Synopsis
The dystonias are a group of disorders characterized by excessive involuntary muscle contractions leading to abnormal postures and/or repetitive movements. There are many different clinical manifestations and many different causes. A careful assessment of the clinical manifestations is helpful for identifying syndromic patterns that focus diagnostic testing on potential causes. If a cause can be identified, specific etiology-based treatments may be available. However, in the majority of cases, a specific cause cannot be identified, and treatments are based on symptoms. Treatment options include counseling and education, oral medications, botulinum toxin injections, and several surgical procedures. A substantial reduction in symptoms and improved quality of life can be achieved in the majority of patients by combining these various options.
Keywords: blepharospasm, botulinum toxin, cervical dystonia, deep brain stimulation, focal hand dystonia, Meige syndrome, oromandibular dystonia, spasmodic dysphonia, torticollis
Introduction
The dystonias are a group of disorders defined by specific types of abnormal movements. The essential feature is over-activity of muscles needed for movement. This over-activity can be expressed as excessive force in the primary muscles used for a movement, overflow activation of additional muscles that are not required for a movement, or co-activation of muscles that antagonize the primary muscles. The clinical expression of dystonia is determined by the severity and distribution of muscles involved. In mild cases dystonic movements appear merely as exaggerations of specific actions. In moderate cases the movements are more clearly abnormal with a quality that is cramped, stiff or twisting. In more severe cases dystonic movements appear as persistent odd postures or fixed deformities.
Dystonic movements are often slow, but they sometimes may be rapid or jerky.1,2 Sometimes the movements may resemble tremor.3–5 They tend to be patterned or stereotyped in individual cases. A recent consensus work group provided the following formal definition for the dystonias:6
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation.
Virtually any region of the body may be affected, alone or in various combinations. The dystonias may emerge at any age; and once they begin, they rarely remit. Some remain relatively static, while others are progressive or intermittent. Dystonia may occur in isolation, or it may be combined with other clinical problems. The many different clinical manifestations are classified according to four dimensions (Table 1) including the region of the body affected, the age at onset, temporal aspects, and whether there are associated clinical problems.6 Each of these dimensions has implications for diagnosis and treatment.
Table 1.
Classification of the dystonias according to clinical features
| Dimension for classification | Subgroups |
|---|---|
| Age at onset | Infancy (birth to 2 years) |
| Childhood (3–12 years) | |
| Adolescence (13–20 years) | |
| Early adulthood (21–40 years) | |
| Late adulthood (40 years and older) | |
| Body distribution | Focal (one isolated region) |
| Segmental (2 or more contiguous regions) | |
| Multifocal (2 or more non-contiguous regions) | |
| Hemidystonia (half the body) | |
| Generalized (trunk plus 3 other sites) | |
| Temporal pattern | Disease course (static vs progressive) |
| Short-term variation (persistent, action-specific, diurnal, paroxysmal) | |
| Associated features | Isolated (with or without tremor) |
| Combined (with other neurological or systemic features) |
In addition to the widely varying clinical manifestations of the dystonias, there also are many different causes.7 Some dystonias are inherited, others are acquired (Table 2). Some dystonias have no apparent pathology in the nervous system, while others are associated with defects that can be detected by neuroimaging or post-mortem histopathological studies. At the molecular level, multiple genes have been discovered for rare subtypes of dystonia,8,9 and they are involved in diverse biochemical processes.9–11 At the anatomical level, several brain regions have been implicated, leading to the concept that dystonia does not arise from dysfunction of a single brain region, but rather from dysfunction of a motor network.12–14 Physiologically, many forms of dystonia have evidence for impaired inhibitory processes in the nervous system, abnormal sensory feedback, and/or maladaptive neural plasticity.15–17 Determining how these diverse processes relate to one another to produce the motor syndrome we know as dystonia is a major current focus of research.11,18
Table 2.
Classification of the dystonias according to etiology
| Dimension for classification | Subgroups |
|---|---|
| Nervous system pathology | Degenerative |
| Structural (typically static) | |
| No evidence for degenerative or structural lesions | |
| Heritability | Inherited (autosomal dominant, autosomal recessive, mitochondrial, etc…) |
| Acquired (brain injury, drugs/toxins, vascular, neoplastic, etc…) | |
| Idiopathic | Sporadic |
| Familial |
Diagnosis
Because there are so many different clinical manifestations and causes, there are no simple algorithms for diagnosis that address all dystonias. A shotgun approach in which all possible disorders are evaluated in a “dystonia test battery” is not recommended. Available genetic test batteries are very expensive, they include only a small fraction of known causes, and the probability of finding a positive result in sporadic cases with dystonia is <1%. Another strategy sometimes recommended follows a “red flag” approach in which diagnostic testing is guided by the identification of telltale clinical features, such as a corneal Kayser-Fleischer ring or liver disease in Wilson’s disease.19,20 This strategy is not ideal because most dystonic disorders lack red flags. Another strategy sometimes recommended is to test only for disorders where there are specific treatments that target underlying etiologies, such as Wilson’s disease where copper-lowering therapies are life saving. This strategy also is untenable, because recent progress in dystonia research has led to a long list of treatable disorders that grows every year (Table 3).
Table 3.
Disorders with dystonia that have specific disease-modifying therapies
| Disorder | Typical age at onset1 | Typical characteristics of dystonia | Other typical clinical features2 | Treatment |
|---|---|---|---|---|
| Abetalipoproteinemia (Bassen-Kornzweig) | childhood to early adulthood | progressive oromandibular or generalized dystonia | ataxia, chorea, retinitis pigmentosa, fat malabsorption | vitamin E, reduced fat diet |
| Aromatic amino acid decarboxylase deficiency | infancy | generalized dystonia | developmental delay, hypotonia, oculogyric crises, autnomic dysfunction | dopamine agonists, monoamine oxidase inhibitors |
| Ataxia with vitamin E deficiency | childhood to early adulthood | rare patients present with dystonia instead of ataxia | ataxia, neuropathy | vitamin E |
| Autoimmune movement disorders | any age | focal or generalized dystonia | systemic signs of autoimmune disease | address autoimmune process |
| Biotinidase deficiency | infancy | generalized dystonia | developmental delay, encephalopathy, seizures, sensory defects, skin rash | biotin |
| Cerebral folate deficiency | early childhood to adolescence | progressive dystonia | developmental delay, neuropsychiatric syndromes, seizures | folinic acid |
| Cerebrotendious xanthomatosis | late childhood to adulthood | oromandibular or limb dystonia | neurocognitive defects, spasticity, myoclonus, tendon xanthomas | chenodeoxycholic acid |
| Cobalamin deficiencies (inherited subtypes A-G) | infancy | generalized dystonia | developmental delay, ataxia, spasticity, seizures, bone marrow defects | cobalamin derivatives and/or protein restriction |
| CoEnzyme Q10 deficiency | any age | some cases present with dystonia and ataxia | varied phenotypes, most often progressive ataxia or encephalopathy | coenzyme Q10 |
| Cerebral creatine deficiency type 3 | infancy | generalized dystonia | developmental delay, myopathy | creatine |
| Dopa-responsive dystonia, classic | early childhood to late adulthood | generalized dystonia | Parkinsonism | levodopa |
| Dopa-responsive dystonia, complicated | infancy to adolescence | generalized dystonia | hypokinetic-rigid syndrome, oculogyric crises, autonomic dysfunction | levodopa, 5-hydroxytryptophan, and/or tetrahydrobiopterin |
| Dystonia with brain manganese accumulation | childhood | progressive generalized dystonia | Parkinsonism, liver disease, polycythemia | chelation therapy |
| Galactosemia | childhood to early adulthood | mild focal or generalized dystonia | ataxia, tremor, food intolerance | lactose restriction |
| GLUT1 deficiency | childhood to adolescence | paroxysmal exertional dystonia | developmental delay, seizures | ketogenic diet |
| Glutaric aciduria type 1 | early childhood to early adulthood | static generalized dystonia following encephalopathic crisis | developmental delay, encephalopathic crisis | avoid or treat aggressively any intercurrent illness, lysine restriction |
| Homocystinuria | childhood | generalized or paroxysmal dystonia | neurocognitive dysfunction, myopia, ectopic lens | methionine restriction |
| Guanidinoacetate methyltransferase deficiency | infancy | progressive generalized dystonia | developmental delay, seizures | arginine restriction, creatine and ornithine |
| Maple syrup urine disease | childhood | focal or paroxysmal dystonia | neonatal encephalopathy, ataxia | leucine restriction ±thiamine |
| Methylmalonic aciduria | childhood | static generalized dystonia following encephalopathic crisis | developmental delay, encephalopathic crisis, renal insufficiency, pancytopenia | avoid or treat aggressively any intercurrent illness, protein restriction |
| Molybdenum cofactor deficiency (sulfite oxidase) | adolescence | rare patients present with dystonia and parkinsonism | developmental delay, encephalopathy, seizures | cyclic pyranopterin monophosphate |
| Niemann Pick type C | early childhood to early adulthood | progressive generalized dystonia | dementia, ataxia, spasticity, seizures, supranuclear gaze palsy | N-butyl-deoxynojirimycin (Miglustat™) |
| Paraneoplastic movement disorders | any age | rapidly progressive focal or generalized dystonia | malignancy, often occult | address underlying malignancy |
| Propionic aciduria | early childhood to adolescence | static generalized dystonia following encephalopathic crisis | developmental delay, encephalopathic crisis, optic atrophy, pancytopenia | avoid or treat aggressively any intercurrent illness, protein restriction |
| Pyruvate dehydrogenase deficiency | infancy | progressive generalized or paroxysmal dystonia | developmental delay, seizures | thiamine, ketogenic diet, dichloroacetate |
| Rapid onset dystonia-Parkinsonism | early childhood to late adulthood | bulbar or generalized dystonia following encephalopathic crisis | psychomotor disability | avoid or treat aggressively any intercurrent illness, protein restriction |
| Wilson’s disease | early childhood to late adulthood | progressive generalized dystonia | neurocognitive dysfunction, liver disease, Kayser-Fleischer rings | zinc, tetrathiomolybdenate |
For most childhood-onset disorders, rare patients may present instead in adulthood.
Some associated clinical features may be attenuated or absent in atypical cases.
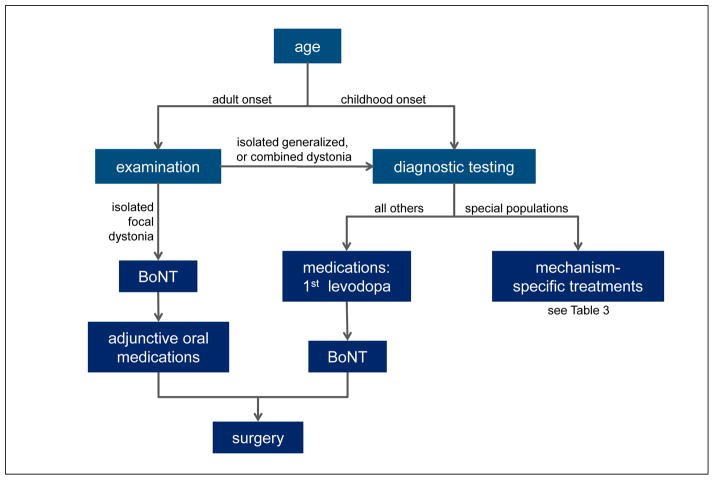
A more methodical strategy for diagnosis is shown in Figure 1. Once a diagnosis of dystonia is suspected based on clinical phenomenology, the first step is to rule out disorders that may mimic dystonia (pseudodystonia), such as those due to orthopedic, neuromuscular or psychogenic processes. The next step is to delineate the clinical syndrome according to the four dimensions used for clinical classification (Table 1). A careful delineation of the syndromic pattern, along with neuroimaging characteristics, is important because it aids in narrowing down the long list of potential etiologies for more targeted diagnostic testing.7
Figure 1.
Methodical strategy for diagnosis of dystonia.
For patients with isolated dystonia, the laboratory workup depends on the age at onset, the body distribution, and whether there are affected family members. In adults with focal or segmental dystonia only, no diagnostic tests are required because they usually are unrevealing.18,21 In adults with hemidystonia or generalized dystonia, neuroimaging is useful because the likelihood of disclosing a structural cause is higher. In sporadic adult-onset isolated dystonias, the chance of finding a genetic cause is less than 1–2%, so genetic testing usually is not cost effective, unless there are other affected family members. The diagnostic approach in younger individuals with isolated dystonia is quite different, because there is a much higher likelihood of disclosing a cause.7 Neuroimaging is important for all early-onset cases, regardless of body distribution. Genetic testing for early-onset isolated dystonias should target the TOR1A gene for DYT1 dystonia and the THAP1 gene for DYT6 dystonia.
For all patients where dystonia is combined with other neurological or systemic features, some additional workup is warranted, regardless of the age at onset or body distribution. The laboratory workup depends on the nature of the associated features and the age at onset. A recent review summarized more than 100 different disorders where dystonia may be combined with other features, organized in 18 tables according to the associated clinical features and age at onset.7 Neuroimaging is useful in virtually all combined dystonias, because it can provide important diagnostic clues. Genetic testing for DYT1 and DYT6 dystonia is not useful in combined dystonia syndromes. Instead, laboratory testing is driven by the syndromic pattern. For example, dystonia combined with Parkinsonism leads to a relatively small list of disorders for more targeted diagnostic testing.22–24
When a specific etiology cannot be determined, it is important to follow patients and revise the diagnosis as additional clinical features are recognized. Many combined dystonic disorders may present first with what appears to be isolated dystonia, and additional clinical features may develop over the following months or years. One of the most common examples is idiopathic Parkinson’s disease and related Parkinsonian syndromes, where 10–15% of patients may present first with isolated dystonia of an arm or leg.25–30 It is not until other clinical features emerge that the diagnosis becomes more obvious.
Treatment
There are many different treatment options that involve counseling and education, oral medications, intramuscular injection of botulinum neurotoxins (BoNT), physical and occupational therapy, and neurosurgical interventions. In the next section these options are summarized individually. Subsequently, some suggestions are offered for how these individual ingredients can be combined for the best outcomes in different types of dystonia.
Education and counseling
Education and counseling are important for several reasons. Patients frequently are misdiagnosed for many years, and many are told they suffering from a psychiatric problem. Even for the most common and readily diagnosed subtypes of dystonia such as cervical dystonia, the mean time from onset of symptoms to diagnosis is 4–6 years.31–33 These delays in reaching a diagnosis often lead to frustration and mistrust of medical providers. Education and counseling are important for regaining trust so that patients are more likely to accept recommendations.
Education and counseling also are important because few therapies are curative. Achieving the best outcome often requires an empirical trial and error approach, which can sometimes amplify existing frustration and mistrust. A frank discussion of treatment options is essential to ensure that expectations are realistic. It is also worth bearing in mind that there is a high rate of psychiatric co-morbidity in the dystonias including depression, anxiety and social withdrawal.34–37 An open discussion of how these factors may influence overall quality of life is important.
Finally, many patients learn about their medical diagnoses and treatment options via the internet, which is not always a reliable source of information. Educating patients about the most reliable online sources of information can help to avoid misunderstandings. Table 4 provides a summary of some internet sites where information is regularly updated, and other educational and research opportunities are available to patients. Most of these groups also provide informational brochures, newsletters and local patient support group meetings where patients can obtain new information.
Table 4.
Online educational resources for patients
| Organization | Internet address |
|---|---|
| American Dystonia Society | dystoniasociety.org |
| Bachmann-Strauss Dystonia & Parkinson Foundation | dystonia-parkinsons.org |
| Benign Essential Blepharospasm Research Foundation | blepharospasm.org |
| Dystonia Coalition | dystoniacoalition.org |
| Dystonia Ireland | dystonia.ie |
| Dystonia Medical Research Foundation | dystonia-foundation.org |
| Dystonia Medical Research Foundation, Canada | dystoniacanada.org |
| Global Dystonia Registry | Globaldystoniaregistry.org |
| National Institute of Neurological Disorders and Stroke | ninds.nih.gov/disorders/dystonias |
| National Spasmodic Dysphonia Association | dysphonia.org |
| National Spasmodic Torticollis Association | torticollis.org |
| ST/Dystonia | spasmodictorticollis.org |
| The Dystonia Society (UK) | dystonia.org.uk |
Physical & Occupational Therapy
Patients frequently ask about the value of exercise and physical therapy, because they seem intuitively helpful for addressing abnormal muscle activity and pain. Although many patients seem to appreciate physical therapy, benefits often are temporary, and there are no large-scale double-blind studies that demonstrate objective benefits to justify regular application.
Several investigators have sought to demonstrate objective improvements using specific methods based on theories regarding the pathophysiology of dystonia. For example, the theory that dystonia results from maladaptive neural plasticity15,38,39 has led to attempts to re-train normal patterns of activity via “constraint-induced” movement training to limit abnormal movements while reinforcing normal ones,40,41 “sensorimotor retuning” with intensive exercises,42 “slow down” therapy,43 active exercise,44 and EMG-biofeedback.45,46 Theories regarding maladaptive plasticity also have led to the opposing strategy of attempting to erase abnormal plasticity via lengthy periods of immobilization.47 Theories relating the pathophysiology of dystonia to defects in sensory processes or sensorimotor integration48 have led to attempts to alter sensory feedback as a treatment strategy. Various methods have been exploited including modification of sensory inputs,49,50 “kinesogenic taping,”51 transcutaneous electrical nerve stimulation,52 and augmentation of somatosensory discrimination by Braille training.53
Despite the enthusiasm for physical therapy in dystonia, systematic reviews have concluded that there is insufficient evidence to recommend any particular strategy.45,46 There are several reasons for the lack of clear guidelines. First, most of the studies have been quite small with outcomes that often were not reproducible, and the larger studies frequently demonstrated wide response variations among patients. Second, the reported benefits often have been quite small, transient, or subjective. The modest benefits of individual techniques have led to attempts to combine treatments using “multimodal” strategies, further obscuring the value of specific interventions. Third, there is a tradition in physical therapy to customize procedures according to the needs of individual patients. As a result, large studies using uniform protocols are scarce. Double-blind and placebo-controlled studies are rare also in part because of the difficulty in designing an appropriate control group to rule out non-specific placebo effects. Finally, many of the methods are cumbersome and time consuming, limiting enthusiasm for clinical application.
Enthusiasm for specific strategies also is blunted by concerns that some well-intentioned designs may be harmful. For example, significant improvements have been reported for patients with hand dystonia following 4–6 weeks of immobilization with a rigid splint.54,55 However, broad adoption has been limited by the long duration of splinting, side effects of hand clumsiness and weakness following splinting, and concerns that prolonged immobilization can trigger more severe fixed dystonia, sometimes as part of the complex regional pain syndrome.56 Similarly, transcutaneous electrical nerve stimulation has been reported to be helpful in some patients,52 yet detrimental to others.57 Despite these limitations, the available studies have provided some promising suggestions that deserve further exploration and development before more general recommendations can be formulated.58
In the absence of solid evidence to guide more specific recommendations, it seems reasonable to incorporate general physical therapy methods according to patient preferences. These may include regular stretching exercises to mitigate against contractures, muscle relaxation methods to attenuate pulling and pain, and strengthening of antagonist muscles to balance abnormal postures. Various assistive devices also are available to allow more significantly disabled patients to function more independently.
Oral medications
There are multiple articles summarizing oral medications for dystonia,59–63 including two systematic evidence-based reviews.64,65 None of the commonly used drugs has been subject to large-scale, double-blinded, placebo-controlled trials. None of them has been FDA approved for treatment of dystonia. Much of the evidence supporting the use of these drugs comes from small controlled trials, non-blinded trials, retrospective reviews, and anecdotal experience (Table 5).
Table 5.
Commonly used oral medications for dystonia
| Class of medication | Examples |
|---|---|
| Anticholinergics | benztropine, biperidin, ethopropazine, ophenadrine, procyclidine, trihexyphenidyl |
| Dopaminergics | levodopa, pramipexole, ropinirole, tetrabenazine |
| GABAergics | alprazolam, baclofen, chlordiazepoxide, clonazepam, diazepam |
| Muscle “relaxants” | baclofen, benzodiazepines, carisoprodol, chlorzoxazone, cyclobenzeprine, metaxolone, methocarbamol, orphenadrine |
| Others | carbamazepine, cannabidiol, cyproheptidine, gabapentin, lithium, mexilitine, nabilone, riluzole, tizanidine, zolpidem |
Acetylcholine-related drugs
One of the most frequently prescribed classes of medications for the dystonias include anticholinergics such as trihexyphenidyl, benztropine, biperidin, ethopropazine, orphenadrine, and procyclidine. These drugs are thought to work by blocking muscarinic acetylcholine receptors in the basal ganglia.66 Their use is supported by multiple retrospective studies,67 and one prospective, double-blind trial of trihexyphenidyl that showed clinically significant improvements in 71% of patients on an mean dose of 30 mg daily.68 However this study included only 31 patients with predominantly isolated dystonia, and a mean age of 19 years. Similar studies of children with dystonia associated with cerebral palsy showed that a significant proportion may worsen with anticholinergics.69 There are no prospective double-blind, placebo-controlled trials of anticholinergics for older adults, who are less likely to tolerate their many side effects.70,71
Despite the limited and sometimes conflicting information, anticholinergics remain in broad use because they seem to be at least partly effective for many types of dystonia, regardless of the underlying etiology. Trihexyphenidyl must be started at a low dose, for example 2 mg twice daily. It can be increased by 2 mg every few days until benefits are observed or side effects emerge. Effective doses range from 6–40 mg daily, divided across 3–4 doses. Typical side effects include memory loss, confusion, restlessness, depression, dry mouth, constipation, urinary retention, blurry vision or worsening of narrow-angle glaucoma
Dopamine-related drugs
Medications that augment or suppress dopaminergic transmission in the basal ganglia may be extraordinarily helpful in select populations of patients with dystonia. Augmenting dopamine transmission with levodopa is dramatically effective in dopa-responsive dystonia, which is most often caused by mutations in the GCH1 gene encoding the enzyme GTP-cyclohydrolase. 72,73 Many patients respond to doses as low as half of a 25/100 mg tablet of carbidopa/levodopa twice daily, although others require larger doses.72 For an adequate trial of levodopa, the dose should be increased slowly to 1000 mg in an adult (or 20 mg/kg for children) divided across three daily doses for one month before concluding it will not be effective. In addition to levodopa, patients with classical dopa-responsive dystonia respond to dopamine agonists and drugs that block dopamine metabolism such as monoamine oxidase inhibitors.
Levodopa is also at least partially effective in other disorders affecting dopamine synthesis that are caused by deficiency of tyrosine hydroxylase, sepiapterin reductase, and others.74 It may also be effective in some other rare disorders such as the dystonia in some cases of spinocerebellar ataxia type 375 or variant forms of ataxia telangiectasia,76 and for dystonia in Parkinson’s disease. Aside from these specific populations, levodopa and dopamine agonists are not broadly useful for other types of dystonia, such as the more common adult-onset isolated focal or segmental dystonias.77
Medications that suppress dopaminergic transmission also may be useful for specific subgroups of patients. Although dopamine receptor antagonists have been used with variable success in small un-blinded studies, their use is generally discouraged because the risk for development of acute dystonic reactions and tardive syndromes may lead to diagnostic confusion. However, depletion of dopamine with tetrabenazine does not carry these same risks, and it may be useful for some patients with dystonia, particularly those with tardive dystonia.78–80 It can be started at half of a 25 mg daily daily, and titrated up by a half tablet every 3–5 days, to a target of 25–100 mg daily. Dose-limiting side effects include drowsiness, parkinsonism, depression, insomnia, nervousness, anxiety, and akathisia.
GABA-related drugs
Another frequently prescribed group of medications is the benzodiazepines such as alprazolam, chlordiazepoxide, clonazepam, and diazepam. They are thought to work by amplifying transmission through GABA receptors. There are no large double-blind and controlled studies of the benzodiazepines in dystonia. Their use is supported by multiple small or retrospective studies. Anecdotal experience suggests they may be most useful for suppressing phasic aspects of dystonia, such as blinking in blepharospasm or tremor-dominant forms of dystonia.67,81,82 They also appear to be useful in the paroxysmal dyskinesias, where dystonia can be a prominent feature.83 Common side effects include sedation, impaired mentation and coordination, and depression. There also is a risk for tachyphylaxis and dependency, so abrupt discontinuation or sudden large decreases in doses should be avoided.
Baclofen is a GABA receptor agonist that also is often used in dystonia. There are no controlled studies to guide recommendations for its use, but several retrospective studies and anecdotal reports suggest it is mot often useful in childhood-onset dystonias, especially those with co-existing spasticity of the lower limbs.67,84 Some adults also may benefit, but most do not. Effective oral doses range from 30–120 mg daily divided across 3–4 doses. Common side effects include sedation, nausea, impaired mentation, dizziness and loss of muscle tone. Abrupt discontinuation or sudden large decreases in doses can be associated with withdrawal reactions that include delirium and seizures.
Baclofen also can be delivered intrathecally via chronically implanted minipumps, where it may be useful in a subpopulation of patients with dystonia.85–87 Here again, it has been most often employed in children where dystonia is combined with spasticity, especially in the lower limbs. The side effects are similar to those listed above for oral administration, with additional complications related to the implanted device. These complications include pump malfunction, catheter obstruction or leaks, or infection of the equipment.
Muscle relaxants
Many patients request “muscle relaxants” because they seem intuitively useful for overactive and sore muscles. This is a broad category of medications with diverse mechanisms of action that include baclofen and benzodiazepines described above, along with carisoprodol, chlorzoxazone, cyclobenzeprine, metaxalone, methocarbamol, and orphenadrine. There are no formal studies to guide recommendations for the use of these drugs in dystonia, and responses vary widely. Nevertheless, many patients derive at least partial benefits, especially those with pain from uncontrolled muscle pulling.
Other medications
A wide variety of other drugs have been advocated for specific forms of dystonia, generally based on small and non-blinded studies or anecdotal experiences. For example, carbamazepine and other anticonvulsants seem particularly useful for dystonic spasms in paroxysmal kinesigenic dyskinesia,88–90 and alcohol is useful in the myoclonus-dystonia syndrome.91 Mexiletine and intravenous lidocaine may be helpful in some cases.92,93 Other options suggested for specific populations include amphetamines, cannabidiol, cyproheptidine, gabapentin, lithium, nabilone, riluzole, tizanidine, and zolpidem.
Botulinum Neurotoxins
Medical BoNTs are derived from a neurotoxic protein produced by the bacterium Clostridium botulinum. The bacterial toxin causes a paralytic disorder known as botulism, but medical grade BoNT is purified and attenuated so that local intramuscular injections suppress overactive muscles in dystonia. There are seven distinct serotypes, A-G. Type A is marketed as onabotulinumtoxinA (Botox™), abobotulinumtoxinA (Dysport™), and incobotulinumtoxinA (Xeomin™). Type B is marketed as rimabotulinumtoxinB (Myobloc™). Their safety and efficacy have been the subject of multiple prior summaries, including several systematic evidence based reviews.64,94,95 They are very effective for many types of dystonia, significantly reducing abnormal movements and associated disability, and improving overall quality of life.
Many detailed resources are available for application of the BoNTs including target muscle selection, dosing, and the use of ancillary procedures for localization such as electromyography and ultrasound.96,97 The technical details associated with administration of BoNTs will not be reviewed here. Instead, the focus is on practical issues faced by physicians who may refer patients for BoNT treatments, and on some of the most common questions regarding their application. The first important issue involves the type of dystonia. The botulinum neurotoxins (BoNTs) are considered the treatment of first choice for most focal and segmental dystonias including blepharospasm, cervical dystonia, oromandibular and laryngeal dystonias, limb dystonias, and others. The benefits from injections usually emerge after 2–7 days, and they last for approximately 3–4 months.98 Most patients return for treatments 3–4 times yearly. BoNTs also can be valuable for patients with broader patterns of dystonia, where they are often under-utilized. For these patients, the goal is to target the regions that cause the most discomfort. For example, patients with dyskinetic cerebral palsy often have generalized dystonia with prominent involvement of the neck, and treatment with BoNT can alleviate this discomfort ant reduce the risk of acquired myelopathy.99
The BoNTs are dramatically effective for most focal dystonias, but it can be challenging to get good results with certain subtypes. Some patients with blepharospasm have co-existing apraxia of eyelid opening, which is more difficult to treat with BoNT.100 Injections into the pretarsal portion of the orbicularis oculi muscles may improve outcomes in these cases.101,102 Among patients with cervical dystonia, those with prominent anterocollis can be more difficult to treat.103 Deep injections into pre-vertebral muscles have been advocated,104,105 although responses vary. For laryngeal dystonias, spasmodic adductor dysphonia responds more predictably than spasmodic abductor dysphonia.106,107 Oromandibular and lingual dystonias can sometimes be challenging to treat, although good outcomes can be achieved in experienced hands.108–112 Because there are so many small muscles that work together for coordinated activities, it can be difficult to achieve satisfactory outcomes for hand dystonias. Some patients enjoy dramatic benefits with very small doses, but achieving the right dose to avoid weakness or involvement of nearby muscles can be difficult to balance.113
Another important issue involves side effects. There are no deleterious long-term side effects even after decades of treatment, apart from a small risk of developing resistance due to antibodies that neutralize the BoNT protein. However, the development of immunologically mediated resistance is rare with current preparations of BoNT.114,115 The short-term side effects depend mostly on local diffusion from the sites of injection. For blepharospasm the most common side effects are ptosis, local hematoma formation, tearing, and rarely blurry vision or diplopia. For cervical dystonia the most common side effects are dysphagia, excessive neck muscle weakness, and occasionally dry mouth. For laryngeal dystonias the most common side effects are hoarsenss or hypophonia, and rarely dysphagia and aspiration. For limb dystonias the most common side effects involve excessive weakening, or weakness of nearby muscles. Systemic side effects are unusual, but a few patients complain of a flu-like syndrome for 3–5 days after their treatments.
A third issue involves the selection of a specific product. Many articles have summarized differences among the BoNTs regarding efficacy, side effects, and formulations. However, there are few scientifically rigorous comparisons, and the similarities are more striking than the differences (Table 6). The choice of product depends largely on the experience and preferences of individual providers.
Table 6.
Comparison of the most common botulinum toxin formulations
| OnabotulinumtoxinA (Botox™) | AbobotulinumtoxinA (Dysport™) | IncobotulinumtoxinA (Xeomin™) | RimabotulinumtoxinB (Myobloc™) | |
|---|---|---|---|---|
| FDA-approved indications for dystonia | blepharospasm, cervical dystonia | cervical dystonia | blepharospasm, cervical dystonia | cervical dystonia |
| Preparation | vacuum dried | freeze dried | powder | liquid |
| Available dose sizes (units) | 100, 200 | 300, 500 | 50, 100 | 1000, 2500, 5000 |
| Storage | refrigerate | refrigerate | room temperature | refrigerate |
| Approximate dose equivalency* | 1 | 2.5 – 3 | 1 | 40 |
As the first FDA-approved BoNT, onabotulinumtoxinA was arbitrarily defined as having a strength of 1. The relative strengths of the others are compared against this value.
Surgical Interventions
Multiple surgical interventions are available for the treatment of the dystonias. Typically these more invasive approaches are reserved for patients who fail more conservative therapies. The most common intervention involves neuromodulation of brain activity via an implanted electrical impulse generator, although focal ablation of select brain areas and peripheral approaches that target nerves or muscles can be applied in some circumstances.
Neuromodulation
Neuromodulation is synonomous with deep brain stimulation (DBS). The term neuromodulation is increasingly preferred because some targets may not be “deep” and “stimulation” implies a mechanism that has not been established. Several extensive reviews on neuromodulation have been published recently,116–118 including a whole issue of the journal Movement Disorders (volume 26, supplement 1, 2011). Here, the focus is on practical issues of relevance to any physician who may council patients regarding these options. The issues include patient selection for best outcomes, long-term expectations, and some ongoing debates.
Some patients with dystonia respond quite well to neuromodulation, while others derive no benefit. Many years of experience have provided some important insights into several factors that predict responses.118,119 Patients with isolated generalized dystonia syndromes (previously “primary” dystonia) tend to respond most consistently, with the most objective blinded studies showing improvements in standardized dystonia rating scales of 40–60%.120,121 Among this patient population best outcomes appear to be associated with younger ages, shorter disease durations, and those who have the common TOR1A mutation for DYT1 dystonia. There is insufficient evidence to predict outcomes in the more recently discovered genes including the THAP1 gene for DYT6. Patients with fixed contractures or scoliosis do not do so well as those with more a more mobile syndrome.
Patients with isolated focal and segmental dystonias also appear to respond well to neuromodulation, although perhaps less predictably than those with isolated generalized dystonia. This group includes patients with relatively localized or segmental patterns involving the neck, face, trunk or limbs. Patients with dystonic syndromes that are combined with other neurological features (previously “dystonia plus” or “secondary” dystonias) respond variably. Some subtypes consistently respond very well, for example myoclonus dystonia and tardive dystonia. Others have a consistently poor outcome (e.g. degenerative disorders) or are less predictable (e.g. cerebral palsy). One of the reasons for the poor outcomes in some populations is that it is difficult to detect a stable benefit for progressive degenerative disorders, or for disorders where dystonia is combined with other motor defects such as spasticity that are not expected to improve with neuromodulation. These populations should be considered for surgery only by very experienced centers after careful counseling, ideally as part of a methodical study aimed at elucidating risk/benefit profiles.
The long-term outcomes of neuromodulation are good,122–124 with benefits sustained for many years, and some studies reporting good outcomes even after 10 years.125 Ongoing access to an experienced center to adjust stimulator settings and address potential complications is essential. Benefits from surgery can be delayed for weeks or even months, requiring frequent visits to adjust stimulator settings for optimal outcomes. Return visits also should be anticipated every 2–4 years for battery replacement. In one study of 47 cases with DYT1 dystonia followed by a very experienced multidisciplinary neuromodulation center for more than 10 years, 8.5% had delayed post-operative infection of equipment requiring antibiotics and/or equipment removal, 8.5% had malfunction of equipment such as impulse generator failure or lead defects, and 4.3% required revisions of lead location.125 These observations indicate that close follow-up by an experienced team is essential for long-term maintenance therapy. This requirement for return visits presents a barrier for some patients who may live far from experienced providers.
There are several unresolved questions that the counseling physician may be asked to address. One is the ideal surgical target.116,118 The internal segment of the globus pallidus (GPi) is the traditional target used by most centers. However, observations that patients with dystonia sometimes develop bradykinesia in unaffected body regions or gait failure have led to interest in other targets such as the subthalamic nucleus. On the other hand, neuromodulation of the subthalamic nucleus has been associated with dyskinesias, weight gain, and psychiatric changes. Others have targeted various regions of the thalamus, usually for focal hand dystonia and those with prominent tremor.126–128 The “ideal” target remains unknown. Whether this target varies according to the subtype of dystonia also remains uncertain. As a result the selection of targets often is driven by the opinions of individual centers.
Another unresolved question involves the expected outcomes for patients with dystonias combined with other neurological features (previously “secondary” dystonias). Aside from tardive dystonia and myoclonus dystonia, there is insufficient information to counsel interested patients regarding expectations. The lack of information should not be viewed as an absolute contraindication for surgery, but patients must be clearly informed about the chances for failure.
Ablative approaches
Making controlled focal lesions in specific parts of the brain was the most common surgical procedure conducted for dystonia patients before neuromodulation became more popular.129 Lesions were made in a variety of locations, most notably the thalamus, globus pallidus and cerebellum. Neuromodulation rapidly became more popular because it is more readily tunable, and because it is reversible in the event that intolerable side effects develop. However, neuromodulation has its own risk of complications related to the hardware, and it is expensive. Therefore, there is still a role for ablative procedures in some circumstances.116,129
Ablative procedures may be useful in developing countries where the cost of equipment for neuromodulation and requirements for regular follow-up are prohibitive. Ablations are also useful for patients with a body habitus that presents a high risk for hardware-related complications, such as those with severe dystonia and fixed contractions, or very young or otherwise small patients. They may be offered to patients who suffer repeated hardware infections, or merely do not wish to have chronically implanted hardware. They may also be appropriate as palliative procedures for patients with severe illness who cannot tolerate surgery to install and maintain the hardware, and for some progressive neurodegenerative disorders.
Peripheral surgeries
Another category of surgeries often offered to patients with dystonia before BoNTs and neuromodulation became more popular involved directly sectioning or destroying overactive muscles or the nerves controlling them. These procedures are far less commonly used today, but they are still offered by some centers. They are covered briefly here for physicians encountering patients who may ask about them.
Selective peripheral denervation may be offered to patients with cervical dystonia who fail oral agents and botulinum toxins. The procedure involves extra-spinal sectioning of nerves to specific muscles, so best outcomes are seen for patients with a limited number of muscles involved (e.g. pure torticollis or pure laterocollis). Success rates are reported from 60–90%.130–135 Side effects may include permanent somatosensory loss or dysesthesia in an isolated region of the neck, cosmetic changes associated with scaring or muscle atrophy, muscles weakness, and dysphagia. Abnormal movements may re-emerge after several weeks or years, a phenomenon that may reflect re-innervation or progression of the underlying disorder.
A variety of procedures are offered to patients with blepharospasm too. They include orbicularis myectomy, frontalis suspension, surgical shortening of the levator palpebrae, and removal of redundant eyelid skin.136–138 None of these procedures has been subject to rigorous trials, so they usually are offered only to patients who fail botulinum toxins. Included are patients who are resistant to the toxins, and those with co-existing apraxia of eyelid opening that may respond poorly to the toxins.
There also are several procedures in use for patients with laryngeal dystonia.139,140 Patients with spasmodic dysphonia may undergo thyroplasty to modify the cartilaginous structure of the larynx or thyro-aretynoid myectomy. The most common approach involves sectioning the recurrent laryngeal nerve to the thyro-arytenoid and annealing the stump to the ansa cervicalis. Complications can include transient or permanent voice impediment, dysphagia, and return of symptoms requiring re-operation.
Strategies for Combining Therapies for Specific Populations
Because there are so many different clinical manifestations and causes of the dystonias, it is not feasible to devise a universal treatment algorithm for all subtypes that combines the various medical and surgical options outlined above. Although treatment plans must be individualized, there are some useful guiding principles. The first step is to delineate the diagnosis (Figure 1). The diagnostic subtype is important for the application of some types of treatments that target underlying etiological mechanisms and substantially modify the course of the disease (Table 3). The next step involves counseling to address expectations from treatment and any psychiatric comorbidities.
For the majority of patients where disease-modifying therapies are not yet available, treatments are symptomatic. For late-onset focal or segmental dystonia, BoNTs are the treatment of first choice. Because symptoms may wax and wane in severity, an oral agent may be offered as adjunctive therapy to use when needed. When BoNTs and oral medications are not adequate, patients with focal and segmental dystonias may undergo more invasive surgical approaches.
For adults with generalized dystonia and all early-onset dystonias regardless of body distribution where diagnostic testing does not reveal a cause with etiology-based treatments, a trial of levodopa is mandatory to address the possibility of dopa-responsive dystonia. There are no data to guide the exact age cut-off where a levodopa trial is essential, but many providers proceed with a levodopa trial for any patient with isolated dystonia who is <40 years old. Following this trial, other oral agents may also be attempted. BoNT may be offered to those where one particular region of the body creates the most disability, and surgical interventions are offered when oral agents and BoNT provide insufficient benefits.
Conclusions
The treatment of patients with dystonia has improved dramatically over recent years. There is a rapidly growing list of specific subtypes with specific treatments that target the underlying etiology. This list is expected to continue to grow, as more is learned about the underlying causes for dystonia. There are four widely available BoNTs preparations. They are highly effective in the treatment of focal and segmental dystonias, and can be of benefit in patients with broader distributions too. Neuromodulation of the brain and peripheral surgeries that target nerve or muscles can also be very useful when more conservative methods fail. Knowing how to mix and match these various treatment modalities for specific populations can be challenging, but significant benefits can be achieved in the vast majority of patients.
Acknowledgments
H.A. Jinnah has received in the past 3 years research grant support from the NIH, Ipsen Pharmaceuticals, Merz Pharmaceuticals, Psyadon Pharmaceuticals, the Atlanta Clinical & Translational Science Institute, the Emory University Research Council, the Lesch-Nyhan Syndrome Children’s Research Foundation, the Dystonia Medical Research Foundation, the Bachmann-Strauss Dystonia & Parkinson’s Foundation, and the Benign Essential Blepharospasm Research Foundation. He is principal investigator for the Dystonia Coalition, which receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Sciences and National Institute of Neurological Disorders and Stroke. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc., Ipsen Biopharm, Medtronics Inc, and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, The Bachmann-Strauss Dystonia and Parkinson Foundation, BeatDystonia, The Benign Essential Blepharospasm Foundation, Dystonia Europe, Dystonia Ireland, The Dystonia Medical Research Foundation, The Dystonia Society, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association). Dr. Jinnah serves on the Scientific Advisory Boards for Cure Dystonia Now, the Dystonia Medical Research foundation, Tyler’s Hope for a Dystonia Cure, the Lesch-Nyhan Syndrome Children’s Research Foundation, and Lesch-Nyhan Action France.
Abbreviations & acronyms
- BoNT
botulinum neurotoxin
- DBS
deep brain stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Fahn S. The varied clinical expressions of dystonia. Neurol Clinics. 1984;2:541–554. [PubMed] [Google Scholar]
- 2.Fahn S. Clinical variants of idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry. 1989;(Suppl):96–100. doi: 10.1136/jnnp.52.suppl.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elble RJ. Defining dystonic tremor. Curr Neuropharmacol. 2013;11:48–52. doi: 10.2174/157015913804999478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erro R, Rubio-Agusti I, Saifee TA, et al. Rest and other types of tremor in adult-onset primary dystonia. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2013-305876. in press. [DOI] [PMC free article] [PubMed]
- 5.Defazio G, Gigante AF, Abbruzzese G, et al. Tremor in primary adult-onset dystonia: prevalence and associated clinical features. J Neurol Neurosurg Psychiatry. 2013;84:404–408. doi: 10.1136/jnnp-2012-303782. [DOI] [PubMed] [Google Scholar]
- 6.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung VS, Jinnah HA, Bhatia K, Vidailhet M. Assessment of the patient with dystonia: An update on dystonia syndromes. Mov Disord. 2013;28:889–898. doi: 10.1002/mds.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeDoux MS. The genetics of dystonias. Adv Genet. 2012;79:35–85. doi: 10.1016/B978-0-12-394395-8.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmann K, Klein C. Genetics of dystonia: What’s known? What’s new? What’s next? Mov Disord. 2013;28:899–905. doi: 10.1002/mds.25536. [DOI] [PubMed] [Google Scholar]
- 10.LeDoux MS, Dauer WT, Warner T. Emerging molecular pathways for dystonia. Mov Disord. 2013;15:968–981. doi: 10.1002/mds.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson VB, Jinnah HA, Hess EJ. Convergent mechanisms in etiologically-diverse dystonias. Expert Opin Ther Targets. 2011;15:1387–1403. doi: 10.1517/14728222.2011.641533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neychev VK, Gross R, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: Current view using neuroimaging. Mov Disord. 2013;28:944–957. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 14.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28:958–967. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojovic M, Parees I, Kassavetis P, et al. Secondary and primary dystonia: pathophysiological differences. Brain. 2013;136:2038–2049. doi: 10.1093/brain/awt150. [DOI] [PubMed] [Google Scholar]
- 17.Hallett M. Pathophysiology of dystonia. J Neural Transm Suppl. 2006;70:485–488. doi: 10.1007/978-3-211-45295-0_72. [DOI] [PubMed] [Google Scholar]
- 18.Jinnah HA, Berardelli A, Comella C, et al. The focal dystonias: Current views and challenges for future research. Mov Disord. 2013;7:926–943. doi: 10.1002/mds.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamelou M, Lai SC, Aggarwal A, et al. Dystonic opisthotonus: a “red flag” for neurodegeneration with brain iron accumulation syndromes? Mov Disord. 2013;28:1325–1329. doi: 10.1002/mds.25490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider SA, Aggarwal A, Bhatt MH, et al. Severe tongue protrusion dystonia: clinical syndromes and possible treatment. Neurology. 2006;67:940–943. doi: 10.1212/01.wnl.0000237446.06971.72. [DOI] [PubMed] [Google Scholar]
- 21.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. doi: 10.1016/B978-0-444-52014-2.00037-9. [DOI] [PubMed] [Google Scholar]
- 22.Schneider SA, Bhatia KP. Secondary dystonia - clinical clues and syndromic associations. Eur J Neurol. 2010;17 (Suppl 1):52–57. doi: 10.1111/j.1468-1331.2010.03051.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider SA, Bhatia KP, Hardy J. Complicated recessive dystonia parkinsonism syndromes. Mov Disord. 2009;24:490–499. doi: 10.1002/mds.22314. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Cazorla A, Wolf NI, Serrano M, et al. Inborn errors of metabolism and motor disturbances in children. J Inherit Metab Dis. 2009;32:618–629. doi: 10.1007/s10545-009-1194-9. [DOI] [PubMed] [Google Scholar]
- 25.Stamelou M, Alonso-Canovas A, Bhatia KP. Dystonia in corticobasal degeneration: a review of the literature on 404 pathologically proven cases. Mov Disord. 2012;27:696–702. doi: 10.1002/mds.24992. [DOI] [PubMed] [Google Scholar]
- 26.Wickremaratchi MM, Knipe MD, Sastry BS, et al. The motor phenotype of Parkinson’s disease in relation to age at onset. Mov Disord. 2011;26:457–463. doi: 10.1002/mds.23469. [DOI] [PubMed] [Google Scholar]
- 27.Lalli S, Albanese A. The diagnostic challenge of primary dystonia: evidence from misdiagnosis. Mov Disord. 2010;25:1619–1626. doi: 10.1002/mds.23137. [DOI] [PubMed] [Google Scholar]
- 28.McKeon A, Matsumoto JY, Bower JH, Ahlskog JE. The spectrum of disorders presenting as adult-onset focal lower extremity dystonia. Parkinsonism Relat Disord. 2008;14:613–619. doi: 10.1016/j.parkreldis.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Tolosa E, Compta Y. Dystonia in Parkinson’s disease. J Neurol. 2006;253 (Suppl 7):7–13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- 30.Jankovic J. Dystonia and other deformities in Parkinson’s disease. J Neurol Sci. 2005;229:1–3. doi: 10.1016/j.jns.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Tiderington E, Goodman EM, Rosen AR, et al. How long does it take to diagnose cervical dystonia? J Neurol Sci. 2013;335:72–74. doi: 10.1016/j.jns.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jog M, Chouinard S, Hobson D, et al. Causes for treatment delays in dystonia and hemifacial spasm: a Canadian survey. Can J Neurol Sci. 2011;38:704–711. doi: 10.1017/s0317167100012270. [DOI] [PubMed] [Google Scholar]
- 33.Charles PD, Adler CH, Stacy M, et al. Cervical dystonia and pain: characteristics and treatment patterns from CD PROBE (Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy) J Neurol. 2014 doi: 10.1007/s00415-014-7343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zurowski M, Marsh L, McDonald W. Psychiatric comorbidities in dystonia: Emerging concepts. Mov Disord. 2013;28:914–920. doi: 10.1002/mds.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM. Nonmotor manifestations of dystonia: a systematic review. Mov Disord. 2011;26:1206–1217. doi: 10.1002/mds.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabbrini G, Berardelli I, Moretti G, et al. Psychiatric disorders in adult-onset focal dystonia: a case-control study. Mov Disord. 2010;25:459–465. doi: 10.1002/mds.22983. [DOI] [PubMed] [Google Scholar]
- 37.Lewis L, Butler A, Jahanshahi M. Depression in focal, segmental and generalized dystonia. J Neurol. 2008;255:1750–1755. doi: 10.1007/s00415-008-0020-x. [DOI] [PubMed] [Google Scholar]
- 38.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quartarone A, Siebner HR, Rothwell JC. Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci. 2006;29:192–199. doi: 10.1016/j.tins.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Candia V, Schafer T, Taub E, et al. Sensory motor retuning: a behavioral treatment for focal hand dystonia of pianists and guitarists. Arch Phys Med Rehabil. 2002;83:1342–1348. doi: 10.1053/apmr.2002.35094. [DOI] [PubMed] [Google Scholar]
- 41.Uswatte G, Taub E. Constraint-induced movement therapy: a method for harnessing neuroplasticity to treat motor disorders. Prog Brain Res. 2013;207:379–401. doi: 10.1016/B978-0-444-63327-9.00015-1. [DOI] [PubMed] [Google Scholar]
- 42.Zeuner KE, Shill HA, Sohn YH, et al. Motor training as treatment in focal hand dystonia. Mov Disord. 2005;20:335–341. doi: 10.1002/mds.20314. [DOI] [PubMed] [Google Scholar]
- 43.Sakai N. Slow-down exercise for the treatment of focal hand dystonia in pianists. Med Probl Perform Artists. 2006;21:25–28. [Google Scholar]
- 44.Boyce MJ, Canning CG, Mahant N, Morris J, Latimer J, Fung VS. Active exercise for individuals with cervical dystonia: a pilot randomized controlled trial. Clin Rehabil. 2012;27:226–235. doi: 10.1177/0269215512456221. [DOI] [PubMed] [Google Scholar]
- 45.Delnooz CC, Horstink MW, Tijssen MA, van de Warrenburg BP. Paramedical treatment in primary dystonia: a systematic review. Mov Disord. 2009;24:2187–2198. doi: 10.1002/mds.22608. [DOI] [PubMed] [Google Scholar]
- 46.De Pauw J, Van der Velden K, Meirte J, et al. The effectiveness of physiotherapy for cervical dystonia: a systematic literature review. J Neurol. 2014 doi: 10.1007/s00415-013-7220-8. [DOI] [PubMed] [Google Scholar]
- 47.Cogiamanian F, Barbieri S, Priori A. Novel nonpharmacologic perspectives for the treatment of task-specific focal hand dystonia. J Hand Ther. 2009;22:156–161. doi: 10.1016/j.jht.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Tinazzi M, Fiorio M, Fiaschi A, Rothwell JC, Bhatia KP. Sensory functions in dystonia: insights from behavioral studies. Mov Disord. 2009;24:1427–1436. doi: 10.1002/mds.22490. [DOI] [PubMed] [Google Scholar]
- 49.Karnath HO, Konczak J, Dichgans J. Effect of prolonged neck muscle vibration on lateral head tilt in severe spasmodic torticollis. J Neurol Neurosurg Psychiatry. 2000;69:658–660. doi: 10.1136/jnnp.69.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leis AA, Dimitrijevic MR, Delapasse JS, Sharkey PC. Modification of cervical dystonia by selective sensory stimulation. J Neurol Sci. 1992;110:79–89. doi: 10.1016/0022-510x(92)90013-b. [DOI] [PubMed] [Google Scholar]
- 51.Pelosin E, Avanzino L, Marchese R, et al. kinesiotaping reduces pain and modulates sensory function in patients with focal dystonia: a randomized crossover pilot study. Neurorehabil Neural Repair. 2013;27:722–731. doi: 10.1177/1545968313491010. [DOI] [PubMed] [Google Scholar]
- 52.Tinazzi M, Farina S, Bhatia K, et al. TENS for the treatment of writer’s cramp dystonia: a randomized, placebo-controlled study. Neurology. 2005;64:1946–1948. doi: 10.1212/01.WNL.0000163851.70927.7E. [DOI] [PubMed] [Google Scholar]
- 53.Zeuner KE, Bara-Jimenez W, Noguchi PS, Goldstein SR, Dambrosia JM, Hallett M. Sensory training for patients with focal hand dystonia. Ann Neurol. 2002;51:593–598. doi: 10.1002/ana.10174. [DOI] [PubMed] [Google Scholar]
- 54.Pesenti A, Barbieri S, Priori A. Limb immobilization for occupational dystonia: a possible alternative treatment for selected patients. Adv Neurol. 2004;94:247–254. [PubMed] [Google Scholar]
- 55.Priori A, Pesenti A, Cappellari A, Scarlato G, Barbieri S. Limb immobilization for the treatment of focal occupational dystonia. Neurology. 2001;57:405–409. doi: 10.1212/wnl.57.3.405. [DOI] [PubMed] [Google Scholar]
- 56.Okun MS, Nadeau SE, Rossi F, Triggs WJ. Immobilization dystonia. J Neurol Sci. 2002;201:79–83. doi: 10.1016/s0022-510x(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 57.Meunier S, Bleton JP, Mazevet D, et al. TENS is harmful in primary writing tremor. Clin Neurophysiol. 2011;122:171–175. doi: 10.1016/j.clinph.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 58.van den Dool J, Visser B, Koelman JH, Engelbert RH, Tijssen MA. Cervical dystonia: effectiveness of a standardized physical therapy program; study design and protocol of a single blind randomized controlled trial. BMC Neurol. 2013;13:85. doi: 10.1186/1471-2377-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jankovic J. Medical treatment of dystonia. Mov Disord. 2013;28:1001–1012. doi: 10.1002/mds.25552. [DOI] [PubMed] [Google Scholar]
- 60.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–872. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 61.Bhidayasiri R, Tarsy D. Treatment of dystonia. Expert Rev Neurother. 2006;6:863–886. doi: 10.1586/14737175.6.6.863. [DOI] [PubMed] [Google Scholar]
- 62.Goldman JG, Comella CL. Treatment of dystonia. Clin Neuropharmacol. 2003;26:102–108. doi: 10.1097/00002826-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Cloud LJ, Jinnah HA. Treatment strategies for dystonia. Expert Opin Pharmacother. 2010;11:5–15. doi: 10.1517/14656560903426171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albanese A, Barnes MP, Bhatia KP, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: repor of an EFNS/MDS-ES task force. Eur J Neurol. 2006;13:433–444. doi: 10.1111/j.1468-1331.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 65.Balash Y, Giladi N. Efficacy of pharmacological treatment of dystonia: evidence-based review including meta-anaylsis of the effect of botulinum toxin and other cure options. Eur J Neurol. 2004;11:361–370. doi: 10.1111/j.1468-1331.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 66.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Greene P, Shale H, Fahn S. Experience with high dosages of anticholinergic and other drugs in the treatment of torsion dystonia. Adv Neurol. 1988;50:547–556. [PubMed] [Google Scholar]
- 68.Burke RE, Fahn S, Marsden CD. Torsion dystonia: a double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology. 1986;36:160–164. doi: 10.1212/wnl.36.2.160. [DOI] [PubMed] [Google Scholar]
- 69.Sanger TD, Bastian A, Brunstrom J, et al. Prospective open-label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. J Child Neurol. 2007;22:530–537. doi: 10.1177/0883073807302601. [DOI] [PubMed] [Google Scholar]
- 70.Fahn S. High dosage anticholinergic therapy in dystonia. Neurology. 1983;33:1255–1261. doi: 10.1212/wnl.33.10.1255. [DOI] [PubMed] [Google Scholar]
- 71.Taylor AE, Lang AE, Saint-Cyr JA, Riley DE, Ranawaya R. Cognitive processes in idiopathic dystonia treated with high-dose anticholinergic therapy: implications for treatment strategies. Clin Neuropharmacol. 1991;14:62–77. doi: 10.1097/00002826-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Nygaard TG, Marsden CD, Fahn S. Dopa-responsive dystonia: long-term treatment response and prognosis. Neurology. 1991;41:174–181. doi: 10.1212/wnl.41.2_part_1.174. [DOI] [PubMed] [Google Scholar]
- 73.Segawa M, Nomura Y, Nishiyama N. Dopa-responsive dystonia. In: Stacey MA, editor. Handbook of dystonia. Yew York: Informa Healthcare, Inc; 2008. pp. 219–244. [Google Scholar]
- 74.Kurian MA, Gissen P, Smith M, Heales S, Jr, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–733. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- 75.Wilder-Smith E, Tan EK, Law HY, Zhao Y, Ng I, Wong MC. Spinocerebellar ataxia type 3 presenting as an L-DOPA responsive dystonia phenotype in a Chinese family. J Neurol Sci. 2003;213:25–28. doi: 10.1016/s0022-510x(03)00129-1. [DOI] [PubMed] [Google Scholar]
- 76.Charlesworth G, Mohire MD, Schneider SA, Stamelou M, Wood NW, Bhatia KP. Ataxia telangiectasia presenting as dopa-responsive cervical dystonia. Neurology. 2013;81:1148–1151. doi: 10.1212/WNL.0b013e3182a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang AE. Dopamine agonists and antagonists in the treatment of idiopathic dystonia. Adv Neurol. 1988;50:561–570. [PubMed] [Google Scholar]
- 78.Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34:1487–1504. doi: 10.1016/j.clinthera.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Jankovic J, Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48:358–362. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 80.Jankovic J, Orman J. Tetrabenazine therapy of dystonia, chorea, tics, and other dyskinesias. Neurology. 1988;38:391–394. doi: 10.1212/wnl.38.3.391. [DOI] [PubMed] [Google Scholar]
- 81.Fasano A, Bove F, Lang AE. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:759–769. doi: 10.1136/jnnp-2013-305532. [DOI] [PubMed] [Google Scholar]
- 82.Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13:402–411. doi: 10.1002/ana.410130406. [DOI] [PubMed] [Google Scholar]
- 83.Strzelczyk A, Burk K, Oertel WH. Treatment of paroxysmal dyskinesias. Expert Opin Pharmacother. 2011;12:63–72. doi: 10.1517/14656566.2010.513971. [DOI] [PubMed] [Google Scholar]
- 84.Greene P. Baclofen in the treatment of dystonia. Clin Neuropharmacol. 1992;15:276–288. doi: 10.1097/00002826-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Walker RH, Danisi FO, Swope DM, goodman RR, Germano IM, Brin MF. Intrathecal baclofen for dystonia: benefits and complications during six years of experience. Mov Disord. 2000;15:1242–1247. doi: 10.1002/1531-8257(200011)15:6<1242::aid-mds1028>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 86.Albright AL, Barry MJ, Shafron DH, Ferson SS. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652–657. doi: 10.1017/s0012162201001190. [DOI] [PubMed] [Google Scholar]
- 87.Hou JG, Ondo W, Jankovic J. Intrathecal baclofen for dystonia. Mov Disord. 2001;16:1201–1202. doi: 10.1002/mds.1215. [DOI] [PubMed] [Google Scholar]
- 88.Houser MK, Soland VL, Bhatia KP, Quinn NP, Marsden CD. Paroxysmal kinesigenic choreoathetosis: a report of 26 patients. J Neurol. 1999;246:120–126. doi: 10.1007/s004150050318. [DOI] [PubMed] [Google Scholar]
- 89.Fahn S, Marsden CD. The paroxysmal dyskinesias. In: Marsden CD, Fahn S, editors. Movement disorders 3. Oxford: Butterworth-Heinemann; 1994. pp. 310–347. [Google Scholar]
- 90.Demirkiran M, Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995;38:571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- 91.Kinugawa K, Vidailhet M, Clot F, Apartis E, Grabli D, Roze E. Myoclonus-dystonia: an update. Mov Disord. 2009;24:479–489. doi: 10.1002/mds.22425. [DOI] [PubMed] [Google Scholar]
- 92.Lucetti C, Nuti A, Gambaccini G, et al. Mexiletine in the treatment of torticollis and generalized dystonia. Clin Neuropharmacol. 2000;23:186–189. doi: 10.1097/00002826-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Ohara S, Hayashi R, Momoi H, Miki J, Yanagisawa N. Mexiletine in the treatment of spasmodic torticollis. Mov Disord. 1998;13:934–940. doi: 10.1002/mds.870130612. [DOI] [PubMed] [Google Scholar]
- 94.Hallett M, Benecke R, Blitzer A, Comella CL. Treatment of focal dystonias with botulinum neurotoxin. Toxicon. 2009;54:628–633. doi: 10.1016/j.toxicon.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simpson DM, Blitzer A, Brashear A, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Truong D, Dressler D, Hallet M, Zachary C. Manual of Botulinum Toxin. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 97.Jost W, Valerius KP. Pictoral Atlas of Botulinum Toxin Injection: Dosage, Localization, Application. Berlin: Quintessence Publishing Company; 2008. [Google Scholar]
- 98.Marsh WA, Monroe DM, Brin MF, Gallagher CJ. Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia. BMC Neurol. 2014;14:91. doi: 10.1186/1471-2377-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vidailhet M. Treatment of movement disorders in dystonia-choreoathtosis cerebral palsy. Handb Clin Neurol. 2013;111:197–202. doi: 10.1016/B978-0-444-52891-9.00019-1. [DOI] [PubMed] [Google Scholar]
- 100.Rana AQ, Shah R. Combination of blepharospasm and apraxia of eyelid opening: a condition resistant to treatment. Acta Neurol Belg. 2012;112:95–96. doi: 10.1007/s13760-012-0019-z. [DOI] [PubMed] [Google Scholar]
- 101.Aramideh M, Ongerboer de Visser BW, Brans JW, Koelman JH, Speelman JD. Pretarsal application of botulinum toxin for treatment of blepharospasm. J Neurol Neurosurg Psychiatry. 1995;59:309–311. doi: 10.1136/jnnp.59.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Esposito M, Fasano A, Crisci C, Dubbioso R, Iodice R, Santoro L. The combined treatment with orbital and pretarsal botulinum toxin injections in the management of poorly responsive blepharospasm. Neurol Sci. 2013;35:397–400. doi: 10.1007/s10072-013-1526-2. [DOI] [PubMed] [Google Scholar]
- 103.Waln O, Ledoux MS. Blepharospasm plus cervical dystonia with predominant anterocollis: A distinctive subphenotype of segmental craniocervical dystonia? Tremor Other Hyperkinet Mov. 2011;(1) doi: 10.7916/D8SQ8Z4T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glass GA, Ku S, Ostrem JL, Heath S, Larson PS. Fluoroscopic, EMG-guided injection of botulinum toxin into the longus colli for the treatment of anterocollis. Parkinsonism Relat Disord. 2009;15:610–613. doi: 10.1016/j.parkreldis.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Bhidayasiri R. Treatment of complex cervical dystonia with botulinum toxin: involvement of deep-cervical muscles may contribute to suboptimal responses. Parkinsonism Relat Disord. 2011;17 (Suppl 1):S20–24. doi: 10.1016/j.parkreldis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17 (Suppl 1):28–30. doi: 10.1111/j.1468-1331.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 107.Watts CC, Whurr R, Nye C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst Rev. 2004;3:CD004327. doi: 10.1002/14651858.CD004327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16:438–441. doi: 10.1016/j.parkreldis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 109.Charles PD, Davis TL, Shannon KM, Hook MA, Warner JS. Tongue protrusion dystonia: treatment with botulinum toxin. South Med J. 1997;90:522–525. doi: 10.1097/00007611-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 110.Singer C, Papapetropoulos S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat Disord. 2006;12:115–118. doi: 10.1016/j.parkreldis.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 111.Blitzer A, Brin MF, Greene PE, Fahn S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann Otol Rhinol Laryngol. 1989;98:93–97. doi: 10.1177/000348948909800202. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Alegre P, Schneider RL, Hoffman H. Clinical, etiological, and therapeutic features of jaw-opening and jaw-closing oromandibular dystonias: A decade of experience at a single treatment center. Tremor Other Hyperkinet Mov (N Y) 2014;4:231. doi: 10.7916/D8TH8JSM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lungu C, Karp BI, Alter K, Zolbrod R, Hallett M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750–753. doi: 10.1002/mds.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm. 2013;120:275–290. doi: 10.1007/s00702-012-0893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brin MF, Comella CL, Jankovic J, Lai F, Naumann M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- 116.Moro E, Gross RE, Krauss JK. What’s new in surgical treatments for dystonia? Mov Disord. 2013;28:1013–1020. doi: 10.1002/mds.25550. [DOI] [PubMed] [Google Scholar]
- 117.Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry. 2012;84:1029–1040. doi: 10.1136/jnnp-2011-301714. [DOI] [PubMed] [Google Scholar]
- 118.Mills KA, Starr PA, Ostrem JL. Neuromodulation for dystonia: target and patient selection. Neurosurg Clin N Am. 2014;25:59–75. doi: 10.1016/j.nec.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 119.Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–1389. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- 120.Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 121.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 122.Tagliati M, Krack P, Volkmann J, et al. Long-Term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Mov Disord. 2011;26 (Suppl 1):S54–62. doi: 10.1002/mds.23535. [DOI] [PubMed] [Google Scholar]
- 123.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029–1038. doi: 10.1016/S1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- 124.Reese R, Gruber D, Schoenecker T, et al. Long-term clinical outcome in Meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011;26:691–698. doi: 10.1002/mds.23549. [DOI] [PubMed] [Google Scholar]
- 125.Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86–93. doi: 10.1227/01.neu.0000429841.84083.c8. [DOI] [PubMed] [Google Scholar]
- 126.Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson CE, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88:98–104. doi: 10.1159/000289354. [DOI] [PubMed] [Google Scholar]
- 127.Goto S, Shimazu H, Matsuzaki K, et al. Thalamic Vo-complex vs pallidal deep brain stimulation for focal hand dystonia. Neurology. 2008;70:1500–1501. doi: 10.1212/01.wnl.0000310430.00743.11. [DOI] [PubMed] [Google Scholar]
- 128.Fukaya C, Katayama Y, Kano T, et al. Thalamic deep brain stimulation for writer’s cramp. J Neurosurg. 2007;107:977–982. doi: 10.3171/JNS-07/11/0977. [DOI] [PubMed] [Google Scholar]
- 129.Gross R. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? NeuroRx. 2008;5:281–293. doi: 10.1016/j.nurt.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arce CA. Selective denervation in cervical dystonia. In: Stacey MA, editor. Handbook of dystonia. New York: Informa Healthcare USA, Inc; 2007. pp. 381–392. [Google Scholar]
- 131.Bertrand CM. Selective peripheral denervation for spasmodic torticollis: surgical technique, results, and observations in 260 cases. Surg Neurol. 1993;40:96–103. doi: 10.1016/0090-3019(93)90118-k. [DOI] [PubMed] [Google Scholar]
- 132.Munchau A, Palmer JD, Dressler D, et al. Prospective study of selective peripheral denervation for botulinum toxin resistant patients with cervical dystonia. Brain. 2001;124:769–783. doi: 10.1093/brain/124.4.769. [DOI] [PubMed] [Google Scholar]
- 133.Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, Swenson MA, McClelland RL, Davis DH. Selective peripheral deneration for the treatment of intractable spasmodic torticollis: experience with 168 patients at the Mayo clinic. J Neurosurg. 2003;98:1247–1254. doi: 10.3171/jns.2003.98.6.1247. [DOI] [PubMed] [Google Scholar]
- 134.Chen X, Ma A, Liang J, Ji S, Pei S. Selective denervation and resection of cervical muscles in the treatment of spasmodic torticollis: long-term follow-up results in 207 cases. Stereotact Funct Neurosurg. 2000;75:96–102. doi: 10.1159/000048389. [DOI] [PubMed] [Google Scholar]
- 135.Braun V, Richter HP. Selective peripheral denervation for spasmodic torticollis: 13 year experience with 155 patients. J Neurosurg. 2002;97:207–212. doi: 10.3171/spi.2002.97.2.0207. [DOI] [PubMed] [Google Scholar]
- 136.Georgescu D, Vagefi MR, McMullan TF, McCann JD, Anderson RL. Upper eyelid myectomy in blepharospasm with associated apraxia of lid opening. Am J Ophthalmol. 2008;145:541–547. doi: 10.1016/j.ajo.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 137.Grivet D, Robert PY, Thuret G, et al. Assessment of blepharospasm surgery using an improved disability scale: study of 138 patients. Ophthal Plast Reconstr Surg. 2005;21:230–234. doi: 10.1097/01.iop.0000162429.97307.4d. [DOI] [PubMed] [Google Scholar]
- 138.Pariseau B, Worley MW, Anderson RL. Myectomy for blepharospasm 2013. Curr Opin Ophthalmol. 2013;24:488–493. doi: 10.1097/ICU.0b013e3283645aee. [DOI] [PubMed] [Google Scholar]
- 139.Ludlow CL. Treatment for spasmodic dysphonia: limitations of current approaches. Curr Opin Otolaryngol Head Neck Surg. 2009;17:160–165. doi: 10.1097/MOO.0b013e32832aef6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ludlow CL, Adler CH, Berke GS, et al. Research priorities in spasmodic dysphonia. Oto Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]