Abstract
State-of-the-art Newborn Intensive Care Units (NICUs), instrumental in the survival of high-risk and ever-earlier-born preterm infants, often have costly human repercussions. The developmental sequelae of newborn intensive care are largely misunderstood. Developed countries eager to export their technologies must also transfer the knowledge-base that encompasses all high-risk and preterm infants’ personhood as well as the neuro-essential importance of their parents. Without such understanding, the best medical care, while assuring survival jeopardizes infants’ long-term potential and deprives parents of their critical role. Exchanging the womb for the NICU environment at a time of rapid brain growth compromises preterm infants’ early development, which results in long-term physical and mental health problems and developmental disabilities. The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) aims to prevent the iatrogenic sequelae of intensive care and to maintain the intimate connection between parent and infant, one expression of which is Kangaroo Mother Care. NIDCAP embeds the infant in the natural parent niche, avoids over-stimulation, stress, pain, and isolation while it supports self-regulation, competence, and goal orientation. Research demonstrates that NIDCAP improves brain development, functional competence, health, and life quality. It is cost effective, humane, and ethical, and promises to become the standard for all NICU care.
Keywords: NIDCAP, premature infant, developmental care, Kangaroo Mother Care, NICU, prematurity
INTRODUCTION
Preterm birth, defined as birth before 37 weeks, is a pervasive obstetrical challenge. Globally, 13 million preterm deliveries occur per year, a 9% incidence rate [1]. In developed regions of the world the incidence varies from 5–12%; it may be as high as 40% in less developed, poor areas [2–4]. The incidence, especially in Western countries, is associated with the advent of extensive infertility treatment and women’s increased age at child bearing. Of the 4.3 million live births in the United States annually, 12.7% are premature. For reasons of generational poverty and its associated stresses, including less access to health care, the incidence stands at an all time high of 18.3 % for African-American families [5]. Given significant advances in peri- and neonatology in developed countries, survival rates have dramatically increased even for very low and extremely low birth weight infants. Today, more than 95% of infants born survive even if before 28 weeks gestation, 12 weeks too early and weighing less than 1250 grams. Infants born at 24 weeks have a survival chance of about 50% in modern tertiary care centers. Increasing numbers of countries succeed in assuring survival of infants born at 22 and 23 weeks (Japan and Germany, among others). Major disability rates for infants born between 25 – 27 weeks stands at about 15% while for those born at or below 25 weeks the rate is closer to 25%. While very early born infants comprise only a small percentage of surviving births, they add disproportionately to the morbidity rates, and cost of medical care and long-term educational services [6–8]. Preterm-born infants experience a range of adverse physical, behavioral, and mental health problems. It has been optimistically yet incorrectly proposed that in the absence of major complications, preterm born children would eventually ‘catch up’ to their full- term peers. Recent research suggests that as preterm-born infants mature they remain increasingly disadvantaged on many measures of cognitive function and mental processing, in terms of academic achievement, and in respect to behavior regulation, executive function as well as social and emotional adaptation [9–13] High levels of cerebral palsy (14%), IQ below 85 (38%), poor motor skills (47%), and visual disabilities (10%) [14] and the resultant challenges posed for children and families make it clear that it is no longer good enough to simply assure the survival of preterm born infants, and mandate that assurance of life quality must be paramount for professionals working in newborn intensive care units (NICUs). This goal requires knowledge and understanding of early neurological, affective, and neurobehavioral development and of the effects of the extra-uterine NICU experience on such development. Only with such knowledge will it be possible to restructure NICU care. This change must focus on the quintessential necessity of infant-parent co-regulation to foster long-term integrated functioning and well-adapted development for preterm infants.
UNDERSTANDING THE PRETERM INFANT
Historically fetal infants were thought to function at a neurologically primitive or brain stem level. Studies now recognized preterm infants as complex, responsive, and active in eliciting social and sensory stimulation, while simultaneously attempting to regulate their own thresholds of reaction and response. Neurodevelopmental research provides a framework for understanding the development of preterm infants in the context of their evolutionarily promised milieu with parents as primary and life-long co-regulators. The delivery of care from a neurodevelopmentally supportive perspective will be described as well as the outcomes of such care. Individualized developmental care in the Newborn Intensive Care Unit, originated in the 1980s. This multi-faceted approach, referred to as NIDCAP (Newborn Individualized Developmental Care and Assessment Program) [15] is theory based [16], and increasingly supported by scientific evidence. The model focuses on detailed reading of each individual infant’s behavioral cues. These cues dictate the environmental and care adaptations that are required to support and enhance each infant’s strengths and self-regulation capacities. From an evolutionary perspective, the NICU is a socio-cultural phenomenon engendered by society’s desire to preserve the lives of its smallest and most vulnerable members. By necessity, this extension of the lost womb which evolved to meet the neurodevelopmental expectations of the fragile preterm requires continued infant parent physical and emotional connection as a critically important component. For the human species, parents are the familiar, constant and steady connection in an infant’s life. With their continuous emotional connection, physical contact and nurturance, parents serve as the infant’s best advocate. They assure their infant’s safety, and assure their child’s development of trust and as a whole person. An understanding of individualized, behaviorally-based care along with the training and skill to read the infant’s eloquent behavioral cues allows for comprehensive improved care provision for preterm infants in and in so doing has the potential to improve the long-term outcome of infants and families. The dialogue with the infant and individualization of care are equally relevant and vital whether the infant is supported by mechanical technology and/or is simultaneously held by the parent in kangaroo position, the optimal infant niche.
DEVELOPMENTAL THEORY: PRIMATES OR MAR-SUPIALS?
From the ethologist’s perspective [17–20], for an organism at any stage of development, adaptation to its particular niche is not only species-appropriate but also a species-parsimonious. Since the process of behavioral evolution takes place over generations, many essentials of a species’ behavioral repertoire become hardwired, as experimental animal and human studies document [21]. In the course of a species’ evolution, good-enough organism-environment fit is ensured at the level of the organism’s central nervous system. The more primitive the organism’s nervous system, the more likely behavioral configurations become hardwired on a simple level. In more complex organisms, those requiring sophisticated and flexible behavioral repertoires to ensure species survival, the primary sensory cortex is likely comparatively smaller in relation to the brain regions involved in sensory association and integration, the association cortical regions. Also more likely is brain soft-wiring, which allows for flexibility and complexity of response supported by a system of multiple checks and balances. The more complex and flexible the organism, the larger is the buffering plasticity and organismic Spielraum, the idling or play space of adaptability [22–26].
Ethological studies have identified the importance, complexity, and subtlety of early parent-infant interaction. Being carried, Tragling, in the ventro-ventral configuration [27] is common to many mammals including humans. This posture with skin-to-skin parent-infant contact, widely termed Kangaroo Mother Care (KMC), reinforces the physical and supports the affective closeness between parent and preterm offspring. The term Kangaroo Mother Care (KMC) coined by E. Rey in the late seventies, while somewhat of a misnomer, - the limbs of marsupial mammals, such as the kangaroo, never evolved the adaptation to hold/carry their offspring -, nevertheless conveys well the idea that human fetuses who are separated from the placenta prematurely, just as non-placental metatherian mammals, best complete their fetal development ex-utero when protected from the environment and while assured continuous access to milk. While motorically ineffectual in maintaining the closeness to the parent which is essential for their survival, human infants keep their parents physically and affectively close with elicitors that are evolutionarily assured, positive, and socially relevant. Immediately after birth the connections between the newborn’s eye opening and visual attention and the parent’s affectionate behavior demonstrate a complex homeostatic regulation [28–32]. These behaviors appear to function as releasers for parent and child, launching a complex affective and cognitive interchange which fuels mutual competence well beyond caretaking and provision of nutrition. From the ethological perspective on adaptedness, all of the organism’s behavior at each stage of species adaptation is revealing. The human newborn acts as a socially competent, attending, and active partner in a feedback system with the caregiver. The newborn’s behaviors elicit physiological, motor, state, and attention interactive organization from the caregiver that the newborn requires in order to assure self-actualization. Face-to-face affective interchanges, a prepotent elicitor for maintaining parent closeness, is essential for the human infant for whom prolonged dependence is a distinct feature. This dependence is predicated on the bi-hemispheric, elaborately layered and comparatively heavy brain necessary for the highly differentiated and flexibly adaptive behaviors on which survival in a material culture is predicated. In the surviving preterm, eye-opening and the interplay with the engrossed parent [33] is further supported by KMC, both releasers of maternal (parental) affection, bonding, relaxation and a milk releasing hormonal cascade [34–37]. The evolutionary importance of this earliest extra-uterine social-affective connection is underscored by the simultaneous evolutionary differentiation of facial musculature. The human-specific facial repertoire supports subtle, complex, and flexible expression from birth on. This repertoire of behaviors is co-evolved with the highly differentiated, complex, and flexible social nature of humans, an adaptation assuring species survival [38]. Thus from birth, human newborns are structured for and competent in active elicitation of the affective/cognitive relationship with the caring adult which in turn initiates and supports their own increasing behavioral differentiation [30]. Human newborns, including preterm human newborns, whose very survival is dependent on the human adaptation of medical technology, are active shapers of their own development.
THE HUMAN NEWBORN
The study of the human newborn, guided by the principles of organism-environment transaction, has identified various subsystems of functioning in interplay within the organism. These systems influence the infant’s physiological functioning, motor activity, and state organization as they interact with the care-giving environment. The adaptive tasks for the infant are to achieve phase synchrony between periodicities which characterize these different systems, as well as synchronization between internal events and the environment [39–41]. Stimuli that are poorly timed, too intense, or too complex disrupt all subsystems, while appropriately timed, intense and complex stimuli enhance functional integration and support growth. The task becomes one of identification of synchronous and cohesive functioning, definition of the thresholds to disruption, and determination of the preconditions and requirements of continued differentiated competence.
Two basic physiological types of responses are in constant antagonistic interplay during the mammalian organism’s development towards smooth behavioral integration. These are the exploratory and the avoiding responses; the ‘toward’ and the ‘away’; the reaching out, and the withdrawing [42, 43]. An abrupt switch between the two may occur when the threshold of organism-appropriate stimulation is passed. Balancing these neurobiologically prepotent responses results in the gradual specialization of newborn infants’ central arousal systems leading to increasingly adaptive patterns, for instance, in altricial mammals such as suckling, nipple-grasping, huddling, and others [26, 44–51]. Dual antagonist integration is especially relevant to fetal infants, who find themselves too early in an extra-uterine environment in which they continually experience stimulation at or beyond the threshold of organism-appropriate. The approach/avoidance paradigm aids in understanding the behavioral patterns of preterm infants when assessing thresholds from integration to disorganization. During integrated performance, the ‘toward’ and ‘away’ antagonists modulate each other, bringing about an adaptive response. If an input stimulates the infant’s interest and internal readiness, the infant will approach the input, react and interact with it, and become sensitized to it. If the input exceeds the infant’s capacity to respond, the infant will actively withdraw from it. These responses modulate each another.
The initial overriding issue for the prematurely born human is the stabilization and integration of physiological functions, such as respiration, heart rate, temperature control, digestive function, and elimination, which typically require medical technological support. A healthy preterm’s motor system is a competent fetal motor system [52]. The preterm infant furthermore is well equipped fetally to smell and taste, searching actively for the familiar amniotic fluid [53], and to hear, preferentially seeking the familiar maternal voice [54–56]. State organization and other periodicities, no longer supported by the maternal sleep-wake and rest-activity cycles, nor by the maternal hormonal and nutritional cycles, are now influenced by the rhythms of the NICU. Thus, a model of subsystem differentiation emerges, termed synactive [16] depicted in Fig. (1), which highlights the simultaneity of all subsystems in their interaction with one another and in turn with the environment.
Fig. (1). Model of Synactive Theory.
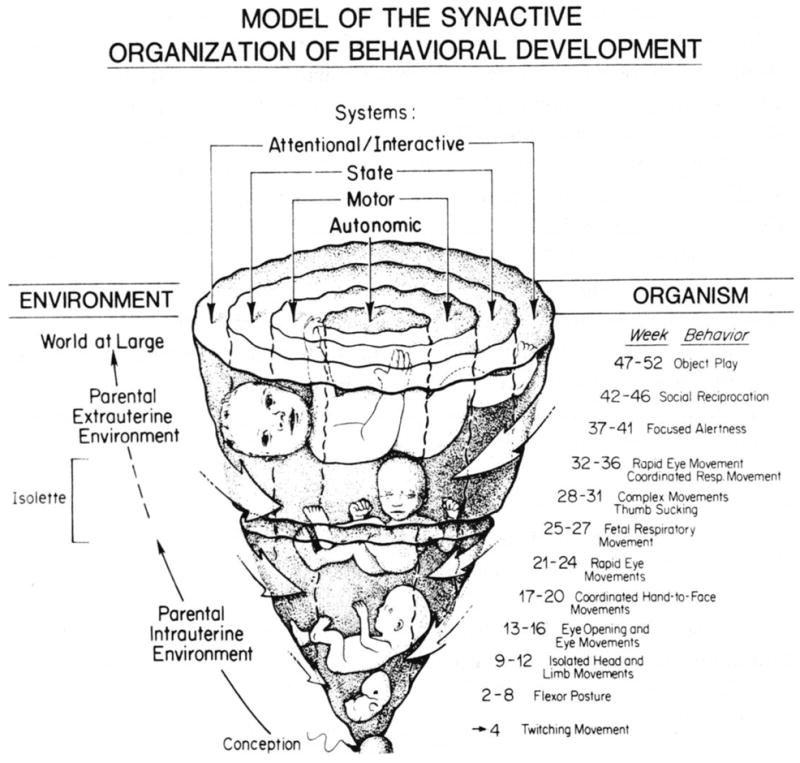
From: Als H. Toward a synactive theory of development: Promise for the assessment of infant individuality. Inf Mental Health J 1982; 3: 229–243. Fig. (1), p 234. Reprinted with permission.
The regulatory closeness of maternal presence is apparent once the infant leaves the enveloping maternal womb environment. The process of development is that of stabilization and integration of subsystems. This supports the differentiation and emergence of next capacities which in turn contribute to a newly integrated more differentiated system. In this model, the entire system continually modulates and differentiates, and progresses to ever more competent, integrated levels of physiological and behavioral organization throughout the lifespan [16, 23, 24, 50, 57, 58]. To paraphrase Erikson [59], self-actualization is a process of interaction/co-regulation with a “minimum of defensive maneuvers and a maximum of activation, a minimum of idiosyncratic distortion and a maximum of joint validation.”
THE PRETERM INFANT IN THE NICU
The biological expectation of the fetal infants is for continual sensory and kinesthetic input from the amniotic fluid and the reactive amniotic sac. Such input influences not only motor system development, but also the development of all the other systems engaged in feedback loops with the continually differentiating motor system. Fetal infants moreover expect maternal diurnal nutritional, sleep-wake-activity and hormonal rhythms, which among other things regulate states of consciousness and their differentiation. They expect muted inputs to the senses, all inevitably changed by the disruption of preterm delivery. The truncation of the parents’ emotional and physical preparation of a fullterm pregnancy further adds to the challenge for preterm infants and their parents. Even in medically low-risk preterm infants these challenges lead to an increase in later developmental difficulties, which include specific learning disabilities, lowered intelligence quotients, disorders of executive function and attention, lowered thresholds to fatigue, as well as a high incidence of visual motor impairments, spatial processing disturbances, language comprehension and speech problems, emotional vulnerabilities, and difficulties with self-regulation and self-esteem [6, 10, 14, 60–62]. These findings underscore the urgency to focus on opportunities to assure more balanced functioning for the preterm infant and to prevent some of the current mal-adaptations experienced in the NICU. The NIDCAP model postulates that an understanding of the neurodevelopmental expectations of the preterm as expressed in the infant’s behavior will provide a reliable basis for the examination, and adaptation of traditionally delivered newborn intensive care, including a realignment of the family eco-niche e.g. by provision of continual KMC in support of the infant while in the NICU.
The human cortex begins its development around the 6th week of gestation, when the embryo is just 1.5 cm in length [63]. At this time, the embryo’s superficial musculature is already developed [64]. Rapidly developing sensitivity in skin areas in and around the mouth, the eyes, palms of the hands, genitalia and soles of the feet is well in place by 12 weeks [65]. These physiological developments initiate experience-feedback loops which build the highly complex human central nervous system. The earliest developing regions of cutaneous sensitivity appear especially difficult for the fetal infant to satisfy behaviorally outside of the womb [16]. Preterm infants are observed to make continual attempts to brace with their feet, grasp with hands and feet, bring their hands to their mouths, search with mouth and tongue, suck, and make efforts to tuck into flexion, as if seeking the contact they could control in the womb environment [52, 66, 67]. These goal-directed behaviors are especially apparent in the first 24–48 hours after delivery, before exhaustion, ensuing from the many medical procedures connected with admission to a NICU, leads to flaccidity and submission. It is during this period that direct skin-to-skin contact in the Kangaroo Position is most crucial [68–70].
In order to appreciate the critical importance of providing brain expectation appropriate experience for the now extra-uterine fetus, it is important to review the rapidity and complexity of early brain development. The cerebral cortex is constructed of an estimated trillion neurons which originate in the germinal lining of the ventricular system. At its peak, the germinal matrix releases as many as 100,000 cortical neurons per day, each of which migrates between the 8th and approximately the 24th week through the cortex to its specific location. Once arrived, each of the 100 billion neurons develops interconnections with other neurons in forming an estimated average of a quintillion synapses, or communication interfaces. The earliest synaptic contacts are established at 7 weeks, many millions are established by 40 weeks, with contacts continuing be established throughout the human life span [71]. The development of axons and dendrites as well as the specificity and form of the synapse development has been shown to be very much influenced and altered by experience [72–79]. By the time the preterm infant enters the extra-uterine environment, a marked increase in brain mass occurs. This is the time when fetal behavior becomes increasingly complex and increasingly individual-specific [80, 81] with sucking on fingers and the hand, grasping, extension, and flexion rotations, increasingly discernible sleep and wake periods, and responses to sound. Highly specialized oligodendrocytes cells begin to deposit myelin in enveloping insulating sheaths around axons and some dendrites, the connective tissue strands between neurons. This allows faster conduction of highly repetitive impulses. Information conduction speeds of myelinated axons are approximately 120 m/sec, compared to un-myelinated axon transmission speeds, which are approximately 0.5 m/sec. Myelination occurs with peak activity in the last trimester and around full-term birth, continues significantly until age 9 years, and perceptibly into the 40s. Synaptic passage of impulses, neuronal transmission, is regulated by chemical neurotransmitters, often released only if multiple different regulatory systems concur in specific configurations. Experience, which is significantly altered once the fetus leaves the womb, influences the development of neurotransmitter receptors. The anatomic vulnerability of the germinal matrix adds architectural fragility to the preterm brain’s sensitivity, aside from the experience altered neurofunctional and structural aspects described. Up to 50% of preterm infants born before 32 weeks show some degree of brain hemorrhage, periventricular leukomalacia (PVL), and/or non-cystic PVL, due to the fragility of the fine blood vessels deep within the germinal matrix. This structure is relatively unsupported once the majority of neurons migrate to form the outer cortical mantle. Given the poorly developed auto-regulation of blood flow velocity and pressure in preterm infants, small surges may burst these fine blood vessels and cause bleeding into the germinal matrix and if more extensive, into brain matter itself. Care giving procedures such a diaper changes and heel sticks with elevated ankles, auscultations, etc. are increasingly being implicated in such inadvertent blood flow changes [82]. The incidence increases with reduction in gestational age [71, 83, 84].
The task, protocol, and schedule-focused environment and care delivery rhythms of the traditional NICU presents sensory overload and absence of neuro-biological rhythms. They stand in stark mismatch to the developing nervous system’s expectation during this exceedingly sensitive time of rapid brain development. Prolonged diffuse sleep states, unattended crying, supine position, routine excessive handling, high ambient sound and light levels, lack of opportunity to suck, and the often poorly timed social and care giving interactions, and the many painful procedures performed on a daily basis all exert deleterious effects upon the immature brain and alter its subsequent development [82, 85–89].
BEHAVIORAL LANGUAGE OF THE PRETERM INFANT
Behavioral observation provides a way to infer the preterm infant’s current developmental goals, to assess current functional competence, and to estimate the most appropriate adaptations for the best developmental outcome. Even very early born and fragile infants display reliably observable behaviors along the four main systems outlined, the autonomic system, the motor system, the state system with special emphasis on the emerging attention system, and the self-regulation system. The autonomic system’s behavioral communication signals include respiration patterns, color fluctuations, and visceral responses such as spitting up, gagging, hiccoughing and bowel movement strains, among others. The motor system’s behavioral communication signals include muscle tone of trunk, extremities and face ranging from with well-modulated, to flaccid or hypertonic; as well as postures and movement patterns, such as finger splays, arching, grimacing, and tucking, among others. The behavioral communication signals of the infant’s state system, which defines the infant’s level of awareness, include the infant’s range of states such as sleeping, wakefulness, and aroused upset, the patterns of transition from state to state, and the robustness and modulation of each of the states. The infant’s self regulation system behaviors indicate how and to what extent the infant makes spontaneous efforts to re-balance and bring into harmony the three other systems when they have moved out of balance; how successful the infant’s own strategies and efforts are in doing so; as well as how easily and to what extent the infant may accept and make use of a caregiver’s or examiner’s facilitation of balance and subsystem harmonization. These reliably observable behavioral communications provide valuable information for the clinician and caregiver in how best to structure and adapt environment, care and interaction, in order to enhance the infant’s own competencies, prevent or at least reduce the infant’s signals of stress, discomfort, and/or pain, and provide more appropriate care for the preterm infant [16]. The well-supported reclining parent who offers KMC by far provides the best bed and support for most care situations, even those that may be technically very challenging, such as extubation [90], as the work conducted in the context of the Newborn Individualized Developmental Care and Assessment Program has demonstrated.
THE NIDCAP MODEL OF CARE
NIDCAP is an individualized developmental approach to support and care based on reading each preterm infant’s behavioral cues, and on formulating of a care plan, which enhances and builds upon the infant’s strengths, and supports the infant in areas of sensitivity and vulnerability (www.nidcap.org) [15]. The goal is the improvement of long-term child and family outcome. The framework applies throughout the infant’s delivery process and admission to the NICU, and continues throughout the infant’s hospital stay, the transition home, and the first few months at home. KMC as a component of family centered, developmentally sensitive care provision supports the premature family with continuous progressive empowerment by respecting, emphasizing and protecting the closeness of the infant-parent dyad, a physical and by extension an emotional closeness that represents the core of the NIDCAP/KMC approach. The comprehensive approach of NIDCAP, in which KMC plays an integral role, was created in an effort to decrease the discrepancy between the immature human brain’s expectation for the all-embracing womb environment and the actual experience of a typical NICU. Thus, the goal of the NIDCAP relationship-based approach is to provide individualized, developmentally supportive, family centered care, which includes KMC, to each prematurely born infant and family in order to support their joint realization of optimal health and development.
The synactive theory proposes that care-implementation, which takes into account the infants’ thresholds to disorganization, is ultimately supportive of the infant’s long-term outcome. NIDCAP is based on the following four assumptions: (1) Detailed observations of infant behavior during daily care giving interactions provide an important basis for recommendations in how best to minimize stress and optimize an infant’s development. (2) Parents and their closest supporters, often family members or friends, provide the optimal co-regulatory support and literal twenty-four-hour bed for the immature infant. (3) Care giving NICU staff benefits from supportive education in implementing the often challenging procedures necessary (e.g. suctioning, extubation, line placements, etc) as well as regularly available emotional support to process their complex feelings and self-doubt about having to give pain while simultaneously understanding the personhood of infant and parent who must trust and rely upon them. (4) Resultant re-envisioning of care will lead to better outcome in infant medical well-being and neurobehavioral functioning, in parent well-being and functioning, and in staff professional and personal development. Fig. (2) depicts the NIDCAP model of NICU care.
Fig. (2). Model of Family Focused Care.
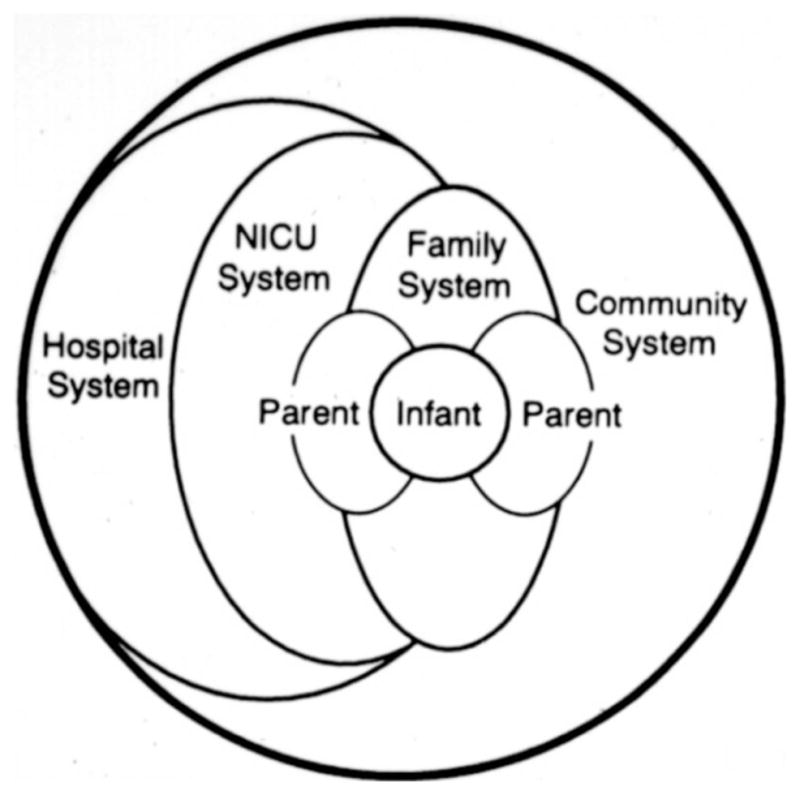
From: Als, H. (1992). Individualized, family-focused developmental care for the very low birthweight preterm infant in the NICU. In S. L. Friedman & M. D. Sigman (Eds.), Advances in Applied Developmental Psychology (Vol. 6, pp. 341–388). Norwood, NJ: Ablex Publishing Company. Fig. (2), p 358 Reprinted with permission.
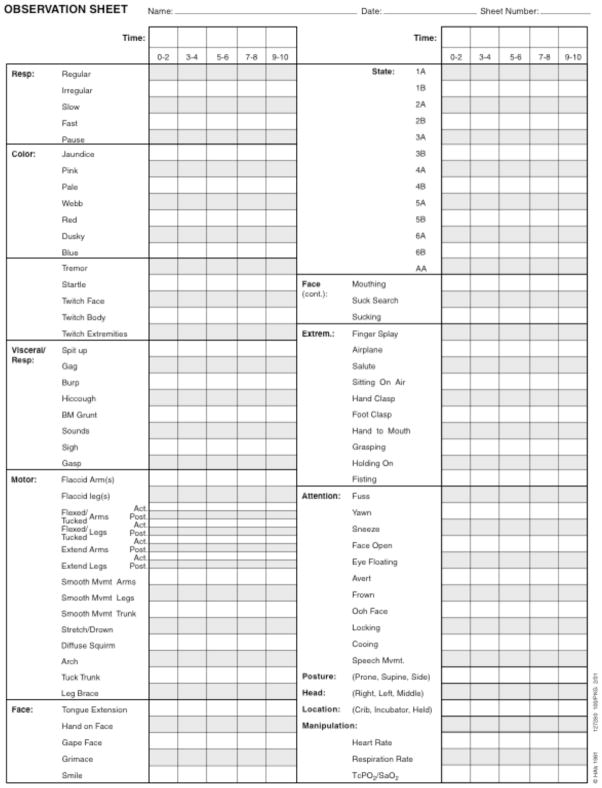
The NIDCAP methodology documents an infant’s ongoing communication through the recording of detailed observation of the infant’s naturally occurring behaviors in the NICU [15]. Fig. (3) shows the NIDCAP observation scan sheet which captures preterm infant’s behaviors as they continue their in-utero development in the face of the challenges of care necessary to assure their survival [91].b
Fig. (3). NIDCAP Observation Sheet.
From Als, H., Lawhon, G., Brown, E., Gibes, R., Duffy, F. H., McAnulty, G. B., et al. (1986). Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: Neonatal Intensive Care Unit and developmental outcome. Pediatrics, 78, 1123–1132. p. 1125. Reprinted with permission.
The naturalistic observation sheet provides for the recording of the individual infant’s behavior with a systematic format for the 2-minute-by-2-minute recording of 91 behaviors. These represent communication signals of the autonomic, motor, state, attention, and self-regulation subsystems. The infant is observed while at rest, ideally in the KMC-position, throughout the duration of the care giving interaction, and as the infant returns to a restful state. Repeated observations yield important information regarding the infant’s robustness as the infant attempts to make the best use of the care provided. These provide the basis for narrative written reports, which describe the infant’s strengths, current sensitivities and apparent goals and thresholds to stress, and the infant’s self-regulatory efforts when at rest, and when in interaction with a caregiver. They form the basis for suggestions regarding care giving and environmental adaptations, and provide care suggestions for infants and families best suited to the infants’ own developmental goals.
The behaviors observed are conceptualized as those which evidence stress and those which evidence competence (Tables 1 and 2) [16].
Table 1.
Stress and Defense Behaviors
|
From: Als H. Toward a synactive theory of development: Promise for the assessment of infant individuality. Inf Mental Health J 1982; 3: 229–243. Table 1, p 237. Reprinted with permission.
Table 2.
Self-Regulatory Behaviors
|
From: Als H. Toward a synactive theory of development: Promise for the assessment of infant individuality. Inf Mental Health J 1982; 3: 229–243. Table 2, p 238. Reprinted with permission.
In these observational reports, supportive opportunities are explored such as adaptations of the infant’s NICU environment, and the more immediate environment in terms of adaptations to Kangaroo position; if in the incubator, suggestions for transfer from the incubator to the parent’s body and into KMC. A number of studies have documented the temperature regulation benefits of time-limited KMC holding. Few have realized NIDCAP’s ultimate goal of the incubator-free infant-family NICU niche as womb-room, where fetal infants and their closest adult co-regulators are cared for and may thrive together. The complementarity of KMC and NIDCAP is evident in support of this goal. KMC developed in a setting of low income, limited technological resources and high infant morbidity/mortality, advocates by necessity, continuous or near continuous physical contact between infant and mother. In so doing, it effectively replaces the ‘incubator’ with the mother’s body. [92, 93] NIDCAP from early on extended this goal into the high-technology NICU, in order to overcome the separation of infant and parent by virtue of the technology employed in the infant’s most acute and vulnerable phase. KMC during the most acute phase may indeed alleviate or at least ameliorate the frequently observed day-2 and day-3 deterioration of the high-risk vulnerable technology-dependent infant. NIDCAP efforts are directed to refine the skills to provide technology while the infant is nurtured and protected in 24-hour KMC. The observational reports may include, as well, adaptations of the social environment in terms of gentleness, supportiveness, and timing of care delivery. Such considerations include appropriate support and nurturance of the infant’s parents and family, who are the primary co-regulators of the infant’s development; appropriate adaptations of the atmosphere and ambiance of nursery space, of care, nurturance, and respect for infant and family in the NICU environment; modifications of the organization and layout of the infant’s care space; the structuring and delivery of specific medical and nursing care procedures and specialty care indicated; and the overall safe-guarding and assurance of a developmental perspective on care and environment. More detailed descriptions of the process are available elsewhere [94–96].
The assurance of the parents as the primary nurturers of their child is crucial to the infant’s developmental outcome. The support and sensitization of the parents to their child’s behavior and its meaning is essential to the appropriate implementation of this model of care. For example, the infant’s hospital space must be recognized as the infant’s and parent’s immediate home. Parents and infants seek respectful, supportive, professional and consistently nurturing environments in the NICU that help them grow in their role as competent parents, and infants, and become well-functioning mutually supportive and trusting families.
STAFF EDUCATION AND SUPPORT
The transitions and transformations that individualized developmentally supportive care demands in the NICU setting entail the movement from a protocol-based task and schedule oriented framework of NICU care to a collaborative, relationship-based individualized framework of care. The key concept of this relationship-based model is the concept of co-regulation. Co-regulation from an evolutionary perspective recognizes the social, i.e. neuro-essentially interconnected nature of human infants and particularly fetal infants and their parents. Implementing a theory-guided rather than procedurally-driven approach is challenging in any setting, even more so in an acute and intensive care setting like that of the NICU. The NICU, by tradition, is oriented to standards, protocols, and strictly enforced rules and care giving routines. In many NICUs, skin-to-skin supported holding or Kangaroo position is yet one more protocol instituted on behest of, albeit, well-meaning staff, and enforced like all other protocols. A co-regulatory frame-work of care requires a rethinking of the components of a successful relationship. It requires sophisticated technical medical expertise, in order to free caregivers of performance anxiety and support them to take pride in being attuned to the other, mindful of the personhood of the infant and the family. The framework requires caregivers to be reflective regarding their own actions and ways of being, while continuing to function effectively in an acute intensive medical care setting. The transformational and practice challenges of the developmental individualized model of care involve considerable staff education and continuous leadership and emotional anticipatory guidance support. The infant’s care involves many procedures, examinations, and intensive interventions delivered by care-giving staff from various disciplines. It must involve, foremost, safeguarding of the infant’s continued best regulation amidst the effective implementation of all care procedures, no matter how complex. The co-regulating parent and/or parent surrogate must be available for each infant and at all times, including on transport to and from the NICU [97]. All care and environmental considerations must be implemented within a developmental framework.
Figs. (4–8) depict examples of parent-infant co-regulatory care in various situations and from various NIDCAP nurseries.
Fig. (4).
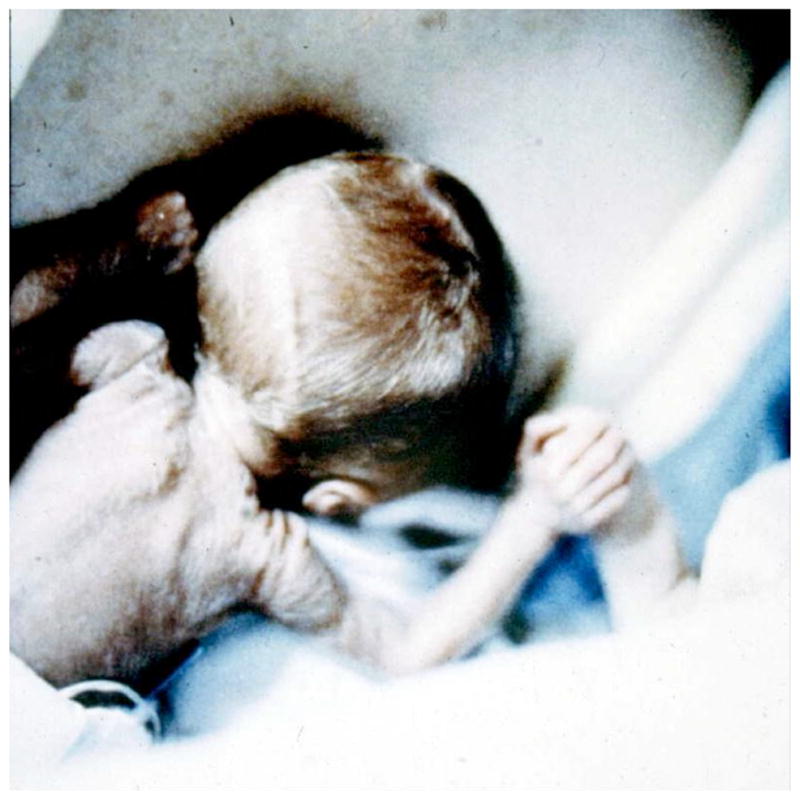
Tiny intubated infant girl on day 2 sleeping on her mother’s chest in KMC. (Brigham and Women’s Hospital Boston, NCRI, 1991).
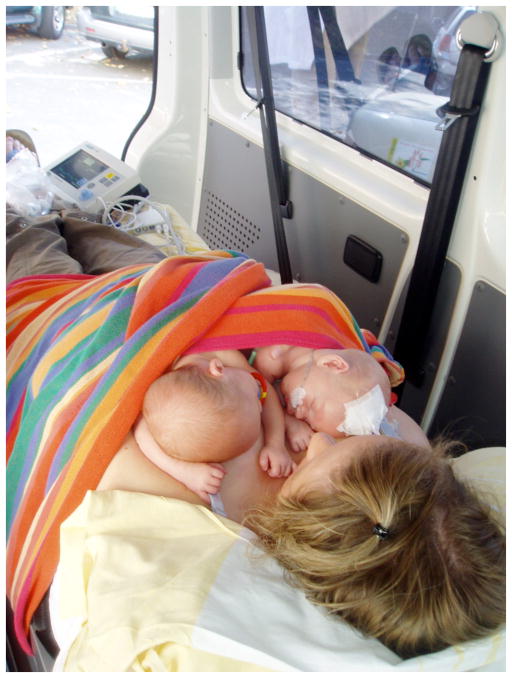
Fig. (8).
Mother and preterm infant twins in KMC during ambulance transport. (Harzer Kinderklinikum, Wernigerode, Germany; Dieter Sontheimer and Kerstin Buch, with permission, 2010).
Transitioning staff to become aware of the infant’s and family’s individuality and goals requires continued support, education and feedback. The aim is to increase staff self-knowledge and insight and to support them to view the infant, the parent and the environment in new ways and with new eyes; letting go of well-practiced beliefs and routines, while staying open to learning new approaches, and engaging in a process of self-reflection and re-definition. Reflection as a framework of practice initially may appear foreign to the action-oriented, intensive care setting. However, the implementation of developmental care demands reflective, self-aware practice coupled with refined relationship engagement skill and paired superior technical skill. NICU work involves intensive human interaction in the context of physical and emotional vulnerability. At its core are the tiny, immature, fully dependent, highly sensitive, and rapidly developing fetal infants and these infants’ hopeful, open, fully dependent, and utterly trusting parents. Both relinquish themselves into the professionals’ skill, attention and investment. This is the challenge and the opportunity of developmental NICU care. The cornerstone of NIDCAP is to nurture the primary and lifelong relationship of the infant and the infant’s family. This may be assured by the biologically prepotent response of the new parent, to hold close to their body their fragile newborn. This may take the form of skin-to-skin contact, be it in the traditionally defined kangaroo-care model or the embracing soothing and cradling care of the parents’ hands to stabilize a tiny infant in the course of and after a difficult procedure.
The introduction of NIDCAP into a nursery involves investment by the system of thought and conceptualization not only in terms of education and physical changes but foremost of the sea change that results in transformation of practice and understanding of nuanced human relationships.
NIDCAP TRAINING AND EDUCATION
NIDCAP training focuses on the partnering, coaching, and education of multi-disciplinary professional teams in NICUs [15]. Introduction of NIDCAP into a system involves considerable investment at all levels of the organization, with the organizational goal of improved long-term development of infants and their families. Developmental care training requires substantive, extensive educational efforts resulting in changes in staff conceptualization and interaction, professional role definition, perception of infant and family, and in the actual practice of care itself. The initial costs are ultimately cost savings as the NICU reaps the benefits over time [98]. NIDCAP requires development in professional self-awareness and the capacity to be present in the moment, to “hold” complex relationships and interactions. The developmentally skilled NICU professional combines highest technical skill embedded in highest relationship skill and greatest personal humbleness. NIDCAP including KMC is highly compelling from an ethical, humanitarian and global health perspective. It is in direct keeping with family-centered care. As such, it is well on its way to become the standard of care for all NICUs. The individualized co-regulatory evolutionary-based developmental approach to newborn intensive care requires leadership, in-depth staff training and broad-based systemwide education. While initially costly it provides unique opportunities for self-reflection and role re-definition that in the long term benefit both the nursery and the health care system in general. The formally established international NIDCAP teaching and training program, safeguarded by the NIDCAP Federation International (NFI), provides for systems education and on-site consultation in support of institutional change, leadership, and the building of reflective process capacity. As such, it requires of a nursery and hospital a strategic plan to bring about the benefits of training and change. Further Training Center contact and information are available on the web (www.nidcap.org).
EVIDENCE IN SUPPORT OF DEVELOPMENTAL CARE AND THE NIDCAP APPROACH
Numerous research studies have tested the effectiveness of the NIDCAP approach in randomized controlled trials, conducted in the US and elsewhere, and have proven the benefits of the NIDCAP model [98–105]. Various sub-components of care modification implemented in the NIDCAP model have also been validated. They document reduced stress during ophthalmologic examination [106], and reduced stress during transfer from incubator to skin-to-skin care with the parent [107]. Improved long-term outcome in infant cognitive, motor system and emotional functioning due to NIDCAP care in the NICU has also been reported. Outcome points studied include corrected ages of nine months [98, 103, 104], 12 to 36 months [108, 109], 6 years [101], and 8 years [110]. Effectiveness in enhanced parent confidence and competence has also been documented [102]. NIDCAP research is increasingly expanding into populations prenatally brain-compromised. These studies assess NIDCAP effectiveness in the compensatory re-alignment of the preterm infant development onto a more optimal trajectory again potentiated by the co-regulatory parent.
NIDCAP furthermore has been shown to significantly reduce healthcare costs [99, 111, 112] While reduced allocations for the early intervention and education services required by graduates of NIDCAP nurseries are difficult to estimate, the hospital cost savings alone far outweigh the initial costs for staff training and compensation for the key developmental professionals required for NICUs which choose to practice in the NIDCAP model. Given these encouraging results, it behooves those responsible for the medical and educational care of preterm born infants in intensive care medical systems to be well informed and educated in the NIDCAP model, and to advocate for and fully support its introduction and sustained implementation. Not to do so, appears irresponsible in the face of the overwhelming evidence.
SUMMARY
This article provides an overview of the changes taking place in Newborn Intensive Care Units (NICUs) and nurseries around the world in relationship to infant developmental outcome, increased knowledge of infant brain development, and implementation of developmentally supportive care, including skin-to-skin contact. It has become clear that reading and trusting the preterm infant’s behavior as meaningful communication, moves traditional newborn intensive care delivery into a collaborative, relationship-based neurodevelopmental framework. Skin-to-skin holding and care, which is at the core of Kangaroo Mother Care Intervention, in its broadest sense is a neuro-developmentally important component of this approach. NIDCAP leads to respect for infants and families as mutually attuned to and invested in one another, and as active structurers of their own developments. It sees infants, parents, and professional caregivers engaged in continuous co-regulation with one another, and in turn with their physical and social environments. It highlights mutually supportive striving to realize developmentally appropriate and individually specific expectations. It fosters increasing differentiation and modulation towards their shared goals, and it improves outcomes. Individualized developmental care is the earliest intervention. It emphasizes the infant’s own strengths and apparent developmental goals, and institutes supports for self-regulatory competence and achievement of these goals. Furthermore, this individualized, behavioral-developmental approach to care as defined in the NIDCAP model, improves outcome not only medically, but also behaviorally, neurophysiologically, and in terms of brain structure. The NIDCAP framework is based in the model of the synactive theory and validated by scientific evidence. The results indicate that increase in support to the infant’s behavioral self-regulation improves developmental outcome. This is likely due to prevention of inappropriate inputs during a highly sensitive period of brain development, by fostering the brain’s receptivity and opportunity for appropriate inputs, and by reliable assurance of the brain’s return to a base of integration, re-balance and re-harmonization of increased differentiation after arousal, activation and often over-stimulation.
Given the scientific evidence supporting the NIDCAP model, which encompasses skin-to-skin holding and care, it behooves those responsible for NICU care to be knowledgeable and proactive in implementing the comprehensive NIDCAP model of care. The futures of infants and families in our NICUs depend on the implementation of individualized, developmentally supportive family centered care. All NICU professionals must warrant the trust that infants and families place in them, and must find the means and ways to provide the proven model of NIDCAP care in order to improve reliably and accountably the futures for infants and families in intensive care.
Fig. (5).
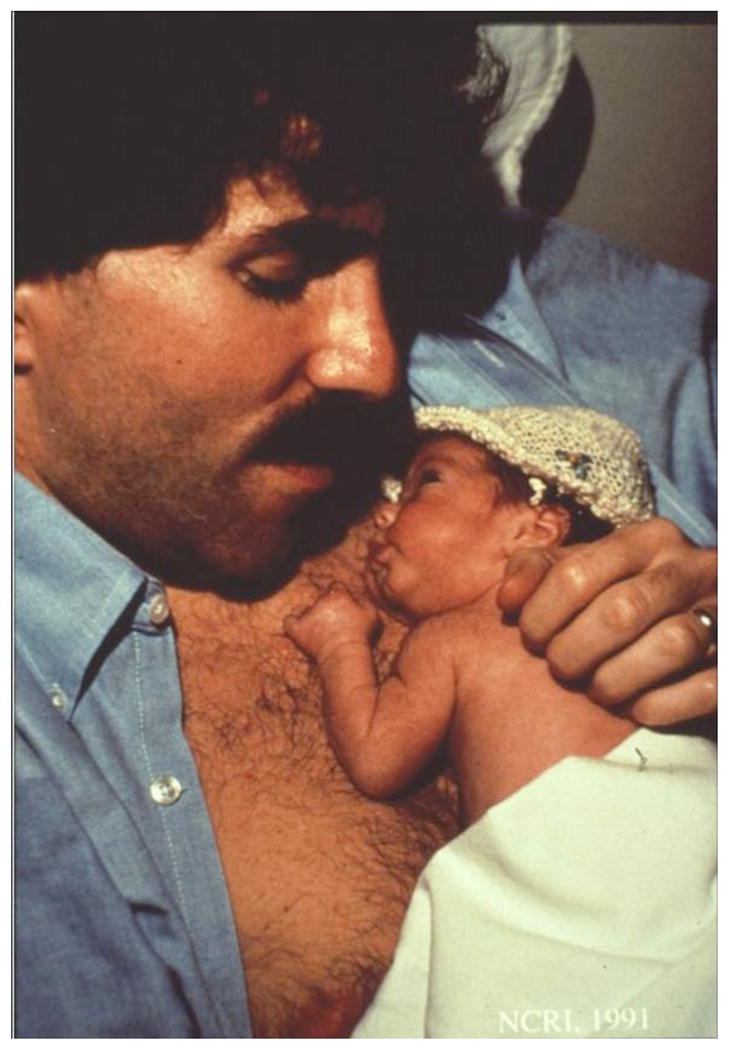
Father holding his preterm infant daughter skin-to-skin in KMC (Brigham and Women’s Hospital Boston, NCRI, 1992).
Fig. (6).

Preterm infant girl with severe fetal growth restriction sleeping on mother’s chest in KMC (Brigham and Women’s Hospital Boston, FGR Study. H. Als, with permission, 2007).
Fig. (7).
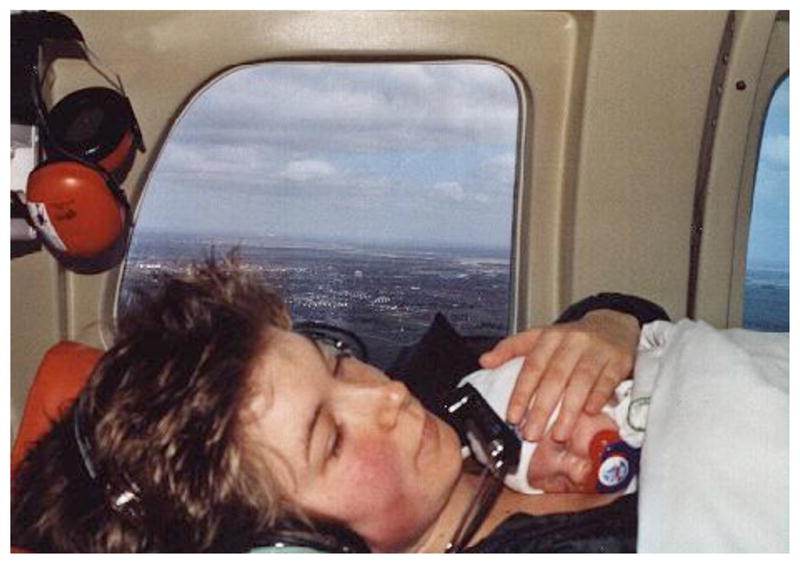
Mother and preterm infant in KMC shown during helicopter transport. (Harzer Kinderklinikum, Wernigerode, Germany; Dieter Sontheimer and Kerstin Buch, with permission, 2010).
Acknowledgments
Supported by National Institutes of Health (NIH) grant R01 HD046855, and a grant from the Irving Harris Foundation to H. Als, PhD; as well as the Children’s Hospital Boston, Intellectual and Developmental Disabilities Research Center (IDDRC) grant P01 HD18655 to Scott Pomeroy, MD, PhD.
References
- 1.Villar J, Merialdi M, Gulmezoglu AM, et al. Characteristics of randomized controlled trials included in systematic reviews of nutritional interventions reporting maternal morbidity, mortality, preterm delivery, intrauterine growth restriction and small for gestational age and birth weight outcomes. J Nutr. 2003;133(5 2):1632–9. doi: 10.1093/jn/133.5.1632S. [DOI] [PubMed] [Google Scholar]
- 2.Creasy RK. Preventing preterm birth. NEJM. 1991;325:727–8. doi: 10.1056/NEJM199109053251009. [DOI] [PubMed] [Google Scholar]
- 3.Savitz DA, Harlow SD. Selection of reproductive health end points for environmental risk assessment. Environ Health Perspect. 1991;90:150–64. doi: 10.1289/ehp.90-1519470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Pediatr Perinatal Epidemiol. 2001;15(2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 5.Heron M, Sutton P, Xu J, Ventura S, Strobino D, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 6.McGrath JM. Developmentally supportive caregiving and technology in the NICU: Isolation or merger of intervention strategies? J Perinat Neonatal Nurs. 2000;14:78–91. doi: 10.1097/00005237-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the national institute of child health and human development neonatal research network, 1993–1994. Pediatrics. 2000;105(6):1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 8.Behrman RE, Stith Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 9.Bohm B, Smedler A-C, Forssberg H. Impulse control working memory and other executive functions in preterm children when starting school. Acta Pediatrica. 2004;93:1363–71. doi: 10.1080/08035250410021379. [DOI] [PubMed] [Google Scholar]
- 10.Saigal S, Stoskopf B, Streiner D, et al. Transition of extremely low-birth-weight infants from adolescence to young adulthood: comparison with normal birth-weight controls. JAMA. 2006;295(6):667–75. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- 11.Marlow N, Hennessy E, Bracewell M, Wolke D, Group ES. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 12.Hack M, Taylor H, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birthweight children at age 8 years. J Devel Behav Ped. 2009;30(2):122–30. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hack M. Care of preterm infants in the Neonatal Intensive Care Unit. Pediatrics. 2009;123(4):1246–47. doi: 10.1542/peds.2009-0122. [DOI] [PubMed] [Google Scholar]
- 14.Hack M, HGT, Drotar D, Schluchter M, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294(3):318–25. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 15.Als H. Program Guide - Newborn Individualized Developmental Care and Assessment Program (NIDCAP): An Education and Training Program for Health Care Professionals. Boston: Copyright, NIDCAP Federation International; 1986. Unpublished Manuscript. rev 2009. [Google Scholar]
- 16.Als H. Toward a synactive theory of development: Promise for the assessment of infant individuality. Inf Mental Health J. 1982;3:229–43. [Google Scholar]
- 17.Babson SG. Growth of low birthweight infants. J Pediatr. 1970;77:11–8. doi: 10.1016/s0022-3476(70)80039-7. [DOI] [PubMed] [Google Scholar]
- 18.Blurton JN. Growing points in human ethology: Another link between ethology and the social sciences. In: Bateson PPG, Hinde RA, editors. Growing Points in Ethology. Cambridge: Cambridge University Press; 1976. pp. 427–51. [Google Scholar]
- 19.Blurton JN. Ethology and early socialization. In: Richards MPM, editor. The Integration of a Child into a Social World. Cambridge: Cambridge University; 1974. pp. 263–95. [Google Scholar]
- 20.Blurton JN. Characteristics of ethological studies of human behavior. In: Blurton Jones N, editor. Ethological Studies of Child Behavior. Cambridge: Cambridge University Press; 1972. pp. 3–37. [Google Scholar]
- 21.Lettvin JY, Maturana H, McCulloch W, Pitts W. What the frog’s eye tells the frog’s brain. Proc I Radio Engineers. 1959;47:1940–51. [Google Scholar]
- 22.Als H, Brazelton TB. A new model of assessing the behavioral organization in preterm and fullterm infants: Two case studies. J Am Acad Child Psychiatry. 1981;20:239–63. doi: 10.1016/s0002-7138(09)60987-0. [DOI] [PubMed] [Google Scholar]
- 23.Als H. The unfolding of behavioral organization in the face of a biological violation. In: Tronick E, editor. Human Communication and the Joint Regulation of Behavior. Baltimore, MD: University Park Press; 1982. pp. 125–60. [Google Scholar]
- 24.Als H, Lester BM, Tronick EZ, Brazelton TB. Towards a research instrument for the assessment of preterm infants’ behavior. In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and Research in Behavioral Pediatrics. New York: Plenum Press; 1982. pp. 35–63. [Google Scholar]
- 25.Als H. Individualized developmental care in the NICU: Estimating expectation for co-regulation. IBD. 1992;15:13. [Google Scholar]
- 26.Als H. Reading the premature infant. In: Goldson E, editor. Developmental Interventions in the Neonatal Intensive Care Nursery. New York: Oxford University Press; 1999. pp. 18–85. [Google Scholar]
- 27.Hassenstein B. Verhaltungsbiologie des Kindes. Muenchen: Piper Verlag; 1973. [Google Scholar]
- 28.Robson K. The role of eye-to-eye contact in maternal-infant attachment. J Child Psychol Psychiatry. 1967;8:13–25. doi: 10.1111/j.1469-7610.1967.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 29.Als H. The human newborn and his mother: An ethological study of their interaction (Doctoral dissertation, University of Pennsylvania, 1975) Dissert Abs Int. 1975;36:5. [Google Scholar]
- 30.Als H. The newborn communicates. J Commun. 1977;27:66–73. doi: 10.1111/j.1460-2466.1977.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 31.Grossman K. Die Wirkung des Augenoffnens von Neugeborenen auf das Verhalten ihrer Mutter. Geburtshilfe und Frauenheilkunde. 1978;38:629–35. [PubMed] [Google Scholar]
- 32.Minde KK, Morton P, Manning D, Hines B. Some determinants of mother-infant interaction in the premature nursery. J Am Ac Child Psych. 1980;19:1–21. doi: 10.1016/s0002-7138(09)60649-x. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg M, Morris N. Engrossment: The newborn’s impact on the father. Am J Orthopsychiatry. 1974;44(4):520–31. doi: 10.1111/j.1939-0025.1974.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 34.Üvnas-Moberg K. The gastrointestinal tract in growth and reproduction. Sci Amer. 1989;261(1):78–83. doi: 10.1038/scientificamerican0789-78. [DOI] [PubMed] [Google Scholar]
- 35.Widstrom AM, Wahlbery V, Matthiesen AS, et al. Short-term effects of suckling and touch of the nipple on maternal behavior. Early Hum Dev. 1990;21:153–63. doi: 10.1016/0378-3782(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 36.Üvnas-Moberg K, Widström AM, Marchini G, Winberg J. Release of GI hormones in mother and infant by sensory stimulation. Acta Paediatr Scand. 1987;76:851–60. doi: 10.1111/j.1651-2227.1987.tb17254.x. [DOI] [PubMed] [Google Scholar]
- 37.Moberg K. The oxycotin factor: Tapping the hormone of calm, lve, and healing. Cambridge, MA: Merloyd Lawrence Books; 2003. [Google Scholar]
- 38.Als H, Duffy FH. The behavior of the fetal newborn: Theoretical considerations and practical suggestions for the use of the APIB. In: Waldstein A, Gilderman D, Taylor-Hershel D, Prestridge S, Anderson J, editors. Issues in Neonatal Care. Chapel Hill, NC: WESTAR/TADS; 1982. pp. 21–60. [Google Scholar]
- 39.Sander LW. Infant and caretaking environment: Investigation and conceptualization of adaptive behavior in a system of increasing complexity. In: Anthony EJ, editor. Explorations in child psychiatry. New York: Plenum Press; 1975. [Google Scholar]
- 40.Sander LW. Investigation of the infant and its environment as a biological system. In: Greenberg SI, Pollock GH, editors. The course of life: psyoanalytic contribution toward understanding personality development. Washington, DC: National Institute of Mental Health; 1980. pp. 177–201. [Google Scholar]
- 41.Sander LW, Stechler G, Burns P, Lee A. Change in infant and caregiver variables over the first two months of life: Integration of action in early development. In: Thomas EB, editor. Origins of the infant’s social responsiveness. Hillsdale, N. J: Lawrence Erlbaum; 1979. [Google Scholar]
- 42.Denny-Brown D. The Basal Ganglia and Their Relation to Disorders of Movement. Oxford: Oxford University Press; 1962. [Google Scholar]
- 43.Denny-Brown D. The Cerebral Control of Movement. Springfield, Ill: Charles C. Thomas; 1966. [Google Scholar]
- 44.Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In: Jones MR, editor. Nebraska Symposium on Motivation. Lincoln: University of Nebraska Press; 1959. pp. 1–42. [Google Scholar]
- 45.Schneirla TC, Rosenblatt JS. Behavioral organization and genesis of the social bond in insects and mammals. Am J Orthopsychiat. 1961;31:223–53. doi: 10.1111/j.1939-0025.1961.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 46.Schneirla TC, Rosenblatt JS. “Critical periods” in the development of behavior. Science. 1963;139:1110–5. doi: 10.1126/science.139.3559.1110. [DOI] [PubMed] [Google Scholar]
- 47.Schneirla TC. Aspects of stimulation and organization in approach and withdrawal processes underlying vertebrate development. Adv Study of Behav. 1965;1:1–74. [Google Scholar]
- 48.Rosenblatt JS. Stages in the early behavioral development of altricial young of selected species of non-primate mammals. In: Bateson PPG, Hinde RA, editors. Growing Points in Ethology. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- 49.Als H, Lester BM, Brazelton TB. Dynamics of the behavioral organization of the premature infant: A theoretical perspective. In: Field TM, Sostek AM, Goldberg S, Shuman HH, editors. Infants Born at Risk. New York: Spectrum Publications; 1979. pp. 173–93. [Google Scholar]
- 50.Als H. The behavior of the fetal newborn: Theoretical considerations and practical suggestions for the use of the APIB. In: Seashore CE, Lewis D, Saetveit DL, editors. Issues in Neonatal Care. WESTAR; New York: 1982. pp. 19–60. [Google Scholar]
- 51.Als H. Cost-effectiveness analysis of developmental care (NIDCAP) in the newborn intensive care unit (NICU) Children’s Hospital; Boston: 1999. Unpublished manuscript. [Google Scholar]
- 52.McCartney G, Hepper P. Development of lateralized behaviour in the human fetus from 12 to 27 weeks gestation. Develop Med Child Neurol. 1999;41(2):83–6. doi: 10.1017/s0012162299000183. [DOI] [PubMed] [Google Scholar]
- 53.Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: Functional status and clinical implications. Clin Perinatol. 2004;31(2):261–85. vi–vii. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Fifer WP, Moon C. Auditory experience in the fetus. In: Smotherman WP, Robinson SR, editors. Behavior of the Fetus. Caldwell, N. J: The Telford Press; 1988. pp. 175–90. [Google Scholar]
- 55.Fifer W, Moon C. Early voice discrimination. In: Euler V, Forssberg CH, Lagercrantz H, editors. Neurobiology of Early Infant Behavior. London: MacMillan Press; 1989. pp. 277–86. [Google Scholar]
- 56.Hepper P. Memory in utero? Develop Med Child Neurol. 1997;39(5):343–6. doi: 10.1111/j.1469-8749.1997.tb07442.x. [DOI] [PubMed] [Google Scholar]
- 57.Als H. Social interaction: Dynamic matrix for developing behavioral organization. In: Uzgiris IC, editor. Social Interaction and Communication in Infancy: New Directions for Child Development. San Francisco, Calif: Jossey-Bass; 1979. pp. 21–41. [Google Scholar]
- 58.Als H. Infant individuality: Assessing patterns of very early development. In: Call J, Galenson E, Tyson RL, editors. Frontiers of Infant Psychiatry. New York: Basic Books; 1983. pp. 363–78. [Google Scholar]
- 59.Erikson EH. Reality and actualization. J Amer Psychoanal Assoc. 1962;10:451–75. [Google Scholar]
- 60.Saigal S, Hoult L, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105(2):325–31. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 61.Hack M, Flanner DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Eng J Med. 2002;346(3):149–57. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 62.Mercuri E, Barnett A, Rutherford M, et al. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics. 2004;113(1):95–100. doi: 10.1542/peds.113.1.95. [DOI] [PubMed] [Google Scholar]
- 63.Marin-Padilla M. Pathogenesis of late-acquired leptomeningeal heterotopias and secondary cortical alterations: A golgi study. In: Galaburda AM, editor. Dyslexia and Development. Cambridge, Mass: Harvard University Press; 1993. pp. 64–89. [Google Scholar]
- 64.Gesell A, Armatruda C. The Embryology of Behavior. Westport CT: Connecticut Greenwood Press; 1945. [Google Scholar]
- 65.Humphrey T. Some correlations between the appearance of human fetal reflexes and the development of the nervous system. Prog Brain Res. 1964;4:93–135. [Google Scholar]
- 66.McCorry NK, Hepper PG. Fetal habituation performance: Gestational age and sex effects. Br J Dev Psychol. 2007;25(2):277–92. [Google Scholar]
- 67.Hepper P, McCartney G, Shannon E. Lateralised behaviour in first trimester human foetuses. Neuropsychologia. 1998;36(6):531–4. doi: 10.1016/s0028-3932(97)00156-5. [DOI] [PubMed] [Google Scholar]
- 68.Nyqvist K, Anderson GC, Bergman N, et al. State of the art and recommendations kangaroo mother care: Application in a high-tech environment. Acta Paediatr. 2010;99:812–9. doi: 10.1111/j.1651-2227.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 69.Nyqvist K, Anderson GC, Bergman N, et al. Towards universal kangaroo mother care: recommensations and report from the first European conference and seventh international workshop on kangaroo mother care. Acta Paediatr. 2010;99:820–6. doi: 10.1111/j.1651-2227.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 70.Lawn J, Mwansa-Kamafwile, Horta B, Barros F, Cousens S. Kangaroo Mother Care to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39(1):i144–i54. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volpe JJ. Neuronal proliferation, migration, organization, and myelination. In: Volpe JJ, editor. Neurology of the Newborn. 4. Philadelphia: WB Saunders; 2001. pp. 45–99. [Google Scholar]
- 72.Duffy FH, Mower GD, Burchfiel JL. Experimental amblyopia production by random monocular shifts of visual inputs. Soc Neurosci Abstracts. 1978;4:625. [Google Scholar]
- 73.Mower GD, Christen WG, Caplain CJ. Very brief visual experience eliminates plasticity in the cat visual cortex. Science. 1983;221:178–80. doi: 10.1126/science.6857278. [DOI] [PubMed] [Google Scholar]
- 74.Duffy FH, Mower GD, Jensen F, Als H. Neural plasticity: A new frontier for infant development. In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and Research in Behavioral Pediatrics. New York: Plenum Press; 1984. pp. 67–96. [Google Scholar]
- 75.Mower GD, Caplan CJ, Christen WG, Duffy FH. Dark rearing prolongs physiological but not anatomical plasticity in the cat visual cortex. J Comp Neurol. 1985;235:448–66. doi: 10.1002/cne.902350404. [DOI] [PubMed] [Google Scholar]
- 76.Mower GD. Role of visual experience in activating critical period in cat visual cortex. J Neurophysiol. 1985;53(2):572–89. doi: 10.1152/jn.1985.53.2.572. [DOI] [PubMed] [Google Scholar]
- 77.Bourgeois JP, Jastreboff PJ, Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: Evidence for intrinsic regulation of synaptic overproduction. Proc NA Sci. 1989;86:4297–301. doi: 10.1073/pnas.86.11.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–8. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 79.Rakic PJ, Bourgeois J, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory and mental illness. In: Von Pelt J, Coiner MA, Uylings HBM, Lopes da Silva PH, editors. The Self-Organizing Brain: From Growth Cones to Functional Networks. Amsterdam: Elsevier Science; 1994. [DOI] [PubMed] [Google Scholar]
- 80.DiPietro J, Allen M. Estimation of gestational age: Implications for developmental research. Child Dev. 1991;62:1184–99. [PubMed] [Google Scholar]
- 81.DiPietro J. Neurobehavioral Assessment. In: Singer L, Zeskind P, editors. Biobehavioral assessment of the infant. New York: The Guilford Press; 2001. pp. 42–80. [Google Scholar]
- 82.Limperopoulos C, Gauvreau K, O’Leary H, et al. Cerebral Hemodynamic Changes During Intensive Care of Preterm Infants. Pediatrics. 2008;122:1006–13. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inder T, Hüppi P, Zientara G, et al. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr. 1999;134:631–4. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 84.Bassan H, Feldman H, Limperopoulos C, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35(2):85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Plotsky P, Meaney M. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 86.Meany MJ, Aitken DH, Berkel CV, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments assoiated with the hippocampus. Science. 1998;239(4841 pt 1):766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 87.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonat. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 88.Bellieni C, Iantorno L, Perrone S, et al. Even routine painful procedures can be harmful for the newborn. Pain. 2009;147:128–31. doi: 10.1016/j.pain.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 89.Kolber B, Boyle M, Wieczorek L, et al. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci. 2010;30(7):2571–81. doi: 10.1523/JNEUROSCI.4470-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conneman N, Oude-Reimer M. Skin to skin holding during medical procedures in the NICU. 19th Annual NIDCAP Trainers Meeting; 2008; Winston-Salem, North Carolina. 2008. [Google Scholar]
- 91.Als H. Manual for the Naturalistic Observation of the Newborn (Preterm and Fullterm). Revision. Boston: The Children’s Hospital; 1984. [Google Scholar]
- 92.Charpak N, Ruiz J, Zupan J, et al. Kangaroo mother care: 25 years after. Acta Paediatr. 2005;94(5):514–22. doi: 10.1111/j.1651-2227.2005.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 93.Hall D, Kirsten G. Kangaroo mother care - a review. Transfus Med. 2008;18:77–82. doi: 10.1111/j.1365-3148.2007.00812.x. [DOI] [PubMed] [Google Scholar]
- 94.Als H. Encyclopedia on Early Childhood Development. Centre of Excellence for Early Childhood Development Website; 2004. Individualized Developmental Care for Preterm Infants. http://www.excellence-earlychildhood.ca/liste_theme.asp. [Google Scholar]
- 95.Als H, Butler S. Die Pflege des Neugeborenen: Die frühe Gehir-nentwicklung und die Bedeutung von frühen Erfahrungen. In: Brisch KH, Hellbrügge T, editors. Der Säugling - Bindung, Neurobiologie und Gene. Stuttgart: Klett-Cotta; 2008. pp. 44–87. [Google Scholar]
- 96.Als H. The Preterm Infant: Brain Development, Early Experience and Implications for Care. In: Sparrow J, Lester B, editors. Nuturing Children and Families: Building on the Legacy of TB. Brazelton: Blackwell Scientific, Wiley; in press. [Google Scholar]
- 97.Sontheimer D, Fischer C, Buch K. Kangaroo transport instead of incubator transport. Pediatrics. 2004;113:920–3. doi: 10.1542/peds.113.4.920. [DOI] [PubMed] [Google Scholar]
- 98.Als H, Lawhon g, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low birthweight preterm infant: Medical and neurofunctional effects. JAMA. 1994;272:853–8. [PubMed] [Google Scholar]
- 99.Fleisher BF, VandenBerg KA, Constantinou J, et al. Individualized developmental care for very-low-birth-weight premature infants. Clin Pediatr. 1995;34:523–9. doi: 10.1177/000992289503401003. [DOI] [PubMed] [Google Scholar]
- 100.Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low-risk preterm infants: Behavioral and electrophysiological evidence. Pediatrics. 1995;96:923–32. [PubMed] [Google Scholar]
- 101.Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized controlled trial to evaluate the effects of the Newborn Individualized Developmental Care and Assessment Program in a Swedish setting. Pediatrics. 2000;105(1):66–72. doi: 10.1542/peds.105.1.66. [DOI] [PubMed] [Google Scholar]
- 102.Als H, Gilkerson L, Duffy FH, et al. A three-center randomized controlled trial of individualized developmental care for very low birth weight preterm infants: Medical, neurodevelopmental, parenting and caregiving effects. J Dev Behav Pediatr. 2003;24(6):399–408. doi: 10.1097/00004703-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Als H, Duffy F, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–57. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 104.McAnulty G, Duffy F, Butler S, et al. Individualized developmental care for a large sample of very preterm infants: Health, neuro-behavior and neurophysiology. Acta Paediatr. 2009;98:1920–6. doi: 10.1111/j.1651-2227.2009.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters K, Rosychuk R, Hendson L, Cote J, McPherson C, Tyebkhan J. Improvement of short- and long-term outcomes for very low birth weight infants: The Edmonton NIDCAP trial. Pediatrics. 2009;124:1009–20. doi: 10.1542/peds.2008-3808. [DOI] [PubMed] [Google Scholar]
- 106.Kleberg A, Westrup B, Wallin L, Lagercrantz H, Wikblad K, Stjernqvist K. Evaluation of the newborn individualized developmental care and assessment program (NIDCAP) in a swedish setting. Prenat Neonatal Med. 1997;2:366–75. [Google Scholar]
- 107.Neu M, Browne JV, Vojir C. The impact of two transfer techniques used during skin-to-skin care on the physiologic and behavioral responses of preterm infants. Nurs Res. 2000;48:215–23. doi: 10.1097/00006199-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) Early Hum Dev. 2002;68:83–91. doi: 10.1016/s0378-3782(02)00014-2. [DOI] [PubMed] [Google Scholar]
- 109.Kleberg A, Westrup B, Stjernqvist K. Developmental outcome, child behavior and mother-child interaction at 3 years of age following Newborn Individualized Developmental Care and Intervention Program (NIDCAP) intervention. Early Hum Dev. 2000;60:123–35. doi: 10.1016/s0378-3782(00)00114-6. [DOI] [PubMed] [Google Scholar]
- 110.McAnulty G, Duffy F, Butler S, Bernstein J, Zurakowski D, Als H. Effects of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) at age eight years: Preliminary data. Clin Pediatr (Phila) 2010;49(3):258–70. doi: 10.1177/0009922809335668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Als H, Duffy FH, McAnulty GB, Blickman JG, Gibes-Grossman R, Lawhon G. Developmental care for very low-birth-weight infants. Reply to letters to the editor. JAMA. 1995;273(20):1577–8. [Google Scholar]
- 112.Petryshen P, Stevens B, Hawkins J, Stewart M. Comparing nursing costs for preterm infants receiving conventional vs. developmental care. Nurs Econ. 1997;15:138–50. [PubMed] [Google Scholar]