Abstract
Mipu1 (myocardial ischemic preconditioning upregulated protein 1) is a novel N-terminal Kruppel-associated box (KRAB)/C2H2 zinc finger superfamily protein, that displays a powerful effect in protecting H9c2 cells from oxidative stress-induced cell apoptosis. The present study aims to investigate the effect of Mipu1 overexpression on oxidized low-density lipoprotein (oxLDL)-induced foam cell formation, cell apoptosis, and its possible mechanisms. New Zealand healthy rabbits were used to establish atherosclerosis model, and serum levels of triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were detected by an automatic biochemical analyzer. Sudan IV staining was used to detect atherosclerotic lesions. The RAW264.7 macrophage cell line was selected as the experimental material. Oil red O staining, high-performance liquid chromatography, and Dil-labeled lipoprotein were used to detect cholesterol accumulation qualitatively and quantitatively, respectively. Flow cytometry was used to determine cell apoptosis. Real-time quantitative polymerase chain reaction (PCR) was used to detect the mRNA expression of the main proteins that are associated with the transport of cholesterol, such as ABCA1, ABCG1, SR-BI, and CD36. Western blot analysis was used to detect the protein expression of Mipu1. There were atherosclerotic lesions in the high-fat diet group with Sudan IV staining. High-fat diet decreased Mipu1 expression and increased CD36 expression significantly at the 10th week compared with standard-diet rabbits. Mipu1 overexpression decreased oxLDL-induced cholesterol accumulation, oxLDL uptake, cell apoptosis, and cleaved caspase-3. Mipu1 overexpression inhibited the oxLDL-induced CD36 mRNA and protein expression, but it did not significantly inhibit the mRNA expression of ABCA1, ABCG1, and SR-BI. Mipu1 overexpression inhibits oxLDL-induced foam cell formation and cell apoptosis. Mipu1 overexpression reduces the lipid intake of macrophages and might be associated with the downregulation of CD36 expression in the presence of oxLDL.
Introduction
The migration of circulating monocytes into the subendothelial arterial space and their differentiation into macrophages are considered to be the essential steps of atherosclerosis (AS) development, which leads to rapid uptake of modified low-density lipoprotein (LDL) and subsequent foam cell formation. Foam cells are the characteristic pathological cells in atherosclerotic plaques and cholesterol accumulation is the most important reason for foam cell formation (Shashkin et al., 2005; Das et al., 2013). A variety of proteins are involved in cholesterol regulation. In particular, ATP-binding cassette transporters ABCA1 and ABCG1 play a pivotal role in cholesterol efflux from macrophage-derived foam cells, as they mediate the efflux of intracellular cholesterol and phospholipids to high-density lipoprotein (HDL) and apoA-I, respectively (Schmitz et al., 2001; Feng and Tabas, 2002; Jessup et al., 2006). In this way, cholesterol from peripheral cells is delivered to the liver for its conversion to bile acids (Rothblat et al., 1999). Cluster of differentiation 36 (CD36) plays an important role in the uptake of oxidized low-density lipoprotein (oxLDL) (Rahaman et al., 2006; Chen et al., 2008), which is a well-known risk factor for AS, as the capacities of oxLDL uptake and foam cell formation are both decreased significantly in CD36−/− mice (Febbraio et al., 1999). SR-BI another scavenger receptor, mediates physiologically relevant, selective cholesterol transport, and plays a key role in controlling plasma lipoprotein and biliary cholesterol concentrations and reverse cholesterol transport (RCT). It has been proved that SR-BI can protect against early-onset AS in SR-BI/apolipoprotein E double homozygous knockout mice (Trigatti et al., 1999; Connelly and Williams, 2004).
Mipu1 (myocardial ischemic preconditioning upregulated protein 1), a novel N-terminal Kruppel-associated box (KRAB)/C2H2 zinc finger superfamily protein, was identified because of its upregulation after myocardial ischemic preconditioning. It has an open reading frame of 1827 bp for encoding 608 amino acids with a KRAB domain and 14 C-terminal C2H2 zinc fingers (Yuan et al., 2004; Jiang et al., 2007). It has been proved that Mipul can inhibit the apoptosis of H9c2 induced by H2O2 and TNF-a, and repress the expression of apoptosis-related genes Fas and Bax (Jiang et al., 2007; Wang et al., 2009). Recently, Wang et al. (2009) reported that hypoxia-inducible factor-1α (HIF-1α) bound to the hypoxia response element within the Mipu1 promoter region and promoted its transcription, the cytoprotection of HIF-1 against H2O2-mediated injury in H9c2 cells partly through the regulation of Mipu1 expression (Wang et al., 2013). However, the role of Mipu1 overexpression on oxLDL-induced lipid accumulation in macrophages has not been elucidated.
In this study, our findings indicate that a high-fat diet downregulated the expression of Mipu1 in aortas of New Zealand healthy rabbits. Mipu1 overexpression modulated lipid uptake and cell apoptosis induced by oxLDL through downregulating CD36 expression.
Materials and Methods
Animals and diets
New Zealand rabbits were obtained from the Experimental Animal Center of Hunan University of Traditional Chinese Medicine, China. The 24 rabbits were maintained under controlled temperature (21°C–23°C), 12-h light–12-h dark cycle and free access to food and water, no restrictions on male or female. All experiments were performed in the experimental animal department of the University of South China, Hengyang, China (certificate number: SCXK2010-0004) according to the institutional and government guidelines and approved by the local council of ethics. The rabbits were randomly divided into two groups: control group and high-fat diet group. The control group was fed with a standard diet, whereas the high-fat diet group was fed with a standard diet containing 2% cholesterol. All the rabbits were fed for 10 weeks.
Metabolic profile analysis
At the end of the 10th week, the rabbits were kept in fasting for at least 8 h before being anesthetized by 10% chloral hydrate, and blood samples were then collected from the ear edge vein. Serum centrifuged from the blood samples was used to measure levels of plasma lipids such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) using an automatic biochemical analyzer (Thermo Electron Co.). Aortas were also collected and quickly frozen in liquid nitrogen, and then stored at −80°C for later analysis.
Detection of atherosclerotic lesion area
For macroscopic analysis of plaque extension, the adventitia was removed from the aortas and fixed overnight in 10% paraformaldehyde. Then the fixed aortas were stained with Sudan IV, mounted, and then photographed by a digital camera.
Generation of stably transfected RAW264.7-vector and RAW264.7-Mipu1 cells
The RAW264.7 macrophages were obtained from the Type Culture Collection of the Chinese Academy of Sciences. The RAW264.7 cells were transfected with pcDNA3.1-Mipu1 or pcDNA3.1-vector (a kind gift of Dr. Xianzhong Xiao, Central South University, Changsha, China) using Lipofectamine® 2000 Transfection Reagent (Invitrogen), following the manufacturer's instructions. Stably transfected RAW264.7-vector and RAW264.7-Mipu1 cells were selected in the culture medium, supplemented with 500 μg/mL geneticin (G418; Invitrogen). RAW264.7 cells were maintained in DMEM (HyClone) supplemented with 10% fetal bovine serum and 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C under 5% CO2.
Mipu1 expression through western blot analysis
Aortic tissues were ground on ice with 300 μL RIPA buffer per 20 mg, and then centrifuged at 10,000 g for 15 min to obtain the aortic protein. RAW264.7-vector and RAW264.7-Mipu1 cells were washed in cold phosphate-buffered saline (PBS) and lysed in RIPA buffer for 30 min at 4°C. SDS-PAGE immunoblotting was used to quantify the expression of Mipu1 (Jiang et al., 2009) and GAPDH (Cell Signaling Technology). Electrophoresis and immunoblotting were employed as described previously (Qu et al., 2010).
Lipids uptake assay
Three different methods were used to detect the uptake of lipids by macrophages. In the first assay, after treatment with oxLDL (all of the oxLDL were commercial, the TBARS content was ranging from 5 to 15 nmol MDA/mg cholesterol), the culture medium was removed, and cells were washed four times with PBS, and fixed with 4% paraformaldehyde for 20 min. Fixed cells were rinsed with PBS and stained with fresh Oil red O (Sigma) working solution (0.5% Oil red O in isopropanol:dH2O 6:4) for 30 min. The nuclei were lightly stained with hematoxylin for 5 min. Stained cells were observed using an inverted microscope. For the second method, RAW264.7 cells were washed with PBS for three times after treatment with oxLDL. Protein content was determined by the BCA kit. Proteins were precipitated with 6% trichloroacetic acid, and neutral lipids were extracted with a mixture of hexane/isopropanol (v:v 4:1). The organic phase containing the lipids was collected in a vacuum freeze drying plant at 65°C and the residues were redissolved in 0.1 ml mixture of isopropanol/n-heptane/acetonitrile (v:v35:12:52). The mixture was centrifuged at 1500 rpm for 5 min. Cholesterol ester content was analyzed by high-performance liquid chromatography (Ma et al., 2009). Briefly, the chromatographer (PerkinElmer, Inc.) system included a PerkinElmer series 600 LINK, a PerkinElmer series 200 vacuum degasser, a PerkinElmer series 200 UV/visible detector, a pump, and a reverse-phase column. Ten microliters of supernatants were injected into the column and eluted with isopropanol/n-heptane/acetonitrile (v:v, 35:12:52) at a flow rate of 1 mL/min. Absorbance at 216 nm was monitored. The products were identified by comparing the retention times and peak areas with authentic external standards. For the third method, oxLDL (v/v, 4:1) was labeled with Dil overnight at 37°C. Seventy-five micrograms Dil-oxLDL was incubated with RAW264.7 cells for 24 h at 37°C; then cells were washed three times with PBS, and then lysed in lysis buffer. The protein concentration was detected by the BCA assay. Dil fluorescence was detected following excitation at 520 nm and emission at 590 nm using a 590 nm cut-off filter (Gemini XS microplate fluorometer). A fluorescence standard curve was prepared by diluting 0, 1, 2, 4, 8, 16, and 32 μg Dil-oxLDL in lysis buffer. Results were normalized to total cell protein concentration.
Apoptosis detection by FACS
The apoptosis of RAW264.7 cells was detected by FACS analysis with the Annexin-V staining (BD Biosciences). RAW264.7 cells were incubated with 75 μg/mL oxLDL for 24 h. The cells were then collected and incubated with PE-Annexin-V, cells were resuspended in PBS (2% paraformaldehyde), and detected by FACS. The percentage of PE-Annexin-V positive cells was quantified as the degree of apoptosis.
Caspase-3 activity assay
After incubation with 75 μg/mL oxLDL for 24 h, cells were then lysed with passive lysis buffer (Promega) and the clear supernatant was used for caspase-3 activity assay after centrifugation according to the manufacturer's instructions. The protein concentration of the lysate was detected by the BCA kit and the relative caspase-3 activities were normalized to protein concentration.
Quantitative real-time reverse transcription PCR
Total RNA was extracted, and cDNA fragments were generated by reverse transcription. A total of 100 ng of cDNA was used in the real-time PCR to measure mRNA expression, whereas 10 ng of cDNA was used in the β-actin control PCR. The primer sequences used for the amplification were designed using the Primer 5.0 software and were evaluated using the Oligo 5.0 software. The primer sequences (mouse) for ABCG-1 were: forward 5′-GGGTCTGAACTGCCCTACCT-3′, reverse 5′-TACTCC CCTGATGCCACTTC-3′ (NM_009593.2, 66 bp); for ABCA1: forward 5′-C CCCACTTTTTTCTTCCCCTTTC-3′, reverse 5′-GACACGAGACCGACGAG GGAG-3′ (NM_013454.3, 128 bp); for CD36: forward 5′-GAACCTATTGAAGGCTT ACATCC-3, reverse 5′-CCCA GTCACTTGTGTTTTGAAC-3′ (NM_001159557.1, 246 bp); for SR-BI: forward 5′-TTTGGAGTGGTAGTAAAAAG GGC-3′, reverse 5′-TGACATCAGGGACTCAGAGTAG-3′ (NM_001205083.1, 72 bp); for β-actin: forward 5′-TGGCCGGGACCTGACAGACTA-3′, reverse 5′-ATCTGAAGCTCGTC CTCTACCGG-3′ (NM_007393.3, 142 bp). SYBR Green I fluorescence dye (Takara Biotechnology) was used to bind specifically to the minor groove of double-stranded DNA. All reactions followed the typical sigmoidal reaction profile, and cycle threshold was used as a measurement of amplicon abundance as described previously (Zhu et al., 2011).
Statistical analyses
The results are expressed as mean±SEM. Statistical analyses were performed with the Graphpad Prism V.5 software using the Student's t-test. Significance was taken as p<0.05.
Results
Change of Mipu1 and CD36 protein expression in thoracic aorta
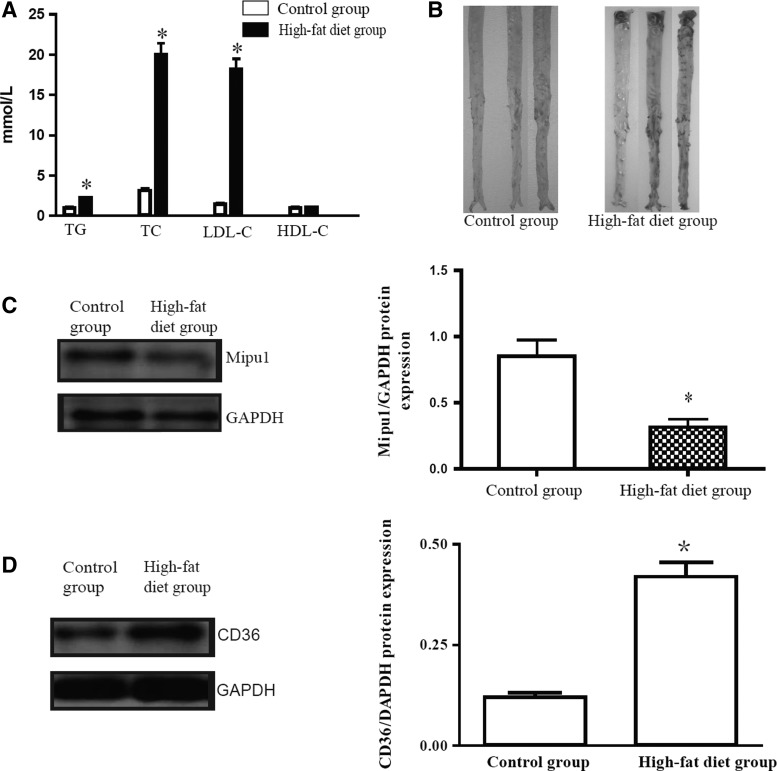
Circulating levels of TC, TG, and LDL-C were remarkably increased in the high-fat diet group of New Zealand rabbits relative to those in the standard-diet fed group, and no difference of serum HDL-C concentration was observed between these two groups p>0.05 (Fig. 1A). Gross examination of the aorta revealed atherosclerotic lesion in the high-fat diet group with Sudan IV staining (Fig. 1B). As shown in Figure 1C, the high-fat diet decreased Mipu1 and increased CD36 expression significantly at the 10th week compared with the standard-diet rabbits p<0.05 (Fig. 1D).
FIG. 1.
Change of Mipu1 (myocardial ischemic preconditioning upregulated protein 1) and CD36 expression in high-fat diet induced atherosclerosis in rabbits. (A) Plasma levels of total triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) after 10 weeks of high-fat diet feeding. (B) Plaque quantification of thoracic aorta stained with Sudan IV. Protein expression of Mipu1 (C) and CD36 (D) in the aortic tissue (n=4 each), values are mean±SEM, *p<0.05 versus control group.
Mipu1 overexpression inhibits oxLDL-induced lipid accumulation in macrophages
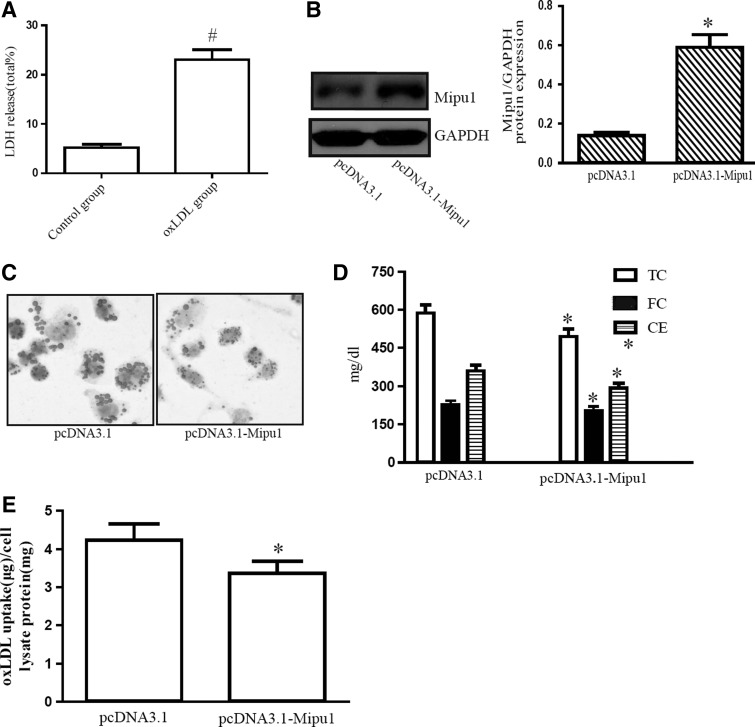
We overexpressed pcDNA3.1-Mipu1 in RAW264.7 cells (Fig. 2A), and used pcDNA3.1 as a control. Since uptake of oxLDL is the event that triggers the formation of lipid-laden foam cells, we examined the effect of Mipu1 overexpression on lipid accumulation in macrophages. The Oil red O staining (Fig. 2B) and intracellular TC quantitative assay (Fig. 2C) indicated that macrophage lipid content in the presence of oxLDL was significantly decreased in cells transfected with pcDNA3.1-Mipu1 compared with those transfected with pcDNA3.1. To further investigate the role of Mipu1 overexpression in lipid accumulation in RAW264.7 cells, we detected the effect of Mipu1 overexpression on oxLDL uptake by macrophages. As shown in Figure 2D, Mipu1 overexpression inhibited the uptake of oxLDL in macrophages. These data suggested that Mipu1 overexpression inhibited the oxLDL-induced lipid accumulation in macrophages.
FIG. 2.
Mipu1 overexpression inhibits oxLDL-induced lipid accumulation in macrophages. (A) LDH release in oxidized low-density lipoprotein (oxLDL)-treated group (75 μg/mL of oxLDL for 24 h) was 23.4%±3.1% (relative to total LDH release), and control group (no oxLDL treatment) was 5.0%±0.7%. (B) Expression of Mipu1 protein in RAW264.7 cells transfected with pcDNA3.1-Mipu1 was analyzed by western blot. (C) RAW264.7-vector and RAW264.7-Mipu1 cells were treated with 75 μg/mL oxLDL for 24 h, and the extent of lipid loading was assessed by Oil red O staining. (D) Under the same conditions as in (C), the intracellular cholesterol content was measured through high-performance liquid chromatography. (E) Dil-oxLDL fluorescence intensity in cells incubated with Dil-labeled oxLDL (75 μg/mL) for 24 h. Data are presented as the mean±SEM of at least four independent experiments. #p<0.05 versus control group; *p<0.05 versus pcDNA3.1 group.
Mipu1 overexpression attenuated oxLDL-induced apoptosis
Overexpression of Mipu1 significantly attenuated oxLDL-induced RAW264.7 cell apoptosis (PE-Annexin-V positive) (p<0.05, n=4) (Fig. 3A). Mipu1 overexpression also significantly reduced caspase-3 activities (Fig. 3B).
FIG. 3.
Mipu1 overexpression inhibited apoptosis of RAW264.7 cells. RAW264.7-vector and RAW264.7-Mipu1 cells were treated with 75 μg/mL oxLDL for 24 h. (A) FACS analysis with PE-Annexin-V staining revealed that Mipu1 overexpression inhibited apoptosis. (B) Caspase-3 activities were measured as described in methods. Data are presented as the mean±SEM of at least four independent experiments. *p<0.05 versus pcDNA3.1 group.
Mipu1 overexpression reduced CD36 expression in the presence of oxLDL
It is known that many key genes are involved in the efflux and uptake of cholesterol by macrophages, and we determined whether Mipu1 overexpression modulates mRNA expression of ABCA1, ABCG1, SR-BI, and CD36 by real-time PCR in RAW264.7 cells. The mRNA levels of ABCA1, ABCG1, SR-BI, and CD36 were similar in RAW264.7-vector versus RAW264.7-Mipu1 cells in the untreated group (Fig. 4). Compared with the control group, the CD36 mRNA/protein expression and ABCA1 mRNA expression were both increased in the oxLDL-treated group (p<0.05), whereas the mRNA expression of SRB1 and ABCG1 showed no significant change. Mipu1 overexpression inhibited the oxLDL-induced expression of CD36 mRNA and protein, whereas the expression of ABCA1 mRNA showed no significant change.
FIG. 4.
Mipu1 overexpression inhibited CD36 expression in the presence of oxLDL. RAW264.7-vector and RAW264.7-Mipu1 cells were incubated with 75 μg/mL oxLDL for 24 h and the amount of mRNA encoding ABCA1, ABCG1, SR-BI, and CD36 was measured by quantitative PCR (A–D) (data show mean fold change relative to untreated RAW264.7-vector±SEM from four independent experiments). (E) Protein expression of CD36 was detected by western blot after oxLDL treatment. #p<0.05 versus untreated group, $p<0.05 versus RAW264.7-vector group.
Discussion
There is evidence showed that hyperglycemia and hypertriglyceridemia have direct effects on the arterial wall and induced endothelial dysfunction (Arcaro et al., 2002). The elevation of TC, TG, and LDL-C was observed in high-fat diet rabbits in our study (Fig. 1A), and there were atherosclerotic lesions in the high-fat diet group and the Mipu1 expression in aortic tissues was much lower in the high-fat diet group (Fig. 1B, C). At the same time, we and others (Yao et al., 2014) demonstrated that CD36 expression in aortic tissues was increased significantly in the high-fat diet group. It has been suggested that CD36 plays an important role in foam cell formation and progression of AS. However, the precise mechanism underlying oxLDL-induced CD36 upregulation has not been completely clarified. Our results in the present study suggested that the capacity of Mipu1 in participating in the process of lipid accumulation might be related to regulation of CD36 expression.
Foam cell formation is a hallmark of the early stages of AS and plays an important role in plaque progression and instability (Fogelman et al., 1980; Boyle et al., 2012). Increasing evidences have indicated that the foam cells found in the atherosclerotic reaction are macrophages that are derived from blood-borne monocytes or smooth muscle cells. The hallmark of these foam cells is that the cholesteryl ester content is over 50% of the total intracellular cholesterol (Fogelman et al., 1980; Boyle et al., 2012). RAW264.7 macrophages are easy to culture and have strong phagocytic and adhesive abilities, so it has been widely used in studies of the pathways of foam cell formation (Kuchibhotla et al., 2008; Steinbusch et al., 2011). In this study, macrophages meet the typical characteristic of foam cells after treatment with 75 μg/mL oxLDL for 24 h. To determine the role of Mipu1 overexpression in foam cell formation, a RAW264.7 cell line, stably expressing Mipu1, was generated. With the use of this cell line, we detected the change of intracellular cholesterol. In this study, we showed that Mipu1 overexpression not only decreased uptake of oxLDL, but also synergistically reduced the oxLDL-induced intracellular cholesterol accumulation and inhibited foam cell formation. Until now, no study has reported the correlation between Mipu1 and AS. Our study is the first to discoverer that Mipu1 overexpression inhibited the formation of foam cells.
Both smooth muscle cells and macrophages undergo apoptosis in atherosclerotic plaques (Kockx and Herman, 2000). Macrophage cell apoptosis promotes the development of the necrotic core, which is a key factor in rendering plaques vulnerable to disruption and acute luminal thrombosis (Seimon and Tabas, 2009). Caspase-3 is a key protein in apoptotic pathways (Hutter et al., 2004). Our study proved that overexpression of Mipu1 inhibited the oxLDL-induced RAW264.7 cell apoptosis, as well as the amount of activated caspase-3.
Assessment of foam cell formation evidenced an additional, new role for Mipu1 overexpression by enhancing macrophage lipid storage. Increased cell cholesterol accumulation could be a result of an increased uptake, decreased efflux, or both. Therefore, the efflux of cholesterol was the next factor evaluated as a potential mechanism involved in the macrophage accumulation of lipids induced by Mipu1. Cholesterol efflux from macrophages is mainly directed by members of the ABC transporter family, such as ABCG1 and ABCA1, through active RCT, in a process that requires the presence of apoA-I or HDL as cholesterol acceptors (Neculai et al., 2013; Fu et al., 2013; Daniil et al., 2013). Real-time PCR experiments indicated that the mRNA levels of the transporter ABCG1 and ABCA1 were not changed significantly in Mipu1-expressing RAW264.7 cells in the presence of oxLDL. Thus, overall, Mipu1 does not seem to play a major role in cholesterol efflux. The uptake of oxLDL leading to focal lipid accumulation in foam cells is intimately involved in the formation of early fatty streak lesions, as well as in the evolution of more complex atherosclerotic plaques (Ashraf and Gupta, 2011; Hutter et al., 2004; Amezaga et al., 2014). In fact, our findings suggest that decreased foam cell formation by Mipu1 overexpression is mainly a result of decreased lipid uptake. Extensive evidence points to a significant role of CD36, the major macrophage scavenger receptor responsible for oxLDL, in the progression of AS and suggests it could be an important target for therapeutic treatment (Febbraio et al., 2000; Kuchibhotla et al., 2008; Ashraf and Gupta, 2011; Steinbusch et al., 2011; Neculai et al., 2013), so we hypothesized that Mipu1 overexpression may be decreasing oxLDL uptake by modulating CD36 expression and/or activity. Indeed, Mipu1 expression induced the downregulation of CD36 mRNA/protein levels in RAW264.7-Mipu1 cells versus RAW264.7-vector cells upon oxLDL treatment. These data suggested that Mipu1 overexpression may inhibit the oxLDL-induced lipid accumulation in macrophages and might be associated with modulation of CD36 expression. It is proposed that there are also other mechanisms in addition to the decrease of CD36 expression that are responsible for the inhibition of lipid accumulation by Mipu1 overexpression.
In summary, our results support the notion that Mipu1 is an anti-atherogenic protein that participates in multiple events of macrophage homeostasis, including macrophage survival, oxLDL uptake, and foam cell formation, which might be associated with the downregulation of CD36 expression. Our findings are of relevance for the understanding of Mipu1 that contribute to the prevention of AS.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81100212, 81170277 and 81100106), the Ph.D. Programs Foundation of Ministry of Education of China (20114324120004 and 20124324110003), the China Postdoctoral Science Foundation (2012M511383), the Scientific Research Fund of Hunan Provincial Education Department (11C1094 and 11C1095), the Science and Technology Project of Hunan Province (2014FJ3014), the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province (2008-244) and the Construct Program of the Key Discipline in Human Province (2011-76).
Disclosure Statement
No competing financial interests exist.
References
- Amezaga N., Sanjurjo L., Julve J., Aran G., Perez-Cabezas B., Bastos-Amador P., Armengol C., Vilella R., Escola-Gil J.C., Blanco-Vaca F., et al. (2014). Human scavenger protein AIM increases foam cell formation and CD36-mediated oxLDL uptake. J Leukoc Biol 95,509–520 [DOI] [PubMed] [Google Scholar]
- Arcaro G., Cretti A., Balzano S., Lechi A., Muggeo M., Bonora E., and Bonadonna R.C. (2002). Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 105,576–582 [DOI] [PubMed] [Google Scholar]
- Ashraf M.Z., and Gupta N. (2011). Scavenger receptors: implications in atherothrombotic disorders. Int J Biochem Cell Biol 43,697–700 [DOI] [PubMed] [Google Scholar]
- Boyle J.J., Johns M., Kampfer T., Nguyen A.T., Game L., Schaer D.J., Mason J.C., and Haskard D.O. (2012). Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 110,20–33 [DOI] [PubMed] [Google Scholar]
- Chen K., Febbraio M., Li W., and Silverstein R.L. (2008). A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 102,1512–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M.A., and Williams D.L. (2004). Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Curr Opin Lipidol 15,287–295 [DOI] [PubMed] [Google Scholar]
- Daniil G., Zannis V.I., and Chroni A. (2013). Effect of apoA-I Mutations in the Capacity of Reconstituted HDL to Promote ABCG1-Mediated Cholesterol Efflux. PLoS One 8,e67993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Ganapathy S., Mahabeleshwar G.H., Drumm C., Febbraio M., Jain M.K., and Plow E.F. (2013). Macrophage gene expression and foam cell formation are regulated by plasminogen. Circulation 127,1209–1218, e1201–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M., Abumrad N.A., Hajjar D.P., Sharma K., Cheng W., Pearce S.F., and Silverstein R.L. (1999). A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274,19055–19062 [DOI] [PubMed] [Google Scholar]
- Febbraio M., Podrez E.A., Smith J.D., Hajjar D.P., Hazen S.L., Hoff H.F., Sharma K., and Silverstein R.L. (2000). Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105,1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., and Tabas I. (2002). ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full NPC1 activity. J Biol Chem 277,43271–43280 [DOI] [PubMed] [Google Scholar]
- Fogelman A.M., Shechter I., Seager J., Hokom M., Child J.S., and Edwards P.A. (1980). Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A 77,2214–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Mukhamedova N., Ip S., D'Souza W., Henley K.J., DiTommaso T., Kesani R., Ditiatkovski M., Jones L., Lane R.M., et al. (2013). ABCA12 regulates ABCA1–dependent cholesterol efflux from macrophages and the development of atherosclerosis. Cell Metab 18,225–238 [DOI] [PubMed] [Google Scholar]
- Hutter R., Valdiviezo C., Sauter B.V., Savontaus M., Chereshnev I., Carrick F.E., Bauriedel G., Luderitz B., Fallon J.T., Fuster V., et al. (2004). Caspase-3 and tissue factor expression in lipid-rich plaque macrophages: evidence for apoptosis as link between inflammation and atherothrombosis. Circulation 109,2001–2008 [DOI] [PubMed] [Google Scholar]
- Jessup W., Gelissen I.C., Gaus K., and Kritharides L. (2006). Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol 17,247–257 [DOI] [PubMed] [Google Scholar]
- Jiang L., Tang D., Wang K., Zhang H., Yuan C., Duan D., and Xiao X. (2007). Functional analysis of a novel KRAB/C2H2 zinc finger protein Mipu1. Biochem Biophys Res Commun 356,829–835 [DOI] [PubMed] [Google Scholar]
- Jiang L., Zhang B., Wang G., Wang K., and Xiao X. (2009). Expression, purification and characterization of rat zinc finger protein Mipu1 in Escherichia coli. Mol Cell Biochem 328,137–144 [DOI] [PubMed] [Google Scholar]
- Kockx M.M., and Herman A.G. (2000). Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res 45,736–746 [DOI] [PubMed] [Google Scholar]
- Kuchibhotla S., Vanegas D., Kennedy D.J., Guy E., Nimako G., Morton R.E., and Febbraio M. (2008). Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res 78,185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Hu Y.W., Mo Z.C., Li X.X., Liu X.H., Xiao J., Yin W.D., Liao D.F., C., and Tang K. (2009). NO-1886 up-regulates Niemann-Pick C1 protein (NPC1) expression through liver X receptor alpha signaling pathway in THP-1 macrophage-derived foam cells. Cardiovasc Drugs Ther 23,199–206 [DOI] [PubMed] [Google Scholar]
- Neculai D., Schwake M., Ravichandran M., Zunke F., Collins R.F., Peters J., Neculai M., Plumb J., Loppnau P., Pizarro J.C., Seitova A., Trimble W.S., Saftig P., Grinstein S., and Dhe-Paganon S. (2013). Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 504,172–176 [DOI] [PubMed] [Google Scholar]
- Qu S., Zhu H., Wei X., Zhang C., Jiang L., Liu Y., Luo Q., and Xiao X. (2010). Oxidative stress-mediated up-regulation of myocardial ischemic preconditioning up-regulated protein 1 gene expression in H9c2 cardiomyocytes is regulated by cyclic AMP-response element binding protein. Free Radic Biol Med 49,580–586 [DOI] [PubMed] [Google Scholar]
- Rahaman S.O., Lennon D.J., Febbraio M., Podrez E.A., Hazen S.L., and Silverstein R.L. (2006). A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab 4,211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblat G.H., de la Llera-Moya M., Atger V., Kellner-Weibel G., Williams D.L., and Phillips M.C. (1999). Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res 40,781–796 [PubMed] [Google Scholar]
- Schmitz G., Langmann T., and Heimerl S. (2001). Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res 42,1513–1520 [PubMed] [Google Scholar]
- Seimon T., and Tabas I. (2009). Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res 50,SupplS382–S387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkin P., Dragulev B., and Ley K. (2005). Macrophage differentiation to foam cells. Curr Pharm Des 11,3061–3072 [DOI] [PubMed] [Google Scholar]
- Steinbusch L.K., Luiken J.J., Vlasblom R., Chabowski A., Hoebers N.T., Coumans W.A., Vroegrijk I.O., Voshol P.J., Ouwens D.M., Glatz J.F., and Diamant M. (2011). Absence of fatty acid transporter CD36 protects against Western-type diet-related cardiac dysfunction following pressure overload in mice. Am J Physiol Endocrinol Metab 301,E618–E627 [DOI] [PubMed] [Google Scholar]
- Trigatti B., Rayburn H., Vinals M., Braun A., Miettinen H., Penman M., Hertz M., Schrenzel M., Amigo L., Rigotti A., and Krieger M. (1999). Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A 96,9322–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zuo X., Liu J., Jiang L., Liu Y., Zheng Y., Zhang B., and Xiao X. (2009). Expression of Mipu1 in response to myocardial infarction in rats. Int J Mol Sci 10,492–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Lei J., Zou J., Xiao H., Chen A., Liu X., Liu Y., Jiang L., Xiao Z., and Xiao X. (2013). Mipu1, a novel direct target gene, is involved in hypoxia inducible factor 1-mediated cytoprotection. PLoS One 8,e82827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S., Miao C., Tian H., Sang H., Yang N., Jiao P., Han J., Zong C., and Qin S. (2014). Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J Biol Chem 289,4032–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Zhang H., Liu Y., Wang Q., and Xiao X. (2004). Cloning and characterization of a new gene Mip1 up-regulated during myocardial ischemia-reperfusion. Prog Biochem Biophys 31,231–236 [Google Scholar]
- Zhu H.L., Wei X., Qu S.L., Zhang C., Zuo X.X., Feng Y.S., Luo Q., Chen G.W., Liu M.D., Jiang L., Xiao X.Z., and Wang K.K. (2011). Ischemic postconditioning protects cardiomyocytes against ischemia/reperfusion injury by inducing MIP2. Exp Mol Med 43,437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]