Abstract
The triadic neural systems model is a heuristic tool, which was developed with the goal of providing a framework for neuroscience research into motivated behaviors. Unlike dual models that highlight dynamics between approach systems centered on striatal function and control systems centered on prefrontal cortex, the triadic model also includes an avoidance system, centered on amygdala-related circuits. A first application of this model has been to account for adolescent behavior.
Keywords: Systems model, Motivation, Regulation, Reward, Avoidance, Cognitive control striatum
1. Adolescent motivated behavior, and brain development
1.1. Adolescent behavior
Adolescence is a distinct transition period from childhood to adulthood, with unique characteristics and behaviors. This period represents both a time of opportunity for building the roots of a successful and fulfilling adult life, and a time of vulnerability owing to the adverse consequences of the typical impulsive/risky adolescent behaviors (Arnett, 1999; Dahl, 2004; Ernst & Hardin, 2009; Ernst, Pine, & Hardin, 2006) and a unique vulnerability to mental problems (Costello et al., 2002; Kessler, Berglund, Demler, Jin, & Walters, 2005).
The most commonly recognized characteristics of concern include cognitive impulsivity and emotional intensity and lability (Arnett, 1999; Dahl, 2004; Ernst & Hardin, 2009; Ernst et al., 2006). These cognitive and affective features are thought to place adolescents at an increased risk for engaging in behaviors with deleterious and dangerous consequences, such as tobacco and drug use, risky sexual activity, or reckless driving (Dahl, 2004; Eaton et al., 2006; Hingson, Heeren, Winter, & Wechsler, 2005; Spear, 2000; Steinberg, 2004, 2005). Inter-individual variability within this stereotypical description of the adolescent is large, and can be traced to hormonal changes (e.g., (Bramen et al., 2011; Forbes et al., 2010; Kuhn et al., 2010; Mazzone et al., 2011; Mueller, Ng, et al., 2010; Neufang et al., 2009; Oldehinkel, Verhulst, & Ormel, 2011), early life experience (Andersen & Teicher, 2009; Mueller, Maheu, et al., 2010; Pechtel & Pizzagalli, 2011; Suomi, 2006), genetic make-up (Cohen, 2010; Enoch, 2011; Schwandt et al., 2010), among many other factors. A better understanding of the underlying factors contributing to interindividual variability can be tremendously helpful for identifying targets for future primary and secondary treatment of untoward outcomes related to adolescent behavior. We now turn to the description of changes in brain maturation across this time window.
1.2. Coordinated brain development
Substantial neural development accompanies the rise in impulsivity, emotionality, and risk seeking over the course of adolescence. The normative trajectory of adolescent neural development is becoming well-characterized with the advent of MRI technology. These neural changes are charted at the structural, functional and connectivity level using neuroimaging tools. Most importantly and best illustrated at the structural level, different brain regions and neural circuits seem to mature along distinct trajectories, which may be orchestrated along unique predetermined timelines. The adherence to these predetermined timelines may be critical to the harmonious development of brain function as a whole (see Fig. 1) (Brain Development Cooperative Group, 2012; Casey, Getz, & Galvan, 2008; Giedd et al., 1999; Gogtay et al., 2004; Sowell et al., 2004). Similar coordinated temporal changes may also operate across complementary and interactive units of function, such as across neurotransmitter systems (e.g., dopaminergic vs. serotonergic vs. adrenergic vs. cholinergic systems) (Chambers, Taylor, & Potenza, 2003; Ernst & Fudge, 2009; Spear, 2000; Wahlstrom, Collins, White, & Luciana, 2010; Wahlstrom, White, & Luciana, 2010) or within a given neurotransmitter system (e.g., D2 dopamine receptor vs. D1 dopamine receptor; GABA receptors) (Andersen, 2003; Huppe-Gourgues & O’Donnell, 2012; Lewis, Melchitzky, & Burgos, 2002).
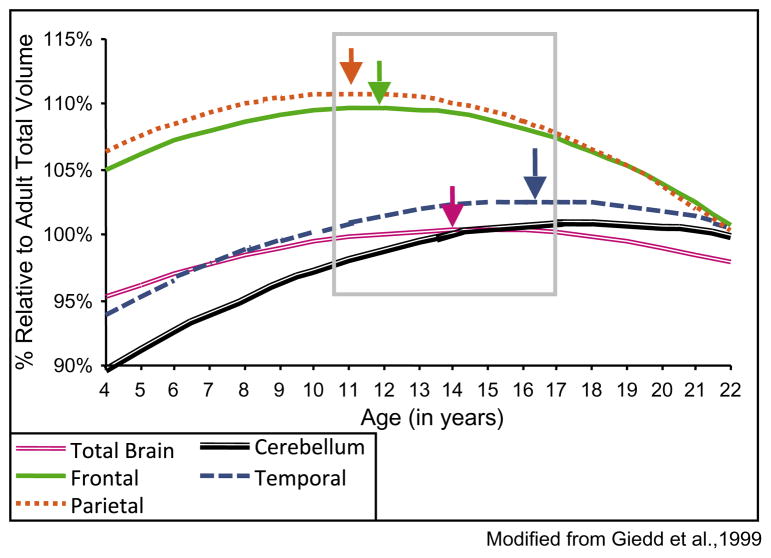
Fig. 1.
Developmental trajectory of individual brain regions. Distinct brain regions reach adult maturity along variable, chronologically determined, time courses. Deviance in the chronology of the brain systems development may affect adolescents’ abilities to successfully recruit and control such systems.
At the histological level, substantial cell, dendrite, and synapse proliferation and then elimination proceed with time (Rubia et al., 2006; Toga, Thompson, & Sowell, 2006) (see Fig. 2). These cellular changes lead to more selective and refined information processing. Together with axonal caliber enlargement, myelination contributes to the age-related decrease in gray matter and increase in white matter (Giedd, 2004; Gogtay et al., 2004; Paus, Keshavan, & Giedd, 2008; Paus et al., 1999). Diffusion tensor imaging (DTI) provides a measure, i.e., fractional anisotropy (FA), thought to reflect the diameter, density and myelination of white matter fibers that connect brain regions (Giorgio et al., 2008; Snook, Paulson, Roy, Phillips, & Beaulieu, 2005). Across adolescence, the increases in FA in major white matter pathways support the active myelination process during this period combined with the increase in axonal diameter (Paus, 2010), and have been associated with improved cognitive function (Fitzgerald et al., 2010; Muetzel et al., 2008; Nagy, Westerberg, & Klingberg, 2004; Olson et al., 2009). Accordingly, myelination speeds up the transmission of information over long distances (e.g., cross-hemispheric projections), and ultimately provides more efficient transmission of information.
Fig. 2.
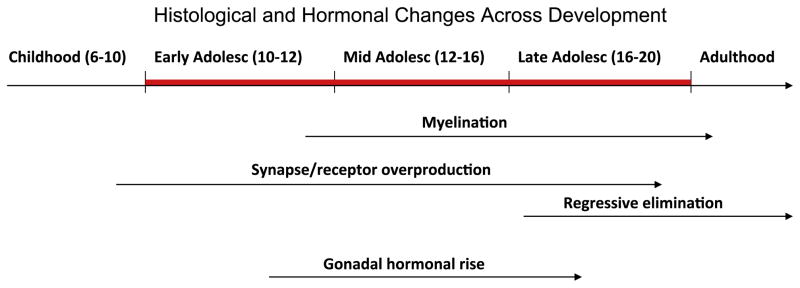
Brain development with age. Several changes occur at the histological and hormonal levels and continue across development. Synapse overproduction begins in mid-to- late childhood and is followed later by regressive elimination in late adolescence. Myelination, associated with the increase of white matter and related decrease in gray matter begins in early adolescence and continues through young adulthood. Gonadal hormonal rise, part of puberty, characterizes this period.
The notion of a predetermined timetable of the progression of various neural changes (e.g., loss of gray matter is last in the superior temporal cortex) (Gogtay et al., 2004) has critical implications. Indeed, important behavioral consequences can result from a disruption within this predetermined order. Behavioral perturbations could emerge from the sub-optimal coordination among nodes or modules organizing behavioral output, and not necessarily from selective local regional abnormalities. This scenario emphasizes interregional influences (i.e., functional connectivity) and the role of potential imbalances in neural maturation across various brain regions, each of which is implicated in specific behavioral patterns. This formulation of functional brain development serves as the foundation of the triadic neural systems model.
2. The triadic model
2.1. Introduction to the triadic neural systems model
The triadic model (see Fig. 3) attributes the determinants of motivated behavior to three functional neural systems, which are distributed networks centered on the prefrontal cortex, striatum and amygdala. These systems are expected to mature along a predetermined order. This coordinated predetermined order is thought to affect the typical age-related changes in behavior. These three neural systems are supported by independent, but overlapping networks, and subsume basic complementary functions (Table 1).
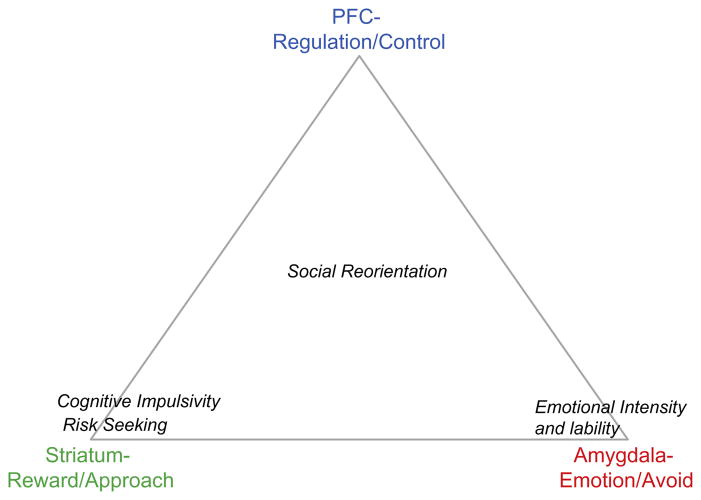
Fig. 3.
The triadic model. The prefrontal cortex (PFC) has a reciprocal relationship with the striatum and amygdala, and the amygdala projects directly to the striatum. Within the triadic model the striatum represents the motivation system, and is associated with approach; the amygdala represents the emotion system, particularly responses to aversive (e.g., fearful) stimuli, and plays a significant role in avoidance; and the prefrontal cortex is the regulatory center, which serves to control approach and avoidance behaviors. Of the four behaviors typically observed in adolescence, the striatum is chiefly responsible for risk seeking and cognitive impulsivity; the amygdala for emotional intensity and lability. Social reorientation involves interactions among all three systems.
Table 1.
Main components and function of the triadic modules.
| Modules of the triadic model | ||
| Approach | Avoidance | Regulation |
| Main structures | ||
| Striatum | Amygdala | Dorsolateral PFC |
| Orbitofrontal cortex | Hippocampus Insula |
Ventromedial Orbital PFC Anterior cingulate cortex |
| Function | ||
| Appetitive stimuli | Aversive stimuli | Salience detection |
| Valence/salience value | Valence/salience value | Executive attention |
| Motivation | Fear responses | Motor control |
| Motor response | Threat avoidance | Conflict detection |
| Positive affect | Negative affect | Conflict monitoring Conflict resolution |
Globally, these three networks are respectively implicated primarily in motivation/approach, emotion/avoidance and regulation. Motivation and emotion are very closely related and interdependent, but they define different constructs.
Motivation: Operationally, motivation determines the energy that fuels behavior, and can be measured by the amount of effort subjects are willing to exert for attaining their goals. Interestingly, most research on motivation has focused on approach behavior in response to incentives. “Motivation to avoid” has rarely been examined as a general aspect of motivation, but rather as part of anxiety disorders. Thus, it is unclear whether different neural circuits underlie motivation to avoid vs. motivation to approach, and this question still needs to be queried. In the triadic model, the neural system coding for approach (dopaminergic, striatal network) is co-labeled with “motivation”, because this dopaminergic mesolimbic system is the most commonly found to be engaged in studies of motivation, rewards, and positive emotions.
Emotion: Emotion defines internal subjective states that influence the direction of subjects’ actions (see Table 2: vignettes 1, 2). Simplistically, positive emotions are associated with approach, and negative emotions with avoidance. At present, self-reports remain the most common measure of emotion. We labeled the neural system associated with avoidance (amygdala-centered) as the “emotion/avoidance system” because this system is most prominently associated with avoidance and [negative] emotions. Here again, this classification may be due to an artifact of the disproportionate amount of research on avoidance associated with this system, rather than a true functional bias towards the coding of negative emotion and associated avoidance behavior. This question will be important to clarify in future work.
Regulation: Regulation refers to the control of the motivational and emotional vectors, at the service of more complex, higher-level behavioral operations (see Table 2: vignette 3). The effectiveness of regulation can be measured as changes in motivation or emotion upon instructions. Measures of behavioral flexibility can be used as proxies of the capacity for regulation.
Table 2.
Vignettes illustrating the influence of emotion and cognitive control on approach, avoidance, and regulation modules.
| Vignette 1: “Happy to be on vacation, John wants to go to the movies and focuses on the films he remembers having read about to finally select, go and enjoy the movie” |
| Comment: In this vignette, the positive emotion facilitates the motivation to work (i.e., attention, memory) for approaching and consuming a goal |
| Vignette 2: “Unhappy about being reprimanded for not taking the trash out, John decides to not do his homework” |
| Comment: In this vignette, the negative emotion is associated with the motivation to avoid a previous goal |
| Vignette 3: “Peter, an older peer, arrives at the party and passes around marijuana. Even though John has a great time (emotion), he tells himself that the party will not be as enjoyable (emotion regulation) and decides to leave (control)” |
| Comment: In this vignette, cognitive regulation is used to avoid a potentially dangerous situation |
A few more considerations regarding the constructs of motivation and emotion need to be addressed. Somewhat consistent with the inter-dependence of the psychological processes of motivation and emotion (e.g., stronger emotions yield stronger motivation), the underlying neural systems are also highly inter-dependent, not only anatomically (see next section), but also functionally. For the sake of a simple, heuristic model, the functional boundaries between these neural systems are presented as finite and unambiguous. However, in reality, significant overlaps characterize these processes from a neural, functional and psychological standpoint. These overlaps become important when considering potential functional biases within these neural systems. Putting aside the potential bias in neuroscience research itself alluded to above, functional neuroimaging studies seem to show that the motivation system, centered on the striatum, shows a bias towards the coding of appetitive processes (reward), whereas the emotion system, centered on the amygdala, shows a bias towards the coding of fear (threat) (Ernst & Fudge, 2009). Of note, these biases complement each other, and the advantage of such functional organization, if real, still remains to be understood. From an evolutionary perspective, these biases may facilitate adaptive behavior geared to the ultimate goal of survival and reproduction (Spear, 2000). In addition, since the different life stages (e.g., childhood, adolescence, adulthood) face unique challenges (e.g., learning, reaching independence, reproducing/producing), these functional biases may also evolve with age. For example, such biases are expected to be affected by the changes in reward-related behaviors described in adolescence (Somerville, Jones, & Casey, 2010; Spear, 2000; Van Leijenhorst et al., 2010), and which have been mapped to changes of striatal function within the motivation node (see below).
2.2. The triadic neural systems model applied to adolescent behavior
The platform of the three modules (1-approach/reward, 2-avoidance/emotion, and 3-control/regulation), which compose the triadic model, provides a basis for studying behavioral responses, and more specifically here, the typical adolescent behaviors that include cognitive impulsivity, risk seeking, emotional intensity and lability, and social reorientation. How these adolescent behaviors can be operationalized along the triadic model template is schematized in Fig. 3.
Cognitive impulsivity (i.e., inability to delay gratification (Christakou, Brammer, & Rubia, 2011; Romer, Duckworth, Sznitman, & Park, 2010) and risk seeking reflect the combination of a hyperactive reward module (serving to approach stimuli or situations) combined with a unique modulation of the emotion-related module (enhanced delay cost, and reduced avoidance of potentially negative stimuli or situations, respectively), as well as a hypo-efficient control region unable to regulate increased reward-seeking.
Emotional intensity and lability indicate poor regulation of emotional responses, a reflection of the poor capacity of the regulatory module to modulate the emotion network. Much work is being conducted to understand the interplay between cognitive regulation and emotion information processing (Pessoa, 2008). This question is particularly salient to research in typical as well as deviant development and psychopathology (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010)
Finally, social reorientation represents a switch in social values, both in terms of magnitude (affective intensity) and quality (switch from familial to peer), and may reflect a re-attribution of positive and negative values to social stimuli (Ernst & Hardin, 2009). This shift in social orientation is likely to originate from the interaction among all three maturing modules of the triadic model, in addition to a reorganization of the social neural circuitry (Blakemore, 2008; Nelson, Leibenluft, McClure, & Pine, 2005).
As briefly described above, four of the distinct features of adolescent behavior, i.e., cognitive impulsivity, risk-seeking, emotional intensity and lability, and social reorientation, can be mapped onto dynamics of the triadic neural systems. These behavioral patterns result from unique and distinct equilibrium states, reached at any given point in time, across the three triadic nodes. The nature of these equilibrium states are age-dependent, and are specific to a given goal/task (e.g., choose between homework and facebook; engage conversation with a peer or leave the room). Most critically however, these equilibrium states are modulated by both transient and sustained factors. Transient factors include individual mental state (e.g., depressed, stressed), physical state (e.g., drug action), or context (e.g., school, home, social, non-social). Sustained factors include individual psychological traits (e.g., inhibited temperament), maturation level (age; puberty), genetic make-up, past experiences (e.g., early deprivation), and gender. These factors are critical as they contribute to the large inter-individual variability in behavioral responses.
2.3. The neural basis of the triadic neural systems model
The specific neural regions central to the model include the striatum given its role in reward, approach, and habitual behaviors (Di Chiara & Bassareo, 2007; Kringelbach, 2005; Wise, 2004); the amygdala for its involvement in emotion, threat, and social information processing (Davis, 2006; LeDoux, 2000; Pine, 2007; Rauch, Shin, & Wright, 2003); and the prefrontal cortex (PFC), which modulates affective and cognitive processes (Amodio & Frith, 2006; Aron, Robbins, & Poldrack, 2004; Bush, Luu, & Posner, 2000; Carter & van Veen, 2007; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). These modules refer to reward, emotion, and regulation from the perspective of their dominant function, and to approach, avoidance, and control from the perspective of their behavioral correlates (see Table 1). We briefly review the neural substrates of the components of the triad (Fig. 4A).
Fig. 4.
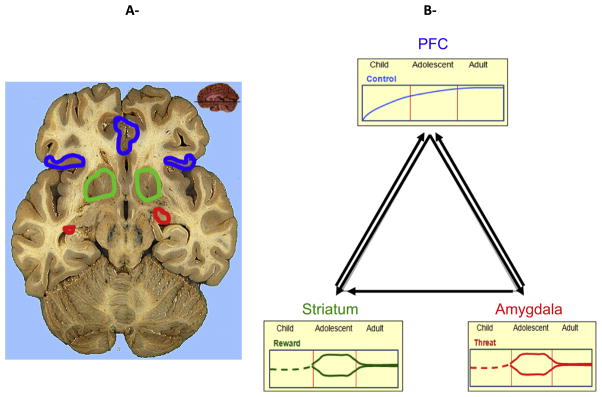
Development pattern of neural substrates of the triadic model. The PFC develops linearly with age, with typical adults possessing the most mature, efficient systems. The striatum and its related systems show a hyper-responsive peak in adolescence in positive contexts, but can also present reduced responsivity in conditions of various cognitive demands. Similarly, the amygdala-centered network has been shown to be hyper-responsive to threat in adolescents, but hyporesponsive in a reward context.
The approach module refers to the reward-related neural system. This neural system comprises subcortical and cortical structures that are major sites of dopamine action, and include primarily the striatum (caudate nucleus, putamen and nucleus accumbens) and the medial and orbital prefrontal cortices (Jensen et al., 2003; Kringelbach, 2005). Behaviorally, appetitive motivational processes seem to follow a curvilinear developmental trajectory, whereby reward sensitivity peaks in adolescence in positively valenced contexts (Ernst & Spear, 2009). However, in other contexts, as in negative contexts or associated with “motivation to act”, the striatum has been found to be less responsive in adolescents than in adults (Bjork, Smith, Chen, & Hommer, 2010; Bjork et al., 2004).
The avoidance module refers to the emotion-related neural system. Although this system is involved in both positive and negative emotions, as indicated above, it seems to be distinctly implicated in threat-related processes (e.g. LeDoux, 2000). This module comprises the amygdala, hippocampus, and insula, which are consistently associated with response to aversive stimuli (Hardin, Pine, & Ernst, 2009; Rauch et al., 2003). Behaviorally, emotion-related processes also seem to follow a curvilinear function, by which emotional responses peak in intensity and lability in adolescence, particularly in negatively valenced contexts (Arnett, 1999; Larson, Moneta, Richards, & Wilson, 2002; Silk, Steinberg, & Morris, 2003; Weinstein, Mermelstein, Hankin, Hedeker, & Flay, 2007). However, in positively valenced context, the amygdala may be less responsive in adolescents than in adults (Ernst et al., 2005).
The control module refers to regulatory processes that modulate subcortical function, (i.e., the approach and the avoidance systems), through “top-down” cognitive regulation. This module relies on prefrontal cortical structures that carry specialized functions, such as inhibition (right inferior prefrontal cortex) (Aron et al., 2004; Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007; Liddle, Kiehl, & Smith, 2001), working memory and cognitive salience detection (dorsolateral prefrontal cortex) (Rubia, Hyde, Halari, Giampietro, & Smith, 2010), conflict detection, monitoring and resolution (anterior cingulate cortex) (Amodio & Frith, 2006; Bush et al., 2000; Carter & van Veen, 2007). Behaviorally, regulatory processes mature linearly with age (Marsh et al., 2006; Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2006).
The next section summarizes work with functional neuroimaging focusing on decision-making and motivation in adolescents. Of note, basic studies with adolescent animal models will not be reviewed, but this topic is fairly well covered in Ernst and Fudge (2009) and Ernst and Spear (2009).
3. Functional neuroimaging of behavioral responses to incentives that can inform the triadic model
The various findings from functional neuroimaging studies comparing different age groups on reward-related processes have been difficult to reconcile. The relatively small number of studies combined with the use of different paradigms across studies is partly responsible for this lack of coherence. Fig. 4B schematizes developmental changes in the function of the triadic systems, with the control system showing linear refinement with age (PFC, blue line); the approach/motivational (striatum, green line) and the avoidance/emotion (amygdala, red line) systems showing quadratic trajectories, with either heightened or dampened reactivity in the adolescence relative to childhood or adulthood, as a function of internal and external conditions. Against this backdrop, we will review the neuroimaging findings organized by type of paradigms, and will begin with a description of three fundamentally different paradigm structures.
3.1. FMRI task structures to study reward-related/motivated behavior
Three basic paradigm structures have been employed: (1) passive receipt of reward/punishment, (2) performance of a task to receive a reward, and (3) task of decision-making among options with different types of rewards.
The first and simplest task consists of the passive exposure to pleasant or unpleasant stimuli. Pleasant stimuli can be the receipt of monetary gains or the presentation of attractive faces. Negative stimuli can be the withdrawal of money or the presentation of angry or fearful faces. In a passive task, no action is required from the subjects. This type of “passive viewing” task is mostly used in research on emotion rather than motivation or reward per se. However, some studies, like the slot-machine task (Van Leijenhorst & Crone, 2009), specifically target reward-related circuits. Another example (Guyer et al. (2008) is a typical emotional probe study, with the inclusion of a passive viewing condition. In general, neural activation can be assessed either during the pre-stimulus period, particularly if a cue precedes the stimulus (anticipation phase), or during the stimulus presentation (feedback or outcome phase). A major drawback of this category of tasks is the absence of behavioral or subjective measures that permit to interpret individual variability in neuroimaging findings.
The second type of task is what we call a reward test-task. For this type of task, participants are asked to complete an action (i.e., pass a test) to obtain a reward, and this action is not directly related to the reward itself. These tests can involve cognitive and/or motor processes that need to be performed correctly to obtain the reward. For example, the test could consist of a memory challenge as in the Pirate’s paradigm (Galvan et al., 2006), or a timed response as in the monetary incentive delay task (Knutson, Fong, Adams, Varner, & Hommer, 2001). Many factors can be manipulated in these paradigms, including the difficulty of the test and the nature of the processes involved (e.g., cognitive, motor, or perceptual challenge). Similar to the passive exposure tasks, these reward test-tasks can include an initial cue (cue stage) informing the nature of the trial to come (e.g., reward vs. loss), or the amount of reward associated with the trial. Whereas some of these reward test-tasks only target reward processes, other studies use these tasks to examine the interactions between reward processes and cognitive/motor processes (Geier et al., 2010; Hardin, Mandell, et al., 2009; Jazbec, McClure, Hardin, Pine, & Ernst, 2005).
Lastly, the third type of task is a reward decision-making task. Here, participants are asked to choose among options. Options may differ as a function of the probability, magnitude or type of associated reward. These tasks are probably the most complex ones because of the number of processes involved in decision-making. The formation of a preference, which guides the decision, is what is unique to these tasks. Of note, the boundary between a test-task and a decision-making task can be subtle, and subject to controversy. For example, we place the Cake Gambling task (Van Leijenhorst, Crone, & Bunge, 2006) in the category of test-tasks, because there is an uncontroversial correct and incorrect response, the correct response being the largest slice of the cake, which corresponds to the highest likelihood of getting a reward. This slice selection, therefore, does not depend on subjects’ preference, but on a perceptual discrimination of size. However, because this test has been framed as making a decision for the largest slice, this task is usually referred to a “reward decision-making task”.
Finally, the inclusion of the three types of task in a single paradigm opens the possibility of assessing the effect of agency on reward processes (Bar-Haim et al., 2009). After a review of the literature along the lines described above (Richards et al., 2013), we present a summary of the findings by type of task.
3.2. Passive exposure tasks
Studies of passive exposure to incentives suggest that when probabilistic rewards are presented passively, the insula, within the emotion module, seems to be more responsive in adolescents than in adults during reward cue appraisal (Guyer et al., 2008).
The reward module (striatum and OFC), on the other hand, shows an effect of age on regional activation in response to stimulus valence: striatal activation is greater in adolescents than in adults in response to appetitive stimuli, but OFC activation is greater in adults than in adolescents in response to aversive stimuli (Van Leijenhorst & Crone, 2009).
In response to negative stimuli, adolescents show less activation of OFC (Van Leijenhorst & Crone, 2009) and greater activation of the amygdala (Guyer et al., 2008) than adults. As often evoked in studies of emotion regulation (Somerville et al., 2010), these findings may reflect a weaker OFC/vPFC recruitment that does not moderate the amygdala response during aversive stimulation in adolescents compared to adults.
3.3. Reward test-task
Tasks including a cognitive/motor performance to obtain a reward are multi-stage. They can include a first stage of cue presentation, which signals the upcoming type of trial (e.g., rewarded, not rewarded), a second stage of performance preparation, a third stage of test execution, and a last stage of the incentive delivery. During the cue-appraisal stage, adolescents activate the striatum less than adults (Bjork et al., 2004, 2010; Geier et al., 2010), but during the performance preparation stage, adolescents activate striatum more than adults, particularly in reward-trials (Geier et al., 2010; Somerville & Casey, 2010). The distinction between these two early stages of reward processes, which has not been explicitly noted in the literature, appears to be quite important, based on the differential relative reliance on striatal function by adolescents and adults. More work is needed to validate this observation. Finally, during positive feedback stage, stronger striatal activation (Cohen et al., 2010), and, during negative feedback, weaker OFC activation (Van Leijenhorst et al., 2006) emerged in adolescents compared to adults. Of interest, these reward testtasks do not seem to modulate amygdala or insula differentially as a function of age.
3.4. Decision-making tasks
Finally, in decision-making tasks, the early stage of cue-appraisal/selection is associated with greater striatal activation in adolescents than adults (Christakou et al., 2011). However, discrepant findings in OFC and mPFC emerged, e.g., greater activation in adults than adolescents in risky vs. non-risky decisions (Eshel, Nelson, Blair, Pine, & Ernst, 2007), and greater activation in adolescents than in adults in more social decisions (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011). More work is warranted to resolve these conflicting results. During the feedback stage, favorable outcomes are associated with greater striatal activation in adolescents than adults (Ernst et al., 2005; Van Leijenhorst et al., 2010), but greater activation in the amygdala in adults than adolescents (Ernst et al., 2005). Thus, studies of decision-making inform the functional contributions of the three neural modules of the triadic model, and reflect the important notion that the equilibrium among these systems depend on the types of cognitive demands and reward conditions associated with the decision-making task (see Research note).
5. Conclusion
This review outlines a neural systems model underlying the coding of motivated behaviors. This model has been used to account for neurodevelopmental changes that accompany the typical behaviors of adolescence, particularly the propensity for risk taking and the onset of psychiatric disorders.
This model posits a functional equilibrium among three distinct but overlapping distributed networks. The functional maturation of these networks follows structural and neurochemical neurodevelopmental trajectories, among which the dopaminergic system occupies a privileged place, owing to its central role in the coding of motivated behavior (e.g., Wahlstrom, Collins, et al., 2010; Wahlstrom, White, et al., 2010). Much more needs to be learned about the contribution of neurochemical and molecular changes to the function and interaction among these neural networks. Finally, but critically, the triadic equilibrium depends on internal and external, as well as sustained and transient factors, and varies across individuals.
The understanding of the mechanisms that regulate this triadic equilibrium across development is slowly emerging. Contradictory findings regarding the relative function of the triadic nodes need to be examined closely. Here, we show how the nature of the paradigms used to probe these nodes can explain distinct results. Many other factors will need to be considered, at the individual (e.g., genetic, early experiences, temperament) and contextual (e.g., social vs. nonsocial, threatening vs. safe, probabilistic vs. certain context) level.
We hope that this review will stimulate integrated and systematic studies of motivated behaviors across adolescence, and in mental illnesses, and help with the choice of judicious and well-controlled paradigms in which the various stages in motivated behaviors are carefully considered.
Research note
Comparison of two decision-making tasks, during the appraisal/decision stage. Christakou et al. (2011) used a delay discounting task which asked participants to choose between receiving a smaller amount of money now and a larger amount of money a week, a month or a year later. They tested 40 healthy males, 12–32 years old. This task only featured stages of appraisal of options, and expression of preference through the selection of one of the options. Across all trials, independent of the type of selection, adults activated more than adolescents the vmPFC and dlPFC (part of the control module). In contrast adolescents activated more than adults vlPFC, ventral striatum, and insula (part of the motivation [ventral striatum] and emotion [insula] modules).
Eshel et al. (2007) used a 2-option gambling task, during which participants (18 adolescents and 16 adults) were asked to choose between a probable smaller amount of money and an improbable larger amount of money. Here, adults showed greater activation in OFC and mPFC (part of the control [mPFC] module and motivation [OFC] module) than adolescents.
These two tasks differed in the manipulation of the reward value, one using a delay, and the other using probability. In both cases, during appraisal/selection, adolescents activated more than adults components of the motivation module, and adults activated more than adolescents components of the control module. However, the identity of these components differed between studies, which may be accounted for by the differing parameters of the tasks. Much more work needs to be done to fully understand the specificity and specialization of the different.
References
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neuroscience and Biobehavioral Reviews. 2009;33(4):516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54(5):317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. The Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 Years: The NIH MRI study of normal brain development. Cerebral Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive Affective and Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience. 2007;19(1):69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Cohen D. Probabilistic epigenesis: An alternative causal model for conduct disorders in children and adolescents. Neuroscience and Biobehavioral Reviews. 2010;34(1):119–129. doi: 10.1016/j.neubiorev.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, et al. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MA, et al. Development and natural history of mood disorders. Biological Psychiatry. 2002;52(6):529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fearpotentiated startle. American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: What dopamine does and doesn’t do. Current Opinion in Pharmacology. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, et al. Youth risk behavior surveillance-United States, 2005. The Journal of School Health. 2006;76(7):353–372. doi: 10.1111/j.1746-1561.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Hardin M. Neurodevelopment underlying adolescent behavior. In: Zelazo PD, Chandler M, Crone E, editors. Developmental Social Cognitive Neuroscience. New York: Psychology Press; 2009. [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Spear L. Reward systems. In: de Haan M, Gunnar M, editors. Handbook of Developmental Social Neuroscience. New York: Guilford Press; 2009. pp. 324–341. [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biological Psychiatry. 2010;68(11):1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(2):162–172. e161–e165. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Journal of Child Psychology and Psychiatry. 2009a;50(12):1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Pine DS, Ernst M. The influence of context valence in the neural coding of monetary outcomes. Neuroimage. 2009;48(1):249–257. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: Changes from 1998 to 2001. Annual Review of Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Huppe-Gourgues F, O’Donnell P. Periadolescent changes of D(2)–AMPA interactions in the rat nucleus accumbens. Synapse. 2012;66(1):1–8. doi: 10.1002/syn.20976. [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biological Psychiatry. 2005;58(8):632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, et al. The emergence of gonadal hormone influences on dopaminergic function during puberty. Hormones and Behavior. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Development. 2002;73(4):1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Burgos GG. Specificity in the functional architecture of primate prefrontal cortex. Journal of Neurocytology. 2002;31(3–5):265–276. doi: 10.1023/a:1024174026286. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Mueller SC, Maheu F, VanRyzin C, Merke DP, Ernst M. Emotional memory in early steroid abnormalities: An fMRI study of adolescents with congenital adrenal hyperplasia. Developmental Neuropsychology. 2011;36(4):473–492. doi: 10.1080/87565641.2010.549866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, et al. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Ng P, Sinaii N, Leschek EW, Green-Golan L, VanRyzin C, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. European Journal of Endocrinology. 2010b;163(5):801–810. doi: 10.1530/EJE-10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel AM, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39(4):1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social reorientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J. Mental health problems during puberty: Tanner stage-related differences in specific symptoms. The TRAILS study. Journal of Adolescence. 2011;34(1):73–85. doi: 10.1016/j.adolescence.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9-to 23-year-olds: A diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition. 2010;72(1):26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd J, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment, Neuroscience. Biobehavioral Reviews. 2013;37(5):976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Romer D, Duckworth AL, Sznitman S, Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prevention Science. 2010;11(3):319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual–spatial attention allocation. NeuroImage. 2010;51(2):817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior Cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Chen S, Higley JD, Suomi SJ, Heilig M, et al. Alcohol response and consumption in adolescent rhesus macaques: Life history and genetic influences. Alcohol. 2010;44(1):67–80. doi: 10.1016/j.alcohol.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence – What changes, and why? Adolescent Brain Development: Vulnerabilities and Opportunities. 2004;1021:51–58. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Crone EA. Paradoxes in adolescent risk taking. In: Zelazo PD, Chandler M, Crone EA, editors. Developmental Social Cognitive Neuroscience. New York: Psychology Press; 2009. [Google Scholar]
- Van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44(11):2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain and Cognition. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein RJ, Hankin BL, Hedeker D, Flay BR. Longitudinal patterns of daily affect and global mood during adolescence. Journal of Research on Adolescence. 2007;17(3):587–599. doi: 10.1111/j.1532-7795.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]