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry D, Bates S, Mann DJ, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. Embo J. 1995;14:503–11. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruas M, Brookes S, McDonald NQ, Peters G. Functional evaluation of tumour-specific variants of p16INK4a/CDKN2A: correlation with protein structure information. Oncogene. 1999;18:5423–34. doi: 10.1038/sj.onc.1202918. [DOI] [PubMed] [Google Scholar]
- 30.Voorhoeve PM, Agami R. The tumor-suppressive functions of the human INK4A locus. Cancer Cell. 2003;4:311–9. doi: 10.1016/s1535-6108(03)00223-x. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1:179–85. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- 32.Ruas M, Gregory F, Jones R, Poolman R, Starborg M, Rowe J, et al. CDK4 and CDK6 delay senescence by kinase-dependent and p16INK4a-independent mechanisms. Mol Cell Biol. 2007;27:4273–82. doi: 10.1128/MCB.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thullberg M, Bartek J, Lukas J. Ubiquitin/proteasome-mediated degradation of p19INK4d determines its periodic expression during the cell cycle. Oncogene. 2000;19:2870–6. doi: 10.1038/sj.onc.1203579. [DOI] [PubMed] [Google Scholar]
- 34.Forget A, Ayrault O, den Besten W, Kuo ML, Sherr CJ, Roussel MF. Differential post-transcriptional regulation of two Ink4 proteins, p18 Ink4c and p19 Ink4d. Cell Cycle. 2008;7:3737–46. doi: 10.4161/cc.7.23.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phelps DE, Hsiao KM, Li Y, Hu N, Franklin DS, Westphal E, et al. Coupled transcriptional and translational control of cyclin-dependent kinase inhibitor p18INK4c expression during myogenesis. Mol Cell Biol. 1998;18:2334–43. doi: 10.1128/mcb.18.4.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell BB, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol. 1998;8:351–4. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 37.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. The tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011 doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A. 1997;94:7245–50. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blais A, Monte D, Pouliot F, Labrie C. Regulation of the human cyclin-dependent kinase inhibitor p18INK4c by the transcription factors E2F1 and Sp1. J Biol Chem. 2002;277:31679–93. doi: 10.1074/jbc.M204554200. [DOI] [PubMed] [Google Scholar]
- 41.Ragione FD, Russo GL, Oliva A, Mercurio C, Mastropietro S, Pietra VD, et al. Biochemical characterization of p16INK4- and p18-containing complexes in human cell lines. J Biol Chem. 1996;271:15942–9. doi: 10.1074/jbc.271.27.15942. [DOI] [PubMed] [Google Scholar]
- 42.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 43.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 44.Kennedy AL, McBryan T, Enders GH, Johnson FB, Zhang R, Adams PD. Senescent mouse cells fail to overtly regulate the HIRA histone chaperone and do not form robust Senescence Associated Heterochromatin Foci. Cell division. 2010;5:16. doi: 10.1186/1747-1028-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon DA, Kim JS, Jean W, Waldman T. Conspirators in a capital crime: co-deletion of p18INK4c and p16INK4a/p14ARF/p15INK4b in glioblastoma multiforme. Cancer Res. 2008;68:8657–60. doi: 10.1158/0008-5472.CAN-08-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason DX, Jackson TJ, Lin AW. Molecular signature of oncogenic ras-induced senescence. Oncogene. 2004;23:9238–46. doi: 10.1038/sj.onc.1208172. [DOI] [PubMed] [Google Scholar]
- 47.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 48.Kotake Y, Zeng Y, Xiong Y. DDB1-CUL4 and MLL1 mediate oncogene-induced p16INK4a activation. Cancer Res. 2009;69:1809–14. doi: 10.1158/0008-5472.CAN-08-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimri GP, Hara E, Campisi J. Regulation of two E2F-related genes in presenescent and senescent human fibroblasts. J Biol Chem. 1994;269:16180–6. [PubMed] [Google Scholar]
- 50.Matushansky I, Radparvar F, Skoultchi AI. Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin-dependent kinases in G1. Proc Natl Acad Sci U S A. 2000;97:14317–22. doi: 10.1073/pnas.250488697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tourigny MR, Ursini-Siegel J, Lee H, Toellner KM, Cunningham AF, Franklin DS, et al. CDK inhibitor p18(INK4c) is required for the generation of functional plasma cells. Immunity. 2002;17:179–89. doi: 10.1016/s1074-7613(02)00364-3. [DOI] [PubMed] [Google Scholar]
- 52.Ogasawara T, Katagiri M, Yamamoto A, Hoshi K, Takato T, Nakamura K, et al. Osteoclast differentiation by RANKL requires NF-kappaB-mediated downregulation of cyclin-dependent kinase 6 (Cdk6) J Bone Miner Res. 2004;19:1128–36. doi: 10.1359/jbmr.2004.19.7.1128. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto T, Anderson K, Jacobsen SE, Nishikawa SI, Nerlov C. Cdk6 blocks myeloid differentiation by interfering with Runx1 DNA binding and Runx1-C/EBPalpha interaction. Embo J. 2007;26:2361–70. doi: 10.1038/sj.emboj.7601675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.