Abstract
Background
Cognitive impairment and dementia among elderly adults is a pressing public health issue in China but research on biomarkers of cognitive decline has been limited.
Aim
Explore the relationship between multiple domains of cognitive functioning and the volume of the left and right hippocampus in healthy elderly adults.
Methods
Structural MRI scanning was performed on 65 community-dwelling healthy participants 65 to 75 years of age using the Siemens 3.0 T Trio Tim with the MPRAGE sequence. The volumes of the left and right hippocampus were determined using Freesurfer software. Cognitive functioning was evaluated using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Both unadjusted and adjusted associations between the hippocampal volumes and cognitive functioning were estimated.
Results
Within this relatively narrow age range, age was significantly associated with most of the cognitive measures assessed in women but was not significantly associated with any of the cognitive measures in men. In both men and women right hippocampal volume was positively associated with delayed memory and left hippocampal volume was positively associated with both immediate memory and delayed memory (though the relationship with delayed memory in women was only at a trend level). After adjustment for age, gender, and years of formal education (the variable that was most strongly associated with all of the cognitive measures), both left hippocampal volume and right hippocampal volume were positively associated with delayed memory, but not with immediate memory. Interestingly, the difference in the volumes of the left and right hippocampi was negatively associated with the score of the RBANS attention subscale, a relationship that was stronger in women than in men.
Conclusions
This study confirms previous work about the relationship of hippocampal volume and memory, identifies a possible relationship between attention and the difference in size of the two hippocampi, and suggests that there may be some differences in these relationships by gender.
Keywords: elderly adults, executive function, AMSTAR, hippocampal volume, China
Abstract
背景
在中国老年人认知功能障碍及痴呆是一个紧迫 的公共卫生问题,但对认知能力下降的生物学指标的 研究极其有限。
目的
探索健康老人多个领域的认知功能与左右侧海 马体积之间的关系。
方法
使用配有MPRAGE 序列的西门子3.0 T Trio Tim 磁共振扫描仪对65 名65 至75 岁的社区健康受试者进 行结构成像扫描。采用Freesurfer 软件来确定左右侧海 马的体积。使用可重复的神经心理状态成套检查(Repeatable Battery for the Assessment of Neuropsychological Status,RBANS)对认知功能进行评价。评估海马体积 与认知功能之间未调整和调整后的相关性。
结果
在这一相对狭窄的年龄段中,女性的年龄与大 多数认知功能评估结果显著相关,但男性中年龄与任 何认知评估结果都不相关。无论是男性还是女性,右 侧海马体积与延迟记忆呈正相关,而左侧海马体积与 即刻记忆和延迟记忆均呈正相关(虽然在女性中与延 迟记忆只有相关的趋势)。经调整年龄、性别和受教 育年数(该变量与所有认知评估结果的相关性最强) 后,左右侧海马体积均与延迟记忆呈正相关,但与即 刻记忆无关联。有趣的是,左右侧海马的体积之差与 RBANS 注意分量表得分呈负相关,女性中该相关性要 强于男性。
结论
本研究证实了先前关于海马体积和记忆之间相 关性的研究工作,提出了注意力和两侧海马体积差异 可能存在关联,并表明海马体积和记忆的相关性存在 一些性别差异。
1. Introduction
Aging of the population is a pressing issue in China. It is estimated that the elderly population will account for 17% of the population by 2020 and 30% of the population by 2050.[1] Cognitive functions such as memory,attention,and processing speed significantly decline with age.[2],[3] From 10 to 15% of the elderly individuals with mild cognitive impairment (MCI) and 1 to 2% of the healthy elderly develop fully blown Alzheimer’s disease (AD) annually; so MCI is considered an important risk factor for AD.[4] Cognitive functioning reflects the general functionality of the brain,[5] so many researchers in the field have focused on structural changes of the hippocampus because of its critical role in learning, memory, and spatial navigation.[6],[7],[8]. For example, in a ten-year follow-up study of 518 elderly individuals without dementia, Heijer and colleagues[9] found that the decline in the hippocampal volume paralleled or predicted the decline in long-term memory. After Bliss and colleagues[10] discovered the phenomenon of long-term potentiation (LTP) of the hippocampus, many studies have explored the role of synaptic plasticity in learning and memory, and the LTP of the hippocampus has been widely accepted as the neural mechanism of memory.[11]
Decrease in the volume of the hippocampus is a key indicator of hippocampal atrophy.[12] Many clinical studies about memory now include assessment of hippocampal volume, thanks to the rapid development of neuroimaging technology.[13],[14] Using the coronal T1 weighted magnetic resonance imaging (MRI), Du and colleagues[13] found reduced volume of the hippocampus among individuals with MCI compared to those with normal cognitive functioning; 70% of the individuals with MCI could be correctly identified based on hippocampal volume. Previous studies reported that the volume of the right hippocampus is usually larger than that of the left hippocampus,[15],[16] but few studies have investigated the functional differences between the left and right hippocampus. To assess the relationship of hippocampal volume to cognitive changes in healthy elders, the current study used MRI to explore the association of bilateral hippocampal volumes with multiple cognitive domains among community-dwelling elder adults without cognitive impairment.
2. Methods
2.1. Participants
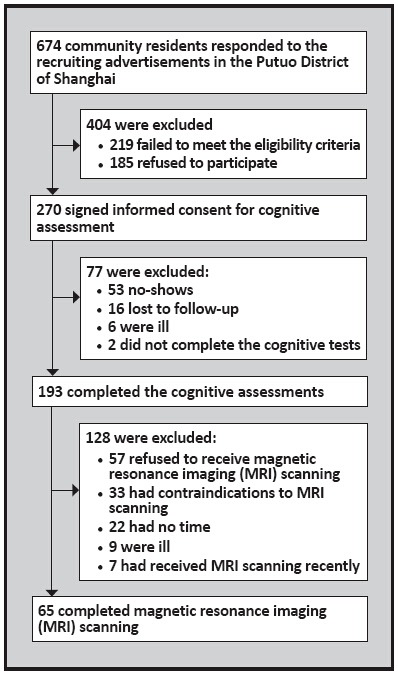
Data used in this report are from the baseline data of a previously published intervention study.[17] As shown in Figure 1, all participants were community dwelling elders living in a neighborhood located in the Putuo District of Shanghai. From March 2008 to April 2008, 674 elders were recruited by local neighborhood committees using posters and radio broadcasts. Eligible participants (a) were 65 to 75 years of age; (b) had no severe physical or mental illnesses; (c) were able to live independently; (d) had no disability, and no difficulties in hearing,vision or communication; (e) had at least one year of formal education; and (f) had an education adjusted normal score on the Chinese version of the Mini-Mental State Examination (MMSE)[18],[19] (>14 for those who had not finished primary school; >19 for those who completed primary school; or >24 for those who had graduated from middle school). Exclusion criteria included: (a) obvious cognitive decline; (b) a diagnosis of AD; or (c) major medical conditions such as cancer, current chemotherapy, or current radiation treatment. Among the 455 eligible elders,185 declined to participate in the cognitive assessment. The remaining 270 elders signed the consent form for the cognitive assessment and 193 completed the cognitive assessment; 65 of these individuals subsequently completed the MRI scan.
Figure 1. Identification of participants.
2.2. Assessment tools
2.2.1. Cognitive functioning
The Chinese version of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)[20], [21] was used to assess cognitive functioning. RBANS consists of 12 tasks (i.e. 12 items) that measure five domains of cognitive functioning: (a) immediate memory was measured using a list learning task (40 points) and a story memory task (24 points); (b) visuospatial and constructional ability was measured using a figure copy task (20 points) and a line orientation task (20 points); (c) language was measured using a picture naming task (10 points) and a semantic fluency task (40 points); (d) attention was measured using a digit span task (16 points) and a coding task (89 points); and (e) delayed memory was measured using a list recall task (10 points),a list recognition task (20 points),a story recall task (12 points) and a figure recall task (20 points).
The original scores (including the subscale scores and the total score) were transformed into scales with a theoretical range in scores from 40 to 160 using the corresponding percentage mapped on to the distribution of the RBANS scores from a previous study.[22] To facilitate comparison of subscale scores, each subscale score is separately scaled by age group to a mean of 100 with a standard deviation of 15; the sum of these five standardized subscale scores is then again adjusted to generate the total score with a mean of 100 and a standard deviation of 15. The Chinese version of the RBANS has demonstrated good reliability and validity among community-dwelling elders.[20] All four evaluators in this study were trained in the administration of the instrument using a standardized protocol. During the pilot phase of the project 7 of the evaluators rated 3 subjects using RBANS; the interrater reliability of the total score was satisfactory - the intraclass correlation coefficients (ICC) of the pairwise comparisons between raters were all greater than 0.75.
2.2.2. Magnetic resonance imaging (MRI)
Structural MRI scanning was performed using the Siemens 3.0 T Trio Tim using the MPRAGE sequence. The parameters were set as follows: repetition time (TR)=1900ms, echo time (TE)=3.43ms, flip angel=9°, slice thickness=1mm without gap,matrix=256×256,scanning 160 slices for 5 minutes.
The MPRAGE data was processed using the Freesurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu) for Mac OS. The data processing took about 30 hours with over 30 steps including stripping, normalization, removal of non-brain structure, flattening and image reconstruction.[24] Upon completion of these data processing steps,the volumes of the left and right hippocampus were obtained.
2.3. Statistical analysis
SPSS software version 17.0 was used for data analyses. Volume difference of the bilateral hippocampus was calculated by subtracting the volume of the left hippocampus from that of the right hippocampus. Pearson correlation coefficient was used to estimate the correlation between the volume variables and cognitive measures. Considering previously reported sex differences in the hippocampal structures[25] and cognitive functioning,[26],[27] the analysis was stratified by sex. Multiple linear regression was used to estimate the association between hippocampal volume and cognitive functions after adjusting for age and the level of education.[28],[29],[30] The statistical significance level was set at p<0.05 for all tests.
3. Results
3.1. Characteristics of study participants
All 65 elders included in the analysis were right-handed. There were 38 males and 27 females with a mean education of 10.1 (3.7) years. The mean volume of the right hippocampus (3962mm3, sd=535mm3) was significantly larger than that of the left hippocampus (3777mm3, sd=526mm3; t=5.33, p<0.001). The overall correlation of the size of the left and right hippocampi was 0.80. The mean total score of the RBANS was 91.7 (14.6), ranging from 55 to 120. The mean score of the immediate memory subscale was 85.3 (15.0), that of the visuospatial and constructional ability subscale was 103.3 (14.8), that of the language subscale was 93.4 (11.2), and that of the attention subscale was 89.2 (17.0).
3.2. Bivariate analysis
As shown in Tables 1 and 2, the factors associated with specific cognitive functions are somewhat different in males and females. Age is not significantly associated with any of the cognitive functions assessed by the RBANS in males but in females it is significantly negatively associated with immediate memory, visuospatial and constructional ability,language,and delayed memory. Among males years of education is only significantly associated with visuospatial and constructional ability and with overall cognitive functioning while among females it is also significantly associated with immediate memory, language, and delayed memory. In males immediate memory,delayed memory, and overall cognitive functioning is positively associated with the volume of the left hippocampus; delayed memory is positively correlated with the volume of the right hippocampus; and none of the cognitive functions are significantly correlated with the difference in the volumes of the two hippocampi. In females immediate memory and overall cognitive functioning are positively correlated with left hippocampus volume; there is a non-significant trend (p=0.54) of a positive correlation between delayed memory and left hippocampal volume; delayed memory is positively correlated with right hippocampus volume; and attention is negatively correlated with the difference in volumes of the right and left hippocampi.
Table 1.
Correlations of Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores with age,educational levels and hippocampal volumes among 38 healthy males 65 to 75 years of age
| Measures | age | years of schooling |
volume of the left hippocampus |
volume of the right hippocampus |
difference in volumes (right-left) of the two hippocampi |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Immediate memory | -0.05 | 0.748 | 0.30 | 0.069 | 0.36 | 0.025 | 0.30 | 0.068 | -0.13 | 0.423 |
| Visuospatial and constructional ability |
-0.12 | 0.480 | 0.47 | 0.003 | 0.26 | 0.110 | 0.19 | 0.246 | -0.15 | 0.366 |
| Language | 0.30 | 0.068 | 0.30 | 0.071 | 0.01 | 0.950 | -0.01 | 0.935 | -0.06 | 0.738 |
| Attention | 0.11 | 0.497 | 0.31 | 0.056 | 0.21 | 0.207 | 0.18 | 0.282 | -0.06 | 0.714 |
| Delayed memory | -0.15 | 0.371 | 0.32 | 0.054 | 0.59 | <0.001 | 0.56 | <0.001 | -0.05 | 0.777 |
| Total score | 0.00 | 0.990 | 0.43 | 0.007 | 0.36 | 0.026 | 0.31 | 0.057 | -0.10 | 0.541 |
Table 2.
Correlations of Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores with age,educational levels and hippocampal volumes among 27 healthy females 65 to 75 years of age
| Measures | age | years of schooling |
volume of the left hippocampus |
volume of the right hippocampus |
difference in volumes (right-left) of the two hippocampi |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Immediate memory | -0.58 | 0.002 | 0.67 | <0.001 | 0.43 | 0.025 | 0.36 | 0.064 | 0.05 | 0.821 |
| Visuospatial and constructional ability |
-0.39 | 0.047 | 0.44 | 0.022 | 0.29 | 0.139 | 0.32 | 0.109 | 0.04 | 0.830 |
| Language | -0.50 | 0.008 | 0.54 | 0.004 | 0.30 | 0.130 | 0.04 | 0.860 | 0.16 | 0.414 |
| Attention | -0.05 | 0.795 | 0.07 | 0.731 | -0.08 | 0.691 | 0.06 | 0.780 | -0.44 | 0.021 |
| Delayed memory | -0.48 | 0.012 | 0.47 | 0.012 | 0.36 | 0.054 | 0.49 | 0.010 | 0.05 | 0.790 |
| Total score | -0.46 | 0.017 | 0.67 | <0.001 | 0.47 | 0.013 | 0.31 | 0.114 | -0.08 | 0.704 |
3.3. Adjusted association between RBANS scores and hippocampal volumes
Six multiple linear regression models were constructed for the total RBANS score and for each of the five RBANS subscale scores. All models included the five potential independent predictors of cognitive functioning: age, gender,years of formal education,volume of right hippocampus,and volume of left hippocampus. As shown in Table 3,after adjusting for all five factors, years of formal education was significantly associated with the total RBANS score and with each of the five RBANS subscale scores. Age and gender were not independently associated with any of the assessed cognitive measures. With the notable exception of the RBANS attention subscale score,the volume of the left and right hippocampi were not significantly associated with any of the other cognitive measures. After adjusting for age,gender,and years of formal education, larger left hippocampus volume was significantly associated with better scores on the RBANS attention subscale and smaller right hippocampus volume was associated (at the trend level) with better scores on the RBANS attention subscale (p=0.059).
Table 3.
Multiple linear regression of factors associated with cognitive functions measured by Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) among 65 healthy community-dwelling adults 65 to 75 years of age
| regression coefficient (B) |
SE | standardized regression coefficient (Beta) |
t | p | adjusted R2 (p-value) |
||
|---|---|---|---|---|---|---|---|
| Immediate memory | 0.119 (0.028) | ||||||
| Female gender | 4.66 | 3.76 | 0.16 | 1.24 | 0.221 | ||
| Age in years | -0.07 | 0.59 | -0.02 | -0.13 | 0.901 | ||
| Years of formal | education | 1.09 | 0.51 | 0.27 | 2.15 | 0.035 | |
| Volume of left hippocampus | 0.01 | 0.01 | 0.26 | 0.99 | 0.325 | ||
| Volume of right hippocampus | 0.00 | 0.01 | 0.00 | 0.02 | 0.988 | ||
| Visuospatial and constructional ability | 0.236 (<0.001) | ||||||
| Female gender | -3.62 | 3.46 | -0.12 | -1.05 | 0.300 | ||
| Age in years | -0.60 | 0.54 | -0.14 | -1.12 | 0.270 | ||
| Years of formal education | 1.74 | 0.47 | 0.43 | 3.74 | <0.001 | ||
| Volume of left hippocampus | 0.00 | 0.00 | 0.11 | 0.44 | 0.660 | ||
| Volume of right hippocampus | -0.00 | 0.01 | -0.03 | -0.12 | 0.900 | ||
| Language | 0.038 (0.201) | ||||||
| Female gender | 4.78 | 2.93 | 0.21 | 1.63 | 0.109 | ||
| Age in years | 0.71 | 0.46 | 0.23 | 1.55 | 0.126 | ||
| Years of formal education | 0.82 | 0.39 | 0.27 | 2.09 | 0.041 | ||
| Volume of left hippocampus | 0.00 | 0.01 | 0.06 | 0.24 | 0.813 | ||
| Volume of right hippocampus | -0.00 | 0.01 | -0.06 | -0.25 | 0.803 | ||
| Attention | 0.152 (0.011) | ||||||
| Female gender 0.57 | 4.19 | 0.02 | 0.14 | 0.893 | |||
| Age in years 0.22 | 0.65 | 0.05 | 0.33 | 0.74 | |||
| Years of formal education | 1.58 | 0.56 | 0.34 | 2.81 | 0.007 | ||
| Volume of left hippocampus | 0.02 | 0.01 | 0.58 | 2.31 | 0.024 | ||
| Volume of right hippocampus | -0.01 | 0.01 | -0.45 | -1.92 | 0.059 | ||
| Delayed memory | 0.341 (<0.001) | ||||||
| Female gender | 6.20 | 3.71 | 0.18 | 1.67 | 0.100 | ||
| Age in years | -0.28 | 0.58 | -0.06 | -0.49 | 0.627 | ||
| Years of formal education | 1.39 | 0.50 | 0.30 | 2.80 | 0.007 | ||
| Volume of left hippocampus | 0.01 | 0.01 | 0.24 | 1.08 | 0.286 | ||
| Volume of right hippocampus | 0.01 | 0.01 | 0.21 | 1.03 | 0.307 | ||
| Total score | 0.261 (<0.001) | ||||||
| Female gender | 3.03 | 3.37 | 0.10 | 0.90 | 0.372 | ||
| Age in years | -0.07 | 0.53 | -0.02 | -0.14 | 0.893 | ||
| Years of formal education | 1.72 | 0.45 | 0.44 | 3.82 | <0.001 | ||
| Volume of left hippocampus | 0.01 | 0.01 | 0.32 | 1.37 | 0.176 | ||
| Volume of right hippocampus | -0.00 | 0.01 | -0.08 | -0.35 | 0.728 |
Given the high correlation in the volume of the right and left hippocampi it is possible that inclusion of both volumes in the regression model obscures their relationship to cognitive functioning. When we repeated the six models only including either the right hippocampus volume or the left hippocampus volume three other significant associations emerged: both the left and right hippocampal volumes were significantly associated with delayed memory (Beta=0.434, p<0.001; Beta=0.400, p<0.001,respectively), and the left hippocampal volume was significantly associated with the RBANS total score (Beta=0.253, p=0.048).
Finally six more multiple regression models were assessed in which the two variables representing the volumes of the right and left hippocampi were replaced with one variable that represents the difference in size of the two hippocampi. The only significant association identified was with attention. After adjusting for age, gender, and years of formal education, the smaller the difference in the volume of the two hippocampi the better the score on the RBANS attention subscale score (Beta=-0.250, p=0.044).
4. Discussion
4.1. Main findings
Consistent with previous studies,[28],[29],[30] we found that age and education were correlated with cognitive functioning. The relationship between years of education and cognitive functioning was evident in both men and women, but within the relatively narrow age range considered in this study (65 to 75) age was significantly correlated with cognitive functioning in women but not in men. Similar to previous studies in other countries,[15],[16] we also found larger right hippocampal volume than left hippocampal volume among healthy adults between 65 and 75 years of age.
The relationship between the hippocampus and memory has been extensively documented. The left hippocampus plays an important role in episodic and autobiographic memory while the right hippocampus is more important for spatial memory.[31] The right hippocampal volume was positively correlated with delayed memory in both men and women, the left hippocampal volume was positively correlated with immediate memory in both men and women, and the left hippocampal volume was also positively correlated with delayed memory in both men and women (though the correlation in women was only at the trend level [p=0.054]).
After adjusting for age,gender,and years of formal education (the strongest predictor of all cognitive functions assessed), both left hippocampal volume and right hippocampal volume were significantly associated with delayed memory, but not with immediate memory. More interestingly, in the multivariate model the difference of the right and left hippocampal volumes is negatively associated with attention; the univariate analysis indicated that this relationship is stronger in women than in men. This suggests that among nondemented elder adults, the greater the difference in the volume of the two hippocampi the poorer the attention. Future studies are needed to repeat this finding and to explore the underlying mechanism.
4.2. Limitations
The accuracy and validity of the assessment of hippocampal volume is critical for studies like this.[14] Manual segmentation is considered the gold standard for measuring hippocampal volume, but it is very time consuming and requires extremely well-trained investigators to achieve high precision. In this study,we used Freesurfer to obtain the automated volumetric measure of the hippocampus. It is simple,fast,and free of human intervention, so it is more replicable than other methods. However the application of automated methods for assessing hippocampal volume is still controversial.[32]
Several factors may affect the external validity of the study. The sample was relatively small,limited to a relatively narrow age range (because the main cognitive training study was focused on persons 65 to 75 years of age), and only included a small proportion of all identified community members who met the inclusion and exclusion criteria for the study. Moreover, the study was cross-sectional so no causal relationships between cognitive functioning and the associated factors is warranted. Finally, the relatively small R2 values for the six regression models (ranging from 0.038 to 0.341) indicate that only a small proportion of the total variance in the cognitive measures assessed was explained by the variables considered (age, gender, years of formal schooling,and hippocampal volume).
4.3. Significance
The measurement of the hippocampal volume using MRI has contributed to the diagnosis and etiology of neuropsychiatric disorders including Alzheimer’s disease, Parkinson’s disease, and major depression. Exploring the association of hippocampal volume and cognitive function can provide valuable information for the prevention and treatment of cognitive impairment among elderly adults. Longitudinal studies with larger sample sizes are needed to further unravel the role of bilateral hippocampal volumes in cognitive functioning.
Biography

Lijuan Jiang graduated from Nanchang University School of Medicine in Jiangxi Province in 2009 and received a Master’s degree from Fudan University in Shanghai in 2012. She is currently working as a researcher in the Department of EEG Source Imaging of the Shanghai Mental Health Center at Shanghai Jiao Tong University School of Medicine. Her current research interests are evidence-based systematic reviews and neuroimaging of mental disorders.
Funding Statement
The study was supported by National Foundation of Natural Science (81371505, 30770769, 81200831), the Shanghai Municipal Commission of Science and Technology (134119a2501), and the Shanghai Mental Health Center (2013-YJ-09).
Acknowledgement: The authors thank Dr. Yi Jin from the Chinese Science Academy for assistance in the data analysis for this article.
Conflict of interest: The authors declare no conflict of interest related to this manuscript.
Ethics approval: This study was approved by the Human Research Ethics Committee of Tongji Hospital in Shanghai, China.
Informed consent: All participants in this study provided written informed consent form.
References
- 1.Office of the National Committee on Ageing[Forecasts ofaging trends in Chinese population] Zhongguo She Hui Bao. 2006;6:1–4. Chinese. [Google Scholar]
- 2.McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: a brief review. Brain Behav Immun. 2004;18(3):214–220. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15(3):93–101. [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severespatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132(1):77–84. doi: 10.1016/S0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 7.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in the rat hippocampus(CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12(7):2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo HM. [Hippocampal structure – from morphology,function to plasticity, aging changes] Shen Jing Jie Pou Xue Za Zhi. 1996;12(2):177–184. Chinese. [Google Scholar]
- 9.Den Heijer T, van der Lijn F, Koudstaal PJ, Hofman A, van derLugt A, Krestin GP, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain. 2010;133(4):1163–1172. doi: 10.1093/brain/awq048. [DOI] [PubMed] [Google Scholar]
- 10.Bliss TV, Collingridge GL. A synaptic model of memory:long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 11.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9(1):65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 12.Gao XG, Ga SN, Li SY, Li S, Gao SJ, Sun WG, et al. [The study of hippocampal volume measurement-MR scan on61 healthy people] Zhongguo Yi Xue Ying Xiang Ji Shu. 2000;16(2):85–87. Chinese. [Google Scholar]
- 13.Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71(4):441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR Jr, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Embong MF, Yaacob R, Abdullah MS, Abdul Karim AH, Ghazali AK, Jalaluddin WM. MR Volumetry of Hippocampus inNormal Adult Malay of Age 50 Years Old and Above. MalaysJ Med Sci. 2013;20(4):25–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21(1):194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, et al. The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med. 2012;10:30. doi: 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li CB, Wu W, Jin H, Zhang X, Xue H, He Y, et al. Successful aging in Shanghai, China: definition, distribution and related factors. Int Psychogeriatr. 2006;18(3):551–563. doi: 10.1017/S1041610205002966. [DOI] [PubMed] [Google Scholar]
- 19.Wu WY, Zhang MY, Li CB, Xiao SF, Zhang X, Jiang SD, et al. [Series studies of successful aging mechanisms:Baseline section] Zhong Hua Jing Shen Ke Za Zhi. 2006;39(1):24–28. doi: 10.3760/j:issn:1006-7884.2006.01.007. Chinese. [DOI] [Google Scholar]
- 20.Cheng Y, Wu W, Wang J, Feng W, Wu X, Li C. Reliabilityand validity of the Repeatable Battery for the Assessment of Neuropsychological Status in Community-dwelling elderly. Arch Med Sci. 2011;7(5):850–857. doi: 10.5114/aoms.2011.25561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status asa screening test in schizophrenia I: sensitivity reliability and validity. Am J Psychiatry. 1999;156(12):1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 22.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status(RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 23.Li CB, He YL, Zhang MY. [Reasonable application of the consistency test methods] Shanghai Arch Psychiatry. 2000;12(4):228–232. Chinese. [Google Scholar]
- 24.Fischl B. Freesurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 26.Herlitz A, Rehnman J. Sex differences in episodic memory. Curr Dir Psychol Sci. 2008;17:52–56. doi: 10.1111/j.1467-8721.2008.00547.x. [DOI] [Google Scholar]
- 27.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 28.Compton DM, Bachman LD, Brand D, Avet TL. Age-associated changes in cognitive function in highly educated adults:emerging myths and realities. Int J Geriatr Psychiatry. 2000;15(1):75–85. doi: 10.1002/(SICI)1099-1166(200001). [DOI] [PubMed] [Google Scholar]
- 29.Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72(5):460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris MC, Scherr PA, Hebert LE, Bennett DA, Wilson RS, Glynn RJ, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21(3):123–130. doi: 10.1159/000054809. [DOI] [PubMed] [Google Scholar]
- 31.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 32.Germeyan SC, Kalikhman D, Jones L, Theodore WH. Automated versus manual hippocampal segmentation in preoperative and postoperative patients with epilepsy. Epilepsia. 2014;25(6) doi: 10.1111/epi.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]


