Abstract
Background
Pill-taking, expectations and therapeutic alliance may account for much of the benefit of medication and placebo treatment for major depressive disorder (MDD).
Aims
To examine the effects of medication, placebo and supportive care on treatment outcome, and the relationships of expectations and therapeutic alliance to improvement.
Method
A total of 88 participants were randomised to 8 weeks of treatment with supportive care alone or combined with double-blind treatment with placebo or antidepressant medication. Expectations of medication effectiveness, general treatment effectiveness and therapeutic alliance were measured (trial registration at ClinicalTrials.gov: NCT00200902).
Results
Medication or placebo plus supportive care were not significantly different but had significantly better outcome than supportive care alone. Therapeutic alliance predicted response to medication and placebo; expectations of medication effectiveness at enrolment predicted only placebo response.
Conclusions
Pill treatment yielded better outcome than supportive care alone. Medication expectations uniquely predicted placebo treatment outcome and were formed by time of enrolment, suggesting that they were shaped by prior experiences outside the clinical trial.
There has been significant debate in recent years about the effectiveness of antidepressant medications. Meta-analyses suggest that medication has modest benefits over placebo treatment in clinical trials for the treatment of major depressive disorder (MDD),1,2 at least for patients with mild-to-moderate symptoms.3 The limited difference detected between drug and placebo may be a result of significant increases in placebo response rates in clinical trials for MDD over the past three decades.4,5 Many factors have been suggested to contribute to high placebo response rates in people with depression, including interpersonal interaction1,5 and the strength of the therapeutic alliance with research personnel,1,6 and expectations of either the efficacy of medication in particular or treatment in general.2,6,7 We performed this study to examine the effects of pill administration, interpersonal interaction and expectation on the placebo response in MDD. Participants were randomly assigned to receive supportive care alone or supportive care along with double-blind treatment with antidepressant medication or placebo, in order to examine the distinct contributions of interpersonal interaction and pill-taking to symptom improvement in MDD. Participants’ expectations of treatment efficacy generally, as well as of medication efficacy specifically, were assessed on three occasions in the first weeks of the study to determine whether expectations formed early in the treatment process affect outcomes. We also examined the effects of the therapeutic alliance on treatment outcome. Medication and treatment expectations, as well as the therapeutic alliance, were examined as independent and additive predictors of symptom improvement in the three treatment conditions.
Method
Participants
A total of 88 participants with MDD, aged 18-65, were recruited through community advertisement for this clinical trial (ClinicalTrials.gov: NCT00200902). Participants were diagnosed as having MDD and no other primary Axis I disorder using the Mini-International Neuropsychiatric Interview (MINI),8 had at least moderate symptoms as measured by the 17-item Hamilton Rating Scale for Depression (HRSD) with a score ⩾179 and were without Axis II disorders that could interfere with their participation in the trial. Users of illicit substances or psychotropic medications were excluded via urine toxicology data. The UCLA Institutional Review Board approved all procedures, and written consent was obtained from all participants prior to participation.
Experimental procedures
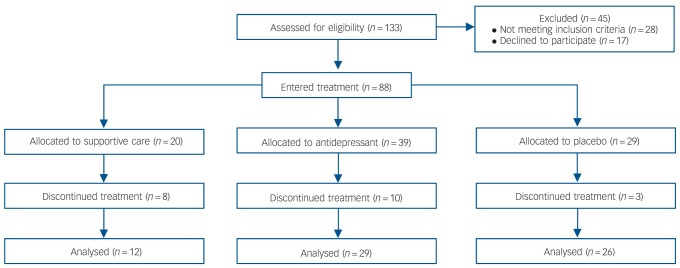
At the baseline visit, participants gave consent and were screened for eligibility. One week later, participants were randomised to one of three treatment arms: supportive care alone (28% likelihood), supportive care plus placebo (28% likelihood) or supportive care plus medication (44% likelihood, divided equally among medications venlafaxine XR, duloxetine or escitalopram) (Fig. 1). The actual percentage of participants in each treatment arm could be affected differently by individuals discontinuing treatment. At study entry, participants were informed that they were most likely to be randomised to double-blind treatment with supportive care plus either medication or placebo, but were not informed of the specific probabilities of any assignment. Supportive care was initiated at the randomisation visit for all groups and consisted of a 30 min session of interpersonal clinical interaction that was repeated 2, 4 and 8 weeks after the initial visit. During these visits, treatment providers assessed risk, side-effects and symptoms, provided support and encouragement, but refrained from engaging in problem-solving that might result in therapeutic effects, as outlined in the National Institute of Mental Health (NIMH) manual for pharmacological clinical management of depression.10 Treatment providers who performed supportive care in this study received specific training in this method and were research coordinators (usually trained in nursing) with at least 5 years of experience in MDD treatment trials. All participants had the same number and length of meetings with research coordinators and physicians, regardless of treatment assignment. Medication and placebo treatment both began with a single-blind 1-week placebo lead-in, which was utilised to maintain consistency with previous studies. This was followed by double-blind administration of the assigned pill. No participants were excluded based upon a response during the placebo lead-in.
Fig. 1.
CONSORT diagram.
Assessment instruments
Diagnoses were established using the MINI,8 and depression severity was assessed each week of the study using the 17-item HRSD.9 History of antidepressant use was determined by structured interview, and was tabulated as a dichotomous variable to indicate whether or not a participant had received treatment with an antidepressant during the present or any previous episode(s) of depression.
Participants’ expectations about treatment were assessed using items from the Patient Attitudes and Expectations Form (PAEF).11 The PAEF contains two separate items that ask participants how helpful they believe that medication in particular, and treatment in general, would be in ameliorating their symptoms of depression, with ratings on a Likert scale ranging from 1, ‘not helpful’ to 7, ‘extremely helpful’. Expectations were measured early in the study, at three time points 1 week apart: at the baseline screening interview, at randomisation to a treatment arm (at which point all patients assigned to pill-taking treatment arms received placebo before beginning their assigned treatment), and at the end of the 1-week placebo lead-in. These measures of expectation therefore could be influenced by participation in the trial, but not the pharmacological effects of current medication.
The California Pharmacotherapy Alliance Scale (CALPAS),12 a measure associated with outcomes of antidepressant pharmacotherapy, was used to measure treatment provider and participants’ perceptions of: (a) participants’ commitment to treatment; (b) participants’ working capacity; (c) treatment providers’ understanding and involvement; and (d) goal and working strategy consensus between participant and treatment provider. Previous work has shown the CALPAS to have good internal consistency (Cronbach’s alpha (α) = 0.83).12 The CALPAS was measured at baseline, randomisation, end of lead in, and weeks 4 and 8. This report focuses on measurements at the first three time points, at which both expectation and therapeutic alliance measurements were available.
Data analysis
Significance level was set at P⩽0.05. Analyses were conducted using IBM SPSS version 21 and Stata12 for Mac. Demographic and clinical characteristics were compared among the treatment groups in the intent-to-treat (ITT) sample for baseline differences using chi-squared, ANOVA, and t-tests as appropriate. Treatment efficacy was compared across the three groups by examining the percentage change in HRSD score from study entry to the final visit 8 weeks later with a one-way ANOVA, using imputation via last-observation-carried-forward (LOCF). In addition, rates of response and remission were compared across the three treatment groups, where response was defined as at least a 50% decrease in HRSD score and remission was defined as a final HRSD score ⩽7.
The trajectories of change in symptoms across the three conditions were compared using a mixed-effects model with repeated measures. The change from baseline in HRSD score measured at each week was entered as the outcome, with time, treatment and the time×treatment interaction as predictors, while covarying for baseline HRSD score. Pattern mixture modelling13 was performed to determine whether drop-out accounted for any differences in symptom change across time.
Following the primary outcome analyses, a path analysis was conducted to examine the relationship between early expectations of medication and symptom change and potential moderation by treatment assignment. Path analysis allows for the use of full information maximum likelihood estimates of variable means, which yields unbiased estimates of missing data under more conditions than does list-wise deletion.14 Following the path analysis, linear regression was used to examine the moderation in more detail. Symptom change was regressed on expectations within each treatment condition (antidepressant medication, placebo), controlling for baseline symptom severity. Linear regression models were also used to examine the relative contributions of expectations and the therapeutic alliance to symptom change within each treatment group.
A measurement model was constructed to assess whether the subscales of the CALPAS represented the same construct over time. Latent variable analysis was used to examine the relationships between CALPAS subscales and outcome, as well as the interaction between CALPAS subscales, treatment group and outcome.
Results
Participant characteristics and clinical outcomes in the ITT sample
There were no differences among the treatment groups in age, gender, baseline severity of depression, history of antidepressant medication treatment, average expectations of receiving benefit from antidepressant medication across the three assessments preceding randomisation, or any of the subscales of the CALPAS (Table 1). Non-specific expectations of treatment, however, were found to differ by treatment assignment (F(2) = 3.79, P<0.05), so that even prior to randomisation, participants later allocated to the supportive care condition had lower expectations of treatment (mean 3.17, 95% CI 2.76-3.57) compared with those later assigned to placebo (mean 3.94, 95% CI 3.56-4.22). Because of these baseline differences in general expectations of treatment, analyses were limited to the measure of anticipated benefit from antidepressant medication.
Table 1.
Demographic characteristics of the intent-to-treat (ITT) sample
| All (n = 88) |
Placebo group (n = 29) |
Medication group (n = 39) |
Support care group (n = 20) |
Statistical tests |
||
|---|---|---|---|---|---|---|
| F (d.f.) | χ2 (d.f.) | |||||
| Age, years: mean (s.d.) | 43.14 (13.42) | 44.24 (13.97) | 42.28 (13.37) | 43.20 (13.28) | 0.17 (2) | |
| Female gender, n | 55 | 18 | 25 | 12 | 0.10 (2) | |
| History of antidepressant use, yes: n | 56 | 16 | 26 | 5 | 1.53 (2) | |
| Baseline HRSD, mean (s.d.) | 21.51 (4.49) | 21.07 (4.61) | 22.16 (4.54) | 20.89 (4.27) | 0.70 (2) | |
| Expectations of medication, mean (s.d.) | 4.65 (1.40) | 5.02 (1.19) | 4.64 (1.37) | 4.13 (1.59) | 2.55 (2) | |
| Expectations of treatment, mean (s.d.) | 3.59 (0.87) | 3.94 (0.61) | 3.55 (0.78) | 3.17 (1.16) | 5.07** (2) | |
| CALPAS subscales, mean (s.d.) | ||||||
| Commitment to treatment | 3.40 (0.74) | 3.39 (0.70) | 3.52 (0.72) | 3.14 (0.85) | 1.32 (2) | |
| Participant working capacity | 2.23 (1.07) | 2.40 (1.14) | 2.11 (1.02) | 2.27 (1.12) | 0.50 (2) | |
| Treatment provider understanding and involvement | 3.12 (0.61) | 3.18 (0.59) | 3.08 (0.67) | 3.10 (0.54) | 0.18 (2) | |
| Goal and working strategy consensus | 2.48 (0.90) | 2.60 (0.82) | 2.53 (0.96) | 2.17 (0.83) | 1.09 (2) | |
HRSD, 17-item Hamilton Rating Scale for Depression; CALPAS, California Pharmacotherapy Alliance Scale.
P<0.01.
Participants assigned to supportive care alone were more likely to discontinue treatment (χ2(1) = 4.40, P<0.05) and spent fewer weeks in treatment (t(86) = –2.97, P<0.01) compared with participants across pill-taking groups. There was no significant difference in drop-out rates or time in treatment between the medication and placebo treatment groups (Table 2).
Table 2.
Treatment outcome in the intent-to-treat sample using last-observation-carried-forward
| All (n = 88) |
Placebo group (n = 29) |
Medication group (n = 39) |
Support care group (n = 20) |
Statistical tests |
||
|---|---|---|---|---|---|---|
| F (d.f.) | χ2 (d.f.) | |||||
| Time in study, weeks: mean (s.d.) | 7.42 (3.08) | 8.31 (2.16) | 7.64 (2.71) | 5.70 (4.16) | 4.83* (2) | |
| Week 8 HRSD, mean (s.d.) | 14.36 (7.94) | 13.48 (8.68) | 12.41 (7.36) | 19.45 (5.76) | 6.01** (2) | |
| Change in HRSD from baseline to week 8, mean (s.d.) | –7.27 (7.56) | –7.59 (7.98) | –10.05 (6.60) | –1.37 (5.27) | 10.13** (2) | |
| % change HRSD from baseline to week 8, mean (s.d.) | –0.34 (0.36) | –0.36 (0.39) | –0.46 (0.31) | –0.05 (0.27) | 9.62** (2) | |
| Responded, n | 29 | 11 | 17 | 1 | 9.53** (2) | |
| Remitted, n | 18 | 9 | 9 | 0 | 7.30* (2) | |
HRSD, 17-item Hamilton Rating Scale for Depression.
P<0.05
P<0.01.
Efficacy and sensitivity assessment
Symptom change did not differ by medication type among those receiving antidepressant medications (F(2) = 0.11, P = 0.90). Medication data therefore were pooled for further analysis. Patients assigned either to antidepressant medication or pill-placebo in addition to supportive care had significantly better symptom improvement (percentage change in HRSD score from baseline to week 8) compared with supportive care alone (F(2) = 9.62, P<0.001) (Table 2). Post hoc Tukey tests showed that the placebo (mean, –0.36, 95% CI –0.49 to –0.24) and medication groups (mean, –0.46, 95% CI –0.57 to –0.35) had significantly better outcomes than supportive care alone (mean -0.05, 95% CI –0.21 to 0.10). Tukey tests showed no other differences in outcome.
The efficacy analysis revealed that the reduction in HRSD symptoms from baseline to week 8 was greater for participants taking placebo and medication compared with those receiving supportive care alone (χ2(1) = 20.85, P<0.0001 and χ2(1) = 37.39, P<0.0001, respectively) (Fig. 2). The change in HRSD score over the course of the study was numerically but not statistically significantly greater in the medication-than in the placebo-treated group (χ2(1) = 3.12, P = 0.08) (Table 2).
Fig. 2.
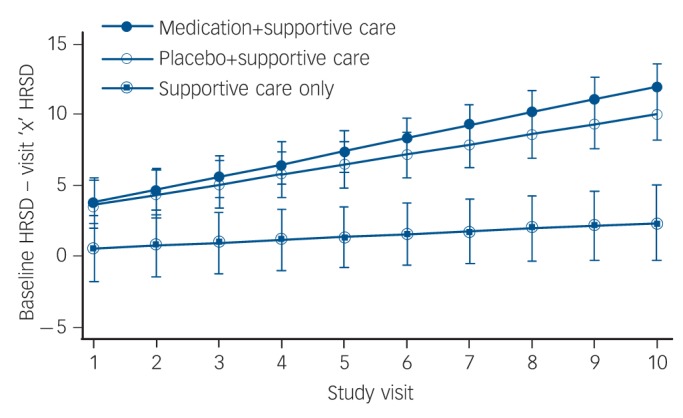
Change in 17-item Hamilton Rating Scale for Depression (HRSD) scores during the study shown separately for each treatment group.
Each point represents change from baseline HRSD at each study visit, as predicted by treatment condition, time, and the interaction of treatment condition and time, while covarying for baseline HRSD score.
There were no significant differences in age (t(86) = 0.33, P = 0.75), gender (χ2(1) = 0.62, P = 0.43) or the patterns of symptom change (χ2(2) = 0.38, P = 0.83) between the full ITT sample (n = 88) and study completers (n = 67) in any of the three treatment groups, after controlling for baseline HRSD. Participants who completed the study did not differ significantly from those who dropped out in baseline medication expectations (t(82) = –0.24, P = 0.81), treatment expectations (t(79) = 0.79, P = 0.43) or HRSD score (t(82) = 0.28, P = 0.78), but had greater commitment to treatment as measured by the CALPAS (t(69) = -2.35, P<0.05). Because the ITT and completer samples were comparable, we conducted the analyses examining the effects of therapeutic alliance and treatment expectations on the completer sample. This allowed us to assess the impact of expectation and therapeutic alliance on placebo and active medication treatment outcome in participants who underwent identical lengths of treatment. Participants receiving only supportive care were excluded from these analyses because of the low response and completion rates in this group.
Expectations and treatment outcome
Repeated measures ANOVAs revealed no change in participants’ expectations of medication in particular (F(2) = 0.24, P = 0.79) or of treatment in general (F(2) = 0.66, P = 0.52) over the first 3 weeks of the study. Expectations therefore were averaged across the three measurement time points to create two variables per participant: average early expectations of treatment in general, and average early expectations of medication. Participants assigned to medication v. placebo did not differ in their average medication expectations (t(52) = –1.00, P = 0.32) or in their average expectations of treatment (mean, 2.11, s.d. = 0.62, t(50) = –1.67, P = 0.10).
Treatment condition was examined as a moderator of the relationship between expectations of medication and the percentage change in HRSD score by regressing the percentage change in HRSD hierarchically on baseline HRSD, expectancy, treatment condition, and the interaction between expectancy and treatment condition. A significant relationship was found between expectations of medication and the percentage change in HRSD score over the course of the 8-week study (β = –0.18, s.e. = 0.06, P<0.01) and treatment assignment acted as a moderator of this relationship (β = 0.16, s.e. = 0.08, P<0.05) over and above the effects of baseline symptom severity (β = 0.00, s.e. = 0.01, P = 0.35). Linear regression models were used to explore the moderation by treatment condition. The percentage change in HRSD score was regressed on expectations of medication separately for participants in the antidepressant and placebo treatment arms. There was a significant relationship between medication expectations and outcome in the placebo group, such that higher expectations predicted a greater decrease in symptoms over and above the effects of baseline symptom severity (β = –0.17, s.e.= 0.06, P<0.01). In the medication group, however, there was no relationship between expectations of medication and the percentage change in HRSD score (β = -0.01, s.e.= –0.04, P= 0.82) (Fig. 3).
Fig. 3.
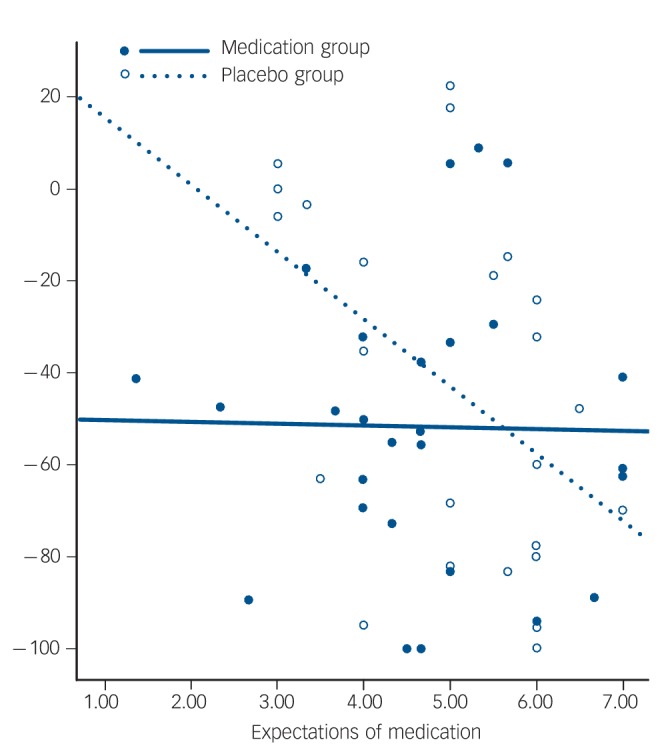
Relationship between expectation and percentage change in 17-item Hamilton Rating Scale for Depression (HRSD) by treatment assignment (antidepressant medication or placebo).
Open circles indicate participants in the placebo group, with the dotted regression line showing the relationship between expectations prior to treatment and reduction in depressive symptoms during the 8-week study (R2(linear) = 2.704E-4). Solid dots indicate participants in the medication group, with the solid regression line showing the relationship between expectations prior to treatment and symptom reduction over the 8-week study (R2(linear) = 0.213).
Therapeutic alliance and treatment outcomes
Latent variable analysis showed that there was no change in the relationship between CALPAS subscale ratings and outcomes over time, and thus a single variable was used to represent the average value of each CALPAS subscale. Of the four CALPAS subscales, only the goal and working strategy consensus (GWSC) between provider and participant predicted symptom change (β = –0.64, P<0.01), whereas participants’ commitment to treatment (β = –0.11, s.e. = 0.14, P = 0.45), working capacity (β = 0.04, s.e. = 0.11, P = 0.74), and view of the treatment provider’s understanding and involvement (β = 0.38, s.e. = 0.21, P = 0.07) were not significant predictors. The GWSC ratings were significant predictors of outcome for participants in both the placebo and medication groups (β = –0.40, P<0.01, and β = –0.25, P<0.01, respectively).
Therapeutic alliance, expectations, and treatment outcomes
When GWSC and mean expectations of medication were entered as simultaneous predictors, along with HRSD, only GWSC remained significant, so that greater consensus regarding the goals and working strategy between practitioner and patient led to greater decreases in HRSD (β = –0.25, P<0.001). Based on this initial finding in the overall sample, separate regressions were conducted within each treatment group to determine the specific effects of the goal and working strategy consensus on the outcome of each treatment. Among participants receiving placebo, expectations of medication and GWSC were each associated with a percentage decrease in HRSD, whereas in the medication group, only higher GWSC was associated with outcome (Table 3).
Table 3.
Relationship between expectations, therapeutic alliance measure, and percentage change in 17-item Hamilton Rating Scale for Depression (HRSD) for completer sample
| Placebo group |
Medication group |
|||
|---|---|---|---|---|
| β | R2 | β | R2 | |
| Model 1 | 0.01 | 0.01 | ||
| Baseline HRSD | 0.01 | 0.01 | ||
| Model 2 | 0.33 | 0.01 | ||
| Baseline HRSD | 0.02 | 0.01 | ||
| Mean expectations | –0.20** | –0.01 | ||
| Model 3 | 0.23 | 0.33 | ||
| Baseline HRSD | 0.01 | 0.01 | ||
| Goal and working strategy consensus | –0.37* | –0.23** | ||
| Model 4 | 0.51 | 0.41 | ||
| Baseline HRSD | 0.03 | 0.01 | ||
| Mean expectations | –0.18** | 0.03 | ||
| Goal and working strategy consensus | –0.35* | –0.26** | ||
P<0.05
P<0.01.
Discussion
Main findings
These results indicate that administration of either antidepressant medication or placebo along with supportive care for the treatment of MDD was superior to supportive care alone for amelioration of depressive symptoms. Participants who received supportive care alone were less likely to respond and more likely to discontinue treatment earlier than those who also received a pill. There was no significant difference, however, between the effectiveness of the medication and placebo treatment conditions. These findings suggest that pill-taking adds significantly to the benefits of supportive care for MDD, and further, that the benefits of placebo treatment cannot be accounted for solely by supportive interaction with research personnel. In fact, the strength of the therapeutic alliance with research personnel was significantly associated with the efficacy of both placebo and medication.
These results additionally suggest a unique role for participants’ medication expectations in engendering a placebo response. Higher expectations of medication effectiveness predicted improvement in the placebo-treated group, in contrast to general treatment expectations, which predicted response to both placebo and medication. There was no change in expectations of medication over the first 2 weeks of the trial for either the medication or placebo groups, and no relationship between expectations and participants’ ratings of the strength of the therapeutic alliance. Expectations regarding medication effectiveness may therefore represent a construct that selectively affects the likelihood of placebo response, and is unaffected by processes that occur early in the clinical trial, or by the quality of the therapeutic relationship.
Findings from other studies
One previous study reported that positive ‘global expectations’ of improvement were associated with increased likelihood of response to four treatment conditions (cognitive-behavioural therapy, interpersonal psychotherapy, imipramine with clinical management, and placebo with clinical management) in a clinical trial for MDD.7 These investigators did not report specifically on medication expectations. In another study, positive expectations of the effectiveness of an experimental antidepressant medication were related to the likelihood of response to the drug.15 There was no placebo control condition in that study, however, and medication expectations may in fact have been predicting placebo response.
Possible explanations for our findings
Placebo response has been characterised as a ‘non-specific’ response to treatment, in contrast to the presumably more specific physiological response to treatment with medications or devices in MDD.16,17 Participants’ medication and general treatment expectations were measured immediately upon entry into the study and on two subsequent occasions in the first 2 weeks, and were found not to change over time. These expectations could have been shaped in part by the consent process, through which participants were informed that they were more likely to receive medication than any other treatment. This process was brief, however, focused on written material explaining the risks and benefits of medication, and did not vary significantly across participants. It is therefore likely that positive medication expectations in placebo responders were formed in large measure prior to the study, measureable at time of entry to the study, and stable over multiple ratings. Previous meta-analyses have suggested that expectations created by participants’ knowledge of a high likelihood of receiving medication were associated with greater clinical improvement during placebo treatment18 and less drug-placebo ‘separation’.19,20 One recent prospective study showed that knowledge of the likelihood of receiving medication affected both drug and placebo response rates,21 although another meta-analysis concluded that response expectancies affect placebo but not active medication treatment.22
Positive expectations towards antidepressant medications that are formed prior to enrolment may reflect both an individual’s past experience with direct exposure to medication(s) and the influence of societal trends. Antidepressant medications were the single most prescribed class of medication in 2011, with 264 million prescriptions filled and six of the ten most prescribed drugs being antidepressants.23 Although placebo response rates have increased over the past 30 years, there has been a roughly commensurate increase in antidepressant medication response rates.22 The widespread and increasing use of antidepressant medication as well as the increase in placebo response rates may reflect in part the influence of direct-to-consumer advertising for antidepressant medications, which represents a large proportion of the more than $10 billion annual direct-to-consumer advertising expenditures.24,25 Direct-to-consumer advertising has been linked to increased patient expectations for the effectiveness of medications26 and increased demand for antidepressants.27 Medication expectations, such as those that were related to placebo response in our study, should be a focus for future studies, both with regard to participants’ treatment experience and within the larger social milieu.
Response expectancies also can be shaped by the therapeutic alliance. Previous research has shown that expectations may interact with the therapeutic alliance in a manner that differentially affects response to placebo and medication.28,29 A meta-analytic study found, however, that although there was a therapeutic impact of more frequent visits in clinical trials for MDD, both placebo and medication response rates increased proportionally.30 This finding is consistent with the present study, in which the strength of the alliance with treatment providers was a significant predictor of response to both medication and placebo treatment, unrelated to expectations, and did not differ between the two pill-taking treatment conditions. It is important to note that this report focuses on the therapeutic alliance at the time of enrolment and shortly thereafter, at a time when it is less likely that a strong relationship with the treatment provider had been established. The nature of the interpersonal interaction at this time probably reflects each participant’s baseline tendency to form a relationship with a new provider, which may vary greatly among individuals. For example, some people might tend to trust and invest in medical providers early on (possibly followed by waning enthusiasm for the relationship later on), whereas others may build a relationship over time, moving from initial ambivalence to deeper trust and involvement. The present results indicate that this very early relationship with providers is associated with differences in outcome, and future studies should examine how the change in this relationship over time is related to treatment response.
It is interesting that despite the significant effect of a therapeutic alliance on treatment outcomes, the supportive care treatment condition alone was significantly less effective than the two treatment conditions that also involved pill administration. This finding suggests limited benefits of a positive therapeutic alliance in a clinical trial in the absence of pill administration. The participants who were randomly assigned to supportive care alone, however, had lower expectations of treatment in general prior to randomisation than those assigned to the pill-taking conditions. This lower baseline level of expectation makes it difficult to draw any firm conclusions regarding the importance of the therapeutic relationship in supportive care. The therapeutic alliance, however, was unrelated to expectations of medication or treatment, suggesting that other factors played a greater role in shaping attitudes towards the clinical trial.
Limitations
The findings of this study should be interpreted within the context of certain limitations. First, participants entering this study had a strong preference for entering a treatment condition that involved administration of a pill, as evidenced by the fact that the treatment condition based on supportive care alone had a higher drop-out rate and was notably less effective than the treatments involving pill-taking. This finding may reflect general social attitudes towards antidepressant medication, the sources of participant recruitment, and other factors. Individuals entering this study were aware that they might be assigned to a treatment condition that did not involve the use of medication, and it is not certain that they are representative of people with MDD who would enter clinical trials for MDD. Future studies should investigate participants’ attitudes and expectations surrounding medication, and how they are formed, in greater detail. Second, we detected an effect of medication expectation but not general treatment expectation on the response to placebo. It is possible that there is a weaker effect of general treatment expectations on placebo response in MDD and that our sample size was not sufficient to detect this effect. Replication of this finding in a larger sample would be useful. Finally, although a numerically higher percentage of participants receiving medication in this study responded to treatment than those receiving placebo, this difference was not statistically significant. The failure to show significant separation between drug and placebo is common in MDD clinical trials, and underscores the importance of this line of investigation. It is possible, however, that a study with a different design or that enrolled more individuals who were more severely depressed, and did show drug-placebo separation, might yield different findings.
Implications
The results of this study extend previous findings regarding the role of expectations and the therapeutic alliance in the placebo response in MDD, and suggest that expectations formed externally to the context of the treatment study are a significant determinant of treatment outcome. Expectations of medication in particular appear to be specifically involved in response to placebo, but not medication, and appear to be stable throughout the first weeks of the clinical trial when treatment assignment is being determined. A variety of factors could help form an individual participant’s expectations, including personality, health attitudes, belief systems, societal factors, as well as previous experiences in treatment. Future studies should systematically investigate whether these and other factors might interact with expectations of medication to help engender the placebo response. Once these factors are better understood, it may be possible to manage medication expectations in clinical trials, prior to the start of treatment, with the goal of better differentiating the effects of placebo from those of antidepressant medication in randomised clinical trials for MDD.
Footnotes
Declaration of interest
A.F.L, within the past 5 years, has received research support from the National Institutes of Health, Wyeth Pharmaceuticals, Novartis Pharmaceuticals, Seaside Therapeutics, Genentech, Shire Pharmaceuticals, Neuronetics, Eli Lilly and Company, and Neurosigma. He has served as a consultant to NeoSync Inc., Brain Cells, Inc., Taisho Pharmaceuticals, Eli Lilly and Company, and Aspect Medical Systems/Covidien. He is Chief Scientific Officer of Brain Biomarker Analytics LLC (BBA). He owns stock options in NeoSync, Inc. and has equity interest in BBA. I.A.C., within the past 5 years, has received research support from Aspect Medical Systems/Covidien, National Institutes of Health, Neuronetics and Shire; he has been on the speakers’ bureau for Neuronetics and the Medical Education Speakers Network; he has been an advisor/consultant/reviewer for Allergan, Covidien, Pfizer, Neuronetics, NeuroSigma, NIH (ITVS), US Department of Defense, US Department of Justice, VA (DSMB); his biomedical intellectual property is assigned to the Regents of the University of California, and he owns stock options in NeuroSigma.
Funding
Research support for this study was received from the National Center for Complementary and Alternative Medicine of the National Institutes of Health (grant number R01 AT002479), Eli Lilly and Company and Wyeth Pharmaceuticals (now a wholly owned subsidiary of Pfizer).
References
- 1. Khan A, Fawcett J, Lichtenberg P, Kirsch I, Brown WA. A systematic review of comparative efficacy of treatments and controls for depression. PLoS One 2012; 7: e41778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan A, Redding N, Brown WA. The persistence of the placebo response in antidepressant clinical trials. J Psychiatr Res 2008; 42: 791–6. [DOI] [PubMed] [Google Scholar]
- 4. Khan A, Bhat A, Kolts R, Thase ME, Brown W. Why has the antidepressant-placebo difference in antidepressant clinical trials diminished over the past three decades? CNS Neurosci Ther 2010; 16: 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial and growing. JAMA 2002; 287: 1840–7. [DOI] [PubMed] [Google Scholar]
- 6. Kirsch I. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd. Psychol Bull 2004; 130: 341–3, discussion 344-5. [DOI] [PubMed] [Google Scholar]
- 7. Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry 1991; 148: 997–1008. [DOI] [PubMed] [Google Scholar]
- 8. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psych 1998; 59 (suppl 20); 22–33. [PubMed] [Google Scholar]
- 9. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management - imipramine/placebo administration manual: NIMH Treatment of Depression Collaboration Research Program. Psychopharmacol Bull 1987; 23: 309–24. [PubMed] [Google Scholar]
- 11. Elkin I, Parloff MB, Hadley SW, Autry JH. NIMH Treatment of Depression Collaborative Research Program. Background and research plan. Arch Gen Psychiatry 1985; 42: 305–16. [DOI] [PubMed] [Google Scholar]
- 12. Weiss M, Gaston L, Propst A, Wisebord S, Zicherman V. The role of the alliance in the pharmacologic treatment of depression. J Clin Psychiatry 1997; 58: 196–204. [DOI] [PubMed] [Google Scholar]
- 13. Su L. A marginalized conditional linear model for longitudinal binary data when informative dropout occurs in continuous time. Biostatistics 2012; 13: 355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enders CK, Badalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling 2001; 8: 430–57. [Google Scholar]
- 15. Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. J Clin Psychiatry 2004; 65: 1174–9. [DOI] [PubMed] [Google Scholar]
- 16. Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One 2009; 4: e4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diederich NJ, Goetz CG. The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology 2008; 71: 677–84. [DOI] [PubMed] [Google Scholar]
- 18. Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom 2009; 78: 172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 2009; 19: 34–40. [DOI] [PubMed] [Google Scholar]
- 20. Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry 2010; 71: 270–9. [DOI] [PubMed] [Google Scholar]
- 21. Rutherford BR, Marcus SM, Wang P, Sneed JR, Pelton G, Devanand D, et al. A randomized, prospective pilot study of patient expectancy and antidepressant outcome. Psychol Med 2013; 43: 975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fountoulakis KN, Möller HJ. Efficacy of antidepressants: a re-analysis and re-interpretation of the Kirsch data. Int J Neuropsychopharmacol 2011; 14: 405–12. [DOI] [PubMed] [Google Scholar]
- 23. Lindsley CW. The top prescription drugs of 2011 in the United States: antipsychotics and antidepressants once again lead CNS therapeutics. ACS Chem Neurosci 2012; 3: 630–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenthal MB, Berndt ER, Donohue JM, Epstein AM, Frank RG. Demand effects of recent changes in prescription drug promotion. In Frontiers in Health Policy Research (Vol 6) (eds Cutler DM, Garber AM.): 1–27 National Bureau of Economic Research, 2003. [Google Scholar]
- 25. Leuchter RK. From cocaine to viagra: a social and economic analysis of the pharmaceutical industry. In Honors Theses-All: 939 Wesleyan University, 2012. [Google Scholar]
- 26. Gilbody S, Wilson P, Watt I. Benefits and harms of direct to consumer advertising: a systematic review. Qual Saf Health Care 2005; 14: 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metzl JM. If direct-to-consumer advertisements come to Europe: lessons from the American marketplace. Lancet 2007; 369: 704–6. [DOI] [PubMed] [Google Scholar]
- 28. Iacoviello BM, McCarthy KS, Barrett MS, Rynn M, Gallop R, Barber JP. Treatment preferences affect the therapeutic alliance: implications for randomized controlled trials. J Consult Clin Psychol 2007; 75: 194–8. [DOI] [PubMed] [Google Scholar]
- 29. Strunk DR, Stewart MO, Hollon SD, DeRubeis RJ, Fawcett J, Amsterdam JD, et al. Can pharmacotherapists be too supportive? A process study of active medication and placebo in the treatment of depression. Psychol Med 2010; 40: 1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Posternak MA, Zimmerman M. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry 2007; 190: 287–92. [DOI] [PubMed] [Google Scholar]