Abstract
The roles of human serum IgA, in contrast to that of mucosal IgA, are relatively unexplored. Previous studies have shown that IgA mediates either pro- or anti-inflammatory effects in innate immune cells. Serum IgA has been shown to interact with many proteins and glycoproteins of which the functions and mechanisms are not fully characterized. Here, we present fresh perspectives into the roles of serum IgA, describing novel IgA–protein interactions, the importance of its glycosylation status in normal functions, and the plausible role of IgA as a driver and regulator of autoimmune diseases/immune overactivation. Other potential roles, including the regulation of cytokines, effector cell function, and homeostasis, are considered in view of the maintenance of immune function. We anticipate future research to uncover new anti-inflammatory or pro-inflammatory roles of human serum IgA in immune functions and dysfunctions, with implications on systemic lupus erythematosus (SLE).
Introduction
Humans produce two major forms of IgA, the monomeric serum IgA and the dimeric mucosal secretory IgA. The total amount of IgA produced far exceeds the combined total of all other antibody classes (Kerr, 1990). Monomeric serum IgA is relatively abundant, second to IgG, the most prevalent circulating antibody (Mestecky et al., 1986). Monomeric serum IgA is produced by plasma cells in the bone marrow, marginal zone B cells, and B1 cells. However, the existence of B1 cells in humans remains controversial (Kerr, 1990; Cerutti et al., 2013; Tangye, 2013). In contrast, the dimeric form of IgA is found mainly in secretions lining the mucosal surfaces, for example, in the gastrointestinal tract, the reproductive tracts, the respiratory epithelium, tears, saliva, and colostrum (Mestecky, 2005). Plasma cells present in the lamina propria of the mucosal surfaces produce polymeric IgA (pIgA), which are monomeric IgA antibodies joined together at the Fc region by a polypeptide called the J chain. pIgA then binds the polymeric immunoglobulin receptor (pIgR) present on the basolateral membrane of epithelial cells and is transported across the epithelium and onto the mucosal surfaces. The pIgR is cleaved to release the pIgA bound by a glycoprotein called the secretory component (SC) derived from the pIgR (Pabst, 2012). This form of dimeric IgA is termed the secretory IgA (sIgA). sIgA performs a plethora of functions, including immune exclusion of pathogens, toxins, or commensal bacteria from crossing the epithelial layer; neutralization of intracellular pathogens; antigen secretion; homeostasis of commensals, and downregulation of pro-inflammatory responses (Corthesy, 2013). Hence, sIgA mainly functions to prevent the invasion of pathogenic and commensal bacteria across the mucosal epithelial layer, thus preventing systemic infection while simultaneously maintaining a physiologically indispensable symbiotic relationship with commensal bacteria.
Hitherto, most of the research on IgA has focused on the mucosa. Literature that expound solely on serum IgA pales in comparison to that on mucosal IgA. Serum IgA receives cursory mention in most texts, before the focus shifts onto the more “important” roles of sIgA in mucosal immunity. This clearly indicates a lack of conclusive evidence or a consensus on functions of serum IgA. The noninflammatory functions of mucosal IgA probably influenced the perception on serum IgA as a neutralizing antibody with poor complement activating and opsonising ability. Pioneer studies into serum IgA largely agreed with this view. Serum IgA has the ability to downregulate the phagocytic ability of polymorphonuclear leukocytes (PMNs) (Wilton, 1978; Nikolova and Russell, 1995). It downregulates pro-inflammatory cytokines or upregulates anti-inflammatory cytokines released by peripheral blood mononuclear cells (PBMCs) (Wolf et al., 1994, 1996; Olas et al., 2005).
Serum from IgA myeloma patients was shown to inhibit chemotaxis and bactericidal activities of PMNs (Van Epps and Williams, 1976; Van Epps et al., 1978). Subsequent studies revealed that this anti-inflammatory function was due to monomeric binding of serum IgA to the Fc alpha receptor (FcαRI), transmitting inhibitory signals through myeloid cells (e.g., monocytes, macrophages, dendritic cells, Kupffer cells, neutrophils, and eosinophils). However, in these studies, it was also found that crosslinking of the FcαRI resulting from IgA binding to pathogens or immune complex formation transmits activating signals leading to phagocytosis, respiratory burst, antibody-dependent cytotoxicity, increased antigen presentation, degranulation, and cytokine release by the abovementioned immune cells (Monteiro, 2010). It was suggested that inhibitory signals occur in the normal physiological state, when IgA titres are lower. During pathogenic infection, IgA binding to its antigen induces crosslinking of FcαRI, which activates immune effector cells to carry out their effector functions (Bakema and van Egmond, 2011). This explains the findings reported by van Egmond et al. (2000), where serum IgA-opsonised bacteria enabled clearance of the pathogen and resolution of the infection by liver Kupffer cells.
Other than binding the FcαRI, IgA has also been found to interact with the Fcα/μ receptors, asialoglycoprotein receptors (ASGP-R), transferrin receptors (CD71), SC receptors, and M-cell receptors. These interactions occur through binding of the IgA Fc region, the glycan chains, the J chain, or SC. The functional implications of these interactions (Stockert et al., 1982; Mostov, 1994; Lamkhioued et al., 1995; Shibuya et al., 2000; Moura et al., 2001; Mantis et al., 2002; Bakema and van Egmond, 2011) have yet to be fully understood and warrant further research. Clearly, the view of serum IgA as a quiescent player in immunity is beginning to change and many more potential functions remain to be ascribed to serum IgA. A comprehensive examination of IgA function is beyond the scope of this article. Instead, here we convey novel perspectives on the potential emerging roles of serum IgA.
IgA Interacts with Other Serum Components
The C-terminal cysteine residue of IgA contains a reactive thiol group that allows covalent bonding with other serum proteins. IgA has been shown to bind through this manner to albumin, α1-antitrypsin, HC-protein, and fibronectin (Kerr, 1990). The IgA–α1-antitrypsin complex inhibits leukocyte elastase, while the IgA–HC complex inhibits directed chemotaxis of neutrophils, suggesting that certain serum IgA complexes can play an anti-inflammatory role (Mendez et al., 1986; Dawes et al., 1987). IgA–fibronectin complexes are present in some individuals suffering primary IgA nephropathy (Kerr, 1990). On the other hand, the bacteriostatic activity of secretory IgA (sIgA) in milk and colostrum has been shown to be increased with the presence of iron-carrier proteins, such as lactoferrin and transferrin, with some sIgA and lactoferrin being covalently bound together (Funakoshi et al., 1982; Dolby and Stephens, 1983; Watanabe et al., 1984). Pathogen-specific serum IgA may bind complement components to enhance chemotaxis or phagocytosis of immune cells. Possessing a reactive thiol group is deemed to confer IgA (both serum IgA and sIgA) with the potential to bind serum components, although some of the complexes may not necessarily be covalent in nature. In fact, it is conceivable that noncovalent interactions between serum IgA and other serum proteins could be more functionally meaningful.
Serum IgA Is a Potential Regulator of Immune Complex Formation and Immune Overactivation
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease in which auto-antibodies produced are directed against self-antigens (e.g., nuclear components), causing widespread immune complex deposition in various tissues in the body (Munoz et al., 2005). IgG has been widely reported as the main player in such immune complexes (Kotzin, 1997; Boes et al., 2000). Patients with SLE appear to suffer from chronic infection-inflammation condition (lowered serum pH levels) and altered levels of serum proteins, such as ficolins, and elevated levels of IgG and IgM (Boes et al., 2000; Mathsson et al., 2007). In addition, SLE patients exhibit poor clearance of immune complexes due to deficient serum complement components, such as C1, C2, and C4 (Truedsson et al., 2007).
Persistent immune complexes induce widespread cytokine release by immune cells, causing a chronic inflammatory condition (Mathsson et al., 2007). Such a condition exacerbates immune complex formation, setting up a vicious cycle that drives continuation of the disease state. The cause of SLE is multifactorial, involving genetics and the environment. It is hard to pin down a single factor as the major cause (Munoz et al., 2005). Regardless of the cause of the disease, certain homeostatic factors would have been lost to allow the disease to progress. A recent discovery that IgG binds ficolin-associated bacteria (Panda et al., 2013) motivated us to make a preliminary study of the immune complexes formed in SLE serum—to examine whether the H-ficolin and IgG are the molecular drivers of immune complex formation, and to tease out which regulatory homeostatic factors may be missing. Studying the immune complexes formed would also allow us to gain an insight on whether IgA is part of such immune complexes, and its potential role(s) in interaction with other serum proteins.
Preliminary studies (Leong et al., 2014) on SLE serum, compared with normal healthy control serum, revealed that the pH was consistently lower, and that IgG levels are elevated in the SLE serum, which agree with earlier findings (Boes et al., 2000; Mathsson et al., 2007). On the other hand, the IgA levels appeared to be lower. Immune complexes in SLE were composed of IgG, mannose binding lectin, H-ficolin, and C-reactive protein whereas, in normal human serum, only IgG and H-ficolin were found in complex but were substantially lower than SLE serum. Interestingly, serum IgA was not found to be part of such immune complexes. These observations indicate that although IgA is present, although in relatively lower amounts in SLE serum, it does not participate in immune complexes, or at best probably forms unstable complexes under infection-inflammation conditions. Conceivably, serum IgA could act as one of the regulatory factors downregulated in SLE and its downregulation may contribute to the exacerbation of SLE. This provoked a further in vitro analysis of the interaction between IgA and H-ficolin in comparison with the more well-known IgG complex formation (Panda et al., 2013, 2014). Comparison of the binding interaction between IgA–H-ficolin and IgG–H-ficolin (under normal or infection-inflammation conditions) showed that IgA had much higher affinity for H-ficolin under normal conditions (14-fold higher affinity compared with IgG–H-ficolin) but a drastically weaker affinity under infection-inflammation conditions (74-fold weaker affinity compared with IgG–H-ficolin). These preliminary observations suggest a potential reciprocal functional relationship between IgG and IgA, perhaps in competition for ficolin, to regulate immune complex formation.
In consideration of greater affinity of IgA for H-ficolin under normal condition, the native serum IgA plausibly competes against IgG for H-ficolin to pre-empt IgG–ficolin immune complex formation and thereby maintains homeostasis. Under infection-inflammation conditions, IgA probably relinquishes its binding to H-ficolin (mechanism unknown), giving way to a greatly increased affinity of IgG for H-ficolin, which would result in the formation of IgG–ficolin complexes that facilitate effective opsonisation and phagocytosis of pathogens (Panda et al., 2013).
At this juncture, it is worth considering selective IgA deficiency (sIgAD). Individuals with sIgAD are normally asymptomatic, although they show increased susceptibility to sinopulmonary and gastrointestinal infections. This has been mainly attributed to compensatory increase in other components, such as secretory IgM (IgM joined by the SC), at the mucosal surfaces or the increase in serum antibodies of other isotypes (Pereira et al., 1997; Cunningham-Rundles, 2001; Saghafi et al., 2008). These individuals also show increased susceptibility to autoimmune diseases (e.g., SLE and rheumatoid arthritis) and allergies, such as allergic conjunctivitis, rhinitis, urticarial, atopic eczema, food allergy, and bronchial asthma (Cunningham-Rundles, 2001; Wang et al., 2011). Furthermore, such individuals possess auto-antibodies (30% harbor IgG antibodies directed against IgA), and deficiency in factors mediating class switching for IgA production, such as APRIL and BAFF (Cunningham-Rundles, 2001; Wang et al., 2011). A parallel can thus be drawn with SLE patients who have lowered levels of IgA, which may compromise the regulation of IgG–immune complex formation, contributing to the exacerbation of the disease. This is also seen in sIgAD. IgA is thus a potential regulator of the immune complex formation and its reduced levels plausibly promote immune complex formation among other antibodies. However, the mechanisms in each case need to be fully explored.
Sugar Residues in Serum IgA: Impact on Its Interactions and Functions
Human serum contains two subclasses of IgA, namely, IgA1 and IgA2, with IgA1 being the dominant subclass (∼84% IgA1 compared with ∼16% IgA2). The opposite is true at the mucosal surfaces (Conley and Delacroix, 1987). IgA is naturally glycosylated post-translationally. IgA1 bears two conserved N-linked glycan chains at the Fc region, on the CH2 and CH3 tailpiece. IgA2 can be further split into three allotypes: IgA2m(1), IgA2m(2), and IgA2(n). The IgA2m(1) contains two additional N-linked glycan chains at the CH1 and CH2 domains compared with IgA1. The IgA2m(2) and IgA2(n) each possesses one additional N-linked glycan in the CH1 domain in comparison to IgA1 and IgA2m(1) (Mattu et al., 1998; Yarema, 2005). In addition, IgA1 is O-glycosylated at three to five sites at the hinge region. The hinge region is missing in all IgA2 allotypes and they are not O-glycosylated. The number and composition of glycan chains at the Fc region or hinge region can show considerable heterogeneity within individuals, as was demonstrated in myeloma patients (Pierce-Cretel et al., 1981, 1989; Mestecky, 2005). The dichotomy of the predominance of the glycosylated forms of IgA1 and IgA2 isotypes in the serum and at the mucosal surfaces is an unsolved enigma worthy of further investigation. Thus far, evidence from studies suggests that (1) O-linked glycans present on IgA1 are required for uptake and catabolism by hepatocytes expressing the ASGP-R and (2) lack of a hinge region in IgA2 confers protection from antibody-cleaving proteases secreted by bacteria at the mucosal surface (Senior et al., 2000; Mestecky, 2005).
Glycan chains on IgA have been reported to be important for interactions between IgA and pathogen or IgA and protein/glycoproteins. For example, removal of carbohydrate chains decorating sIgA or from the free SC resulted in its reduced ability to coat commensal bacteria (Mathias and Corthesy, 2011). Panda et al. (2014) showed that the immune complex between IgG and H-ficolin, which directs the pathogen for phagocytosis, was delineated to the glycosylated CH2–CH3 region of natural IgG Fc and the P-subdomain of ficolin FBG domain. IgA is the most heavily glycosylated isotype of antibodies (IgA1 possesses O-linked glycans at the hinge region and N-linked glycans at the CH2 and CH3 domains), and since H-ficolin is a lectin, it is conceivable that perhaps the glycan chains of IgA would contribute to the IgA–H-ficolin interaction. Indeed, enzymatic removal of the N-linked glycan chains completely abrogated IgA–H-ficolin interaction, and partial removal of glycan chains weakened the IgA–H-ficolin binding affinity (Leong et al., 2014), which suggests that (1) the glycan chains of IgA are important for IgA and H-ficolin interaction, (2) the length of the carbohydrate chains determines the strength of binding to partner proteins, and (3) specific sugar residues are important for binding. However, the exact mechanism of such an interaction has not been characterized and it would be worth exploring.
It follows that glycan chains of IgA may be important for its interaction with other proteins, and thus, for carrying out its normal functions. The importance of its glycosylation is demonstrated most strikingly in the two immune complex diseases, IgA nephropathy and Henoch–Schönlein purpura. In these diseases, IgA is reportedly the major cause of immune complex formation. IgA nephropathy is caused by immune complexes involving IgA depositing in the kidney glomeruli (Wyatt and Julian, 2013). Henoch–Schönlein purpura is caused by immune complexes involving IgA depositing in small blood vessels (Roberts et al., 2007). Patients suffering from such diseases have elevated levels of IgA and, importantly, exhibit aberrant glycosylation of IgA at the O-linked region. Apparently, inappropriate glycosylation of IgA causes conformational changes, leading to increased immune complex formation. Thus, such aberrantly glycosylated IgA antibodies are not only unable to carry out their normal functions but they become drivers of immune complex diseases.
IgG has been shown to be aberrantly glycosylated in SLE patients (Tomana et al., 1992). However, thus far, there is no report on the glycosylation status of IgA in SLE patients, which warrants the need to study the glycosylation status of antibodies in SLE serum, in particular, IgA. Validating that SLE serum IgA is “properly” glycosylated would lend support to the observation that the low propensity of IgA to form immune complexes in SLE patients is attributable to (1) lowered pH due to chronic inflammatory conditions and/or (2) appropriate IgA glycosylation patterns. In such patients, it could be possible that only IgG shows inappropriate glycosylation, adding to the severity of the disease. In contrast, given the differences in antibody isotype trafficking, IgA could still be properly glycosylated. As mentioned before, in diseases like IgA nephropathy and Henoch–Schönlein purpura, inappropriate glycosylation of IgA, which causes conformational deviation, is the cause of IgA immune complex formation. Taken together, all sources of evidence seem to indicate that inappropriate glycosylation of IgA is sufficient for immune complex formation involving IgA. We hasten to add that elevated levels of IgA do not drive IgA immune complex formation (Mestecky and Tomana, 1997; Mestecky et al., 2002).
Serum IgA Function May Be Further Influenced by the Mucosal Immune System
A proportion of serum IgA is derived from marginal zone B cells. Recent evidence suggests that naive B cells activated in the gut-associated lymphoid tissue may home in to the marginal zone of the spleen (Vossenkamper et al., 2013). Therefore IgA, specific for commensal or pathogenic bacteria, produced by plasma cells in the marginal zone may be secreted into the bloodstream. These IgA antibodies may be playing the role of “standby” or “backup” antibodies to guard against systemic infection due to invasion across the mucosal epithelium. It has been shown that B-cells can be activated in a T-cell-independent manner; B cells respond to antigens, such as lipopolysaccharide and polysaccharides, of bacteria (Craxton et al., 2003). The response of marginal zone B cells derived from the gut when they encounter such antigens and the impact on serum IgA levels is an area that remains unexplored. Showing that these cells do respond to gut microbiota would further affirm that marginal zone B cells can indeed be derived from the gut.
Humans are not equipped with the hepatobiliary transport system for secretion of sIgA into the bile and into the gut due to the lack of pIgR expression on hepatocytes, unlike in mice, chickens, and rabbits (Mestecky et al., 1999). This further increases the amount of IgA in systemic circulation that is derived from the gut, which may explain the presence of up to 20% of serum IgA as dimers, trimers, or tetramers (Mestecky, 2005). The ASGP-R expressed by hepatocytes has been shown to be efficient in the uptake of both monomeric and polymeric forms of IgA (Tomana et al., 1985). Whether or not a pathogen-specific pIgA is part of a system that involves clearance by the ASGP-R on hepatocytes has yet to be explored. Any additional physiological roles of these polymeric forms of IgA merit further investigation. Revelation of the two abovementioned scenarios would add to the understanding of the complexity of the relationship between the mucosal and systemic immune systems. Previous studies have shown that exposure to commensal or food antigens at mucosal surfaces is able to suppress or enhance systemic antibody responses and affects immune cell activation (with suppression being predominant); the response is dependent on factors such as species, genetics, age, dosage, and the physical form of the antigen (Mestecky et al., 2005).
Serum-IgA-Mediated Regulation of Leukocyte Function: Shaping of Adaptive Immunity
Pasquier et al. (2005) showed that monovalent binding of FcαRI by monomeric IgA or anti-FcαRI Fab transduced inhibitory signals while crosslinking of FcαRI induced degranulation by human PBMCs. Local concentrations of plasma proteins have been shown to vary in different tissues in rats (Dewey, 1959). As such, local concentrations of IgA in various tissues are likely to be different and dependent on many physiological factors. How this affects the functions of immune cells in the tissue microenvironment before and during a pathogen challenge and the influence of the adaptive immune response is an interesting proposition. The key players being affected in shaping of the adaptive immune response to pathogens are the dendritic cells and macrophages, and the combinations of cytokines released by these cells determine the type of adaptive immune response (Fearon and Locksley, 1996). To illustrate, interleukin 12 released by dendritic cells and macrophages is important for directing the activation of naive CD4 T cells toward the Th1 helper T cell subtype instead of the Th2 subtype (Hsieh et al., 1993).
The mechanisms of FcαRI-mediated inhibitory effects are unclear and are just beginning to be elucidated; it has been postulated that the degree and stability of oligomerization determines the duration or extent of activating or inactivating signals (Blank et al., 2009). The inhibitory effects extend to other receptors, such as FcγR, FcɛRI, TLR4, CCR2, and TNFR (Pasquier et al., 2005; Kanamaru et al., 2008). As such, whether or not changing levels of serum IgA affect the activation threshold of innate immune cells and the sensing of pathogens is an area worth exploring. The sensing of pathogens through innate pathogen-sensing receptors, such as Toll-like receptors (TLRs), induces combinations of cytokines by immune cells. Therefore, serum IgA, through its ability to inhibit other receptors, clearly has a role in the perturbation of the cytokine network crucial in shaping immune responses. But how the network is altered remains unanswered. The knowledge gained from further investigations on serum IgA and its cognate receptors would be vital if intravenous IgA were to be considered as an anti-inflammatory agent (Monteiro, 2010).
Perspectives
Humoral responses to primary and secondary pathogenic challenge are mainly geared toward the production of high-affinity IgG antibodies that efficiently resolves an infection (Cruse and Lewis, 2010). Nevertheless, it is conceivable that the immune system has evolved to attribute different functions to the various antibody isotypes. The frequency of antigen-specific IgA is low, and IgA performs its roles at the mucosal surfaces and serum mainly as an anti-inflammatory antibody that maintains homeostasis under normal conditions (Kerr, 1990). Under infection-inflammation conditions, serum IgA has the potential to strengthen an immune response and aid the resolution of an infection.
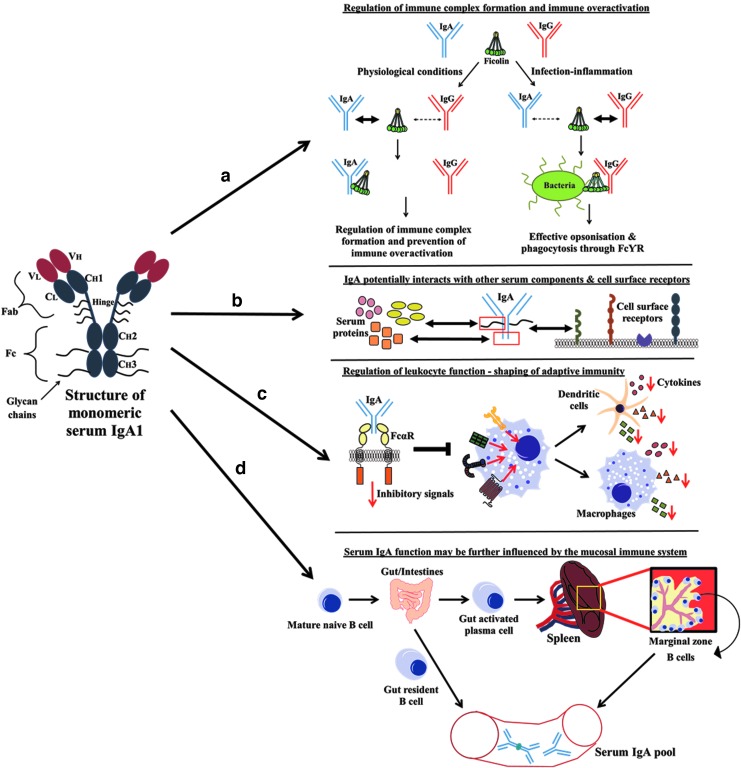
The revelation of IgG effector function and its potency in warding off infection has probably added to the mystery of serum IgA function. Now, with new discoveries being made, IgA should no longer be regarded as the “silent housekeeper,” but as a vital cog in the clockwork of the immune system. Figure 1 illustrates the roles of human serum IgA.
FIG. 1.
Roles of serum IgA. Structure of monomeric serum IgA1 is shown on the left. (a) Role of serum IgA in regulating immune complex formation and immune overactivation (Panda et al., 2013). (b) Serum IgA associates with serum proteins and cell surface receptors (through glycan chains present on various regions of the antibody) to effect various functions (Kerr, 1990). (c) Monovalent binding of FcαRI by monomeric serum IgA Fab transduces inhibitory signals that extend to other receptors, such as FcγR, FcɛRI, TLR4, CCR2, and TNFR, and affects the activation threshold of innate immune cells. Local concentrations of serum IgA in various tissues thus affect the cytokines released by innate immune cells and play a role in the shaping of the adaptive immunity (Dewey, 1959; Fearon and Locksley, 1996; Kanamaru et al., 2008; Pasquier et al., 2005). (d) Mature naive B cells that are activated in the gut may home into the marginal zone of the spleen, contributing IgA antibodies that are specific for gut pathogens/commensal antigens to the serum IgA pool. Gut-resident B cells further contribute to the serum IgA pool in the form dimeric IgA (Vossenkamper et al., 2013; Mestecky et al., 1999). Figure is not drawn to scale.
Acknowledgments
The authors would like to thank Drs. Saswati Panda, Lee Sae Kyung, and Ms. Imelda Winarsih for their advice and assistance with some experiments. This work was supported by grants from the Ministry of Education (MoE Tier 1 and MOE2013-T2-2-007).
Disclosure Statement
The authors declare no competing financial interests exist.
References
- Bakema J.E., and van Egmond M. (2011). The human immunoglobulin A Fc receptor FcalphaRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol 4,612–624 [DOI] [PubMed] [Google Scholar]
- Blank U., Launay P., Benhamou M., and Monteiro R.C. (2009). Inhibitory ITAMs as novel regulators of immunity. Immunol Rev 232,59–71 [DOI] [PubMed] [Google Scholar]
- Boes M., Schmidt T., Linkemann K., Beaudette B.C., Marshak-Rothstein A., and Chen J. (2000). Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A 97,1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A., Cols M., and Puga I. (2013). Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 13,118–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M.E., and Delacroix D.L. (1987). Intravascular and Mucosal Immunoglobulin A: two separate but related systems of immune defense? Ann Intern Med 106,892–899 [DOI] [PubMed] [Google Scholar]
- Corthesy B. (2013). Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 4,185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton A., Magaletti D., Ryan E.J., and Clark E.A. (2003). Macrophage- and dendritic cell—dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101,4464–4471 [DOI] [PubMed] [Google Scholar]
- Cruse J.M., and Lewis R.E. (2010). Atlas of Immunology, 3rd ed. (CRC Press/Taylor & Francis, Boca Raton, FL: ) [Google Scholar]
- Cunningham-Rundles C. (2001). Physiology of IgA and IgA deficiency. J Clin Immunol 21,303–309 [DOI] [PubMed] [Google Scholar]
- Dawes P.T., Jackson R., Shadforth M.F., Lewin I.V., and Stanworth D.R. (1987). The relationship between the complex of immunoglobulin A and alpha-1-antitrypsin, its constituent components and the acute-phase response as measured by C-reactive protein in rheumatoid arthritis treated with gold or D-penicillamine. Br J Rheumatol 26,351–353 [DOI] [PubMed] [Google Scholar]
- Dewey W.C. (1959). Vascular-extravascular exchange of I131 plasma proteins in the rat. Am J Physiol 197,423–431 [DOI] [PubMed] [Google Scholar]
- Dolby J.M., and Stephens S. (1983). Antibodies to Escherichia coli O antigens and the in-vitro bacteriostatic properties of human milk and its IgA. Acta Paediatr Scand 72,577–582 [DOI] [PubMed] [Google Scholar]
- Fearon D.T., and Locksley R.M. (1996). The instructive role of innate immunity in the acquired immune response. Science 272,50–53 [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Doi T., Nakajima T., Suyama T., and Tokuda M. (1982). Antimicrobial effect of human serum IgA. Microbiol Immunol 26,227–239 [DOI] [PubMed] [Google Scholar]
- Hsieh C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., and Murphy K.M. (1993). Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260,547–549 [DOI] [PubMed] [Google Scholar]
- Kanamaru Y., Pfirsch S., Aloulou M., Vrtovsnik F., Essig M., Loirat C., et al. (2008). Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol 180,2669–2678 [DOI] [PubMed] [Google Scholar]
- Kerr M.A. (1990). The structure and function of human IgA. Biochem J 271,285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B.L. (1997). Susceptibility loci for lupus: a guiding light from murine models? J Clin Invest 99,557–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkhioued B., Gounni A.S., Gruart V., Pierce A., Capron A., and Capron M. (1995). Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur J Immunol 25,117–125 [DOI] [PubMed] [Google Scholar]
- Leong K.W., Panda S., and Ding J.L. (2014). Elucidating the role of serum IgA. Undergraduate Research Opportunities Report, National University of Singapore; Available at http://www.dbs.nus.edu.sg/staff/details/ding_pub/LKW_UROPS%20Report_280814.pdf [Google Scholar]
- Mantis N.J., Cheung M.C., Chintalacharuvu K.R., Rey J., Corthesy B., and Neutra M.R. (2002). Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol 169,1844–1851 [DOI] [PubMed] [Google Scholar]
- Mathias A., and Corthesy B. (2011). Recognition of gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J Biol Chem 286,17239–17247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathsson L., Ahlin E., Sjowall C., Skogh T., and Ronnelid J. (2007). Cytokine induction by circulating immune complexes and signs of in-vivo complement activation in systemic lupus erythematosus are associated with the occurrence of anti-Sjogren's syndrome A antibodies. Clin Exp Immunol 147,513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattu T.S., Pleass R.J., Willis A.C., Kilian M., Wormald M.R., Lellouch A.C., et al. (1998). The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem 273,2260–2272 [DOI] [PubMed] [Google Scholar]
- Mendez E., Fernandez-Luna J.L., Grubb A., and Leyva-Cobian F. (1986). Human protein HC and its IgA complex are inhibitors of neutrophil chemotaxis. Proc Natl Acad Sci U S A 83,1472–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. (2005). Mucosal immunology, 3rd ed. (Elsevier Academic Press, Amsterdam; Boston: ) [Google Scholar]
- Mestecky J., Moldoveanu Z., and Elson C.O. (2005). Immune response versus mucosal tolerance to mucosally administered antigens. Vaccine 23,1800–1803 [DOI] [PubMed] [Google Scholar]
- Mestecky J., Novak J., Julian B.A., and Tomana M. (2002). Pathogenic potential of galactose-deficient IgA1 in IgA nephropathy. Nephrology 7,S92–S99 [Google Scholar]
- Mestecky J., Russell M.W., and Elson C.O. (1999). Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 44,2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Russell M.W., Jackson S., and Brown T.A. (1986). The human IgA system: a reassessment. Clin Immunol Immunopathol 40,105–114 [DOI] [PubMed] [Google Scholar]
- Mestecky J., and Tomana M. (1997). Structural heterogeneity of glycans in IgA molecules: implications for IgA nephropathy. Nephrology 3,s653–s657 [Google Scholar]
- Monteiro R. (2010). Role of IgA and IgA Fc Receptors in Inflammation. J Clin Immunol 30,1–9 [DOI] [PubMed] [Google Scholar]
- Mostov K.E. (1994). Transepithelial transport of immunoglobulins. Ann Rev Immunol 12,63–84 [DOI] [PubMed] [Google Scholar]
- Moura I.C., Centelles M.N., Arcos-Fajardo M., Malheiros D.M., Collawn J.F., Cooper M.D., et al. (2001). Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 194,417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz L.E., Gaipl U.S., Franz S., Sheriff A., Voll R.E., Kalden J.R., et al. (2005). SLE—a disease of clearance deficiency? Rheumatology (Oxford, England) 44,1101–1107 [DOI] [PubMed] [Google Scholar]
- Nikolova E.B., and Russell M.W. (1995). Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J Leukoc Biol 57,875–882 [DOI] [PubMed] [Google Scholar]
- Olas K., Butterweck H., Teschner W., Schwarz H.P., and Reipert B. (2005). Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol 140,478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O. (2012). New concepts in the generation and functions of IgA. Nat Rev Immunol 12,821–832 [DOI] [PubMed] [Google Scholar]
- Panda S., Zhang J., Tan N.S., Ho B., and Ding J.L. (2013). Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO J 32,2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Zhang J., Yang L., Anand G.S., and Ding J.L. (2014). Molecular interaction between natural IgG and ficolin—mechanistic insights on adaptive-innate immune crosstalk. Sci Rep 4,3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier B., Launay P., Kanamaru Y., Moura I.C., Pfirsch S., Ruffie C., et al. (2005). Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity 22,31–42 [DOI] [PubMed] [Google Scholar]
- Pereira L.F., Sapina A.M., Arroyo J., Vinuelas J., Bardaji R.M., and Prieto L. (1997). Prevalence of selective IgA deficiency in Spain: more than we thought. Blood 90,893. [PubMed] [Google Scholar]
- Pierce-Cretel A., Decottignies J.P., Wieruszeski J.M., Strecker G., Montreuil J., and Spik G. (1989). Primary structure of twenty three neutral and monosialylated oligosaccharides O-glycosidically linked to the human secretory immunoglobulin A hinge region determined by a combination of permethylation analysis and 400-MHz 1H-NMR spectroscopy. Eur J Biochem 182,457–476 [DOI] [PubMed] [Google Scholar]
- Pierce-Cretel A., Pamblanco M., Strecker G., Montreuil J., and Spik G. (1981). Heterogeneity of the glycans O-glycosidically linked to the hinge region of secretory immunoglobulins from human milk. Eur J Biochem 114,169–178 [DOI] [PubMed] [Google Scholar]
- Roberts P.F., Waller T.A., Brinker T.M., Riffe I.Z., Sayre J.W., and Bratton R.L. (2007). Henoch-Schonlein purpura: a review article. South Med J 100,821–824 [DOI] [PubMed] [Google Scholar]
- Saghafi S., Pourpak Z., Aghamohammadi A., Pourfathollah A.A., Samadian A., Farghadan M., et al. (2008). Selective immunoglobulin A deficiency in Iranian blood donors: prevalence, laboratory and clinical findings. Iranian J Allergy Asthma Immunol 7,157–162 [PubMed] [Google Scholar]
- Senior B.W., Dunlop J.I., Batten M.R., Kilian M., and Woof J.M. (2000). Cleavage of a recombinant human immunoglobulin A2 (IgA2)-IgA1 hybrid antibody by certain bacterial IgA1 proteases. Infect Immun 68,463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya A., Sakamoto N., Shimizu Y., Shibuya K., Osawa M., Hiroyama T., et al. (2000). Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol 1,441–446 [DOI] [PubMed] [Google Scholar]
- Stockert R.J., Kressner M.S., Collins J.C., Sternlieb I., and Morell A.G. (1982). IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci U S A 79,6229–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye S.G. (2013). To B1 or not to B1: that really is still the question! Blood 121,5109–5110 [DOI] [PubMed] [Google Scholar]
- Tomana M., Phillips J.O., Kulhavy R., and Mestecky J. (1985). Carbohydrate-mediated clearance of secretory IgA from the circulation. Mol Immunol 22,887–892 [DOI] [PubMed] [Google Scholar]
- Tomana M., Schrohenloher R.E., Reveille J.D., Arnett F.C., and Koopman W.J. (1992). Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol Int 12,191–194 [DOI] [PubMed] [Google Scholar]
- Truedsson L., Bengtsson A.A., and Sturfelt G. (2007). Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40,560–566 [DOI] [PubMed] [Google Scholar]
- van Egmond M., van Garderen E., van Spriel A.B., Damen C.A., van Amersfoort E.S., van Zandbergen G., et al. (2000). FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med 6,680–685 [DOI] [PubMed] [Google Scholar]
- Van Epps D.E., Reed K., and Williams R.C., Jr. (1978). Suppression of human PMN bactericidal activity by human IgA paraproteins. Cell Immunol 36,363–376 [DOI] [PubMed] [Google Scholar]
- Van Epps D.E., and Williams R.C., Jr. (1976). Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med 144,1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenkamper A., Blair P.A., Safinia N., Fraser L.D., Das L., Sanders T.J., et al. (2013). A role for gut-associated lymphoid tissue in shaping the human B cell repertoire. J Exp Med 210,1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Shen N., Vyse T.J., Anand V., Gunnarson I., Sturfelt G., et al. (2011). Selective IgA deficiency in autoimmune diseases. Mol Med (Cambridge, Mass) 17,1383–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Nagura H., Watanabe K., and Brown W.R. (1984). The binding of human milk lactoferrin to immunoglobulin A. FEBS Lett 168,203–207 [DOI] [PubMed] [Google Scholar]
- Wilton J.M. (1978). Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol 34,423–428 [PMC free article] [PubMed] [Google Scholar]
- Wolf H.M., Fischer M.B., Puhringer H., Samstag A., Vogel E., and Eibl M.M. (1994). Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 83,1278–1288 [PubMed] [Google Scholar]
- Wolf H.M., Hauber I., Gulle H., Samstag A., Fischer M.B., Ahmad R.U., et al. (1996). Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol 105,537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R.J., and Julian B.A. (2013). IgA nephropathy. N Engl J Med 368,2402–2414 [DOI] [PubMed] [Google Scholar]
- Yarema K.J. (2005). Handbook of carbohydrate engineering. (Taylor & Francis, New York: ) [Google Scholar]