Abstract
Sugarcane bagasse (SCB), a lignocellulosic byproduct of juice extraction from sugarcane, is rich in cellulose (40-42%). This could be used as a substrate for the production of cellulase complex. Fermentation conditions were optimized for production of cellulase complex (CMCase, Cellulobiase and FPase) by wild type Trichoderma sp. using sugarcane bagasse as sole carbon source. Alkaline treatment (2% NaOH) of bagasse (AlSCB) was found suitable for the production of reducing sugar over the acidic pretreatment method. After 5 days of incubation period, 5% substrate concentration at pH 5.0 and 400C resulted in maximum production of CMCase (0.622 U), while maximum (3.388 U) production of cellulobiase was obtained at 300C. The CMCase was precipitated and purified to the extent of 59.06 fold by affinity chromatography with 49.09% recovery. On 12% SDS-PAGE, a single band corresponding to 33 kDa was observed. The Km and Vmax for CMCase from Trichoderma was found 507.04 mg/ml and 65.32 mM/min, respectively. The enzyme exhibited maximum activity at 300C at pH-5.0 (0.363 U) and was stable over range of 20-60°C and pH 5.0-7.5.
Background
Lignocellulosic materials are attractive feedstocks for ethanol production since they are abundant and cheap. One of the major lignocellulosic materials to be considered in tropical countries is sugarcane bagasse, the fibrous residue obtained after extracting the juice from sugar cane (Saccharum officinarum) in the sugar production process. India is second largest producer of sugarcane in the world. It occupies an important position in the Indian agriculture scenario covering around 5.06 Mha area. The sugarcane baggase is a byproduct of sugarcane crushing. In this perspective, the present study was aimed at the utilization of agro waste, sugarcane bagasse, as growth substrates for the production of cellulolytic enzymes. Among the agricultural residues, sugarcane bagasse (SCB) is a substrate of high potential for biotechnological processes, which comprises 40–42% cellulose, 24–28 % hemicelluloses, and 10–12 % lignin [1]. This could be used as substrate for the production of cellulase complex, an enzyme which has higher commercial use. Cellulase used in chemicals, fuel, food, animal feed, brewery and wine, textile and laundry, pulp and paper and agro-based industries [2, 3]. Cellulase is used in the fermentation of biomass into biofuels, although this process is relatively experimental at present.
The aim of this study was to optimize the effect of different pretreatment methods, fermentation conditions and to evaluate enzymatic convertibility of the pretreated solids for the enzyme production. Pretreatment of lignocelluloses is an important step, so that the fermentable sugar could be available for the microbial biomass. Pretreatment removes lignin from SCB, reduces crystallinity of cellulose and significantly improve the hydrolysis by avoiding the formation of inhibitors [4]. However, many pretreatment methods have been attempted to hydrolyse the available cellulose. They include mechanical pulverization, pyrolysis, ozonolysis, acid, alkali, oxidative delignification, ionic liquids, ammonia fiber explosion (AFEX), autohydrolysis, microwave assisted, ultrasound assisted, microbial and enzymatic methods. In India, most of the enzymes are imported at huge costs which warrant an absolute need for its commercial indigenous production to reduce the market price [3]. Both bacteria and fungi can produce cellulases for the hydrolysis of lignocellulosic materials. Numbers of aerobic, anaerobic, mesophilic and thermophilic microorganisms have been reported earlier to produce cellulases using various fermentation medium. Bacteria belong to Clostridium, Cellulomanas, Bacillus, Thermomonospora, Ruminococcucs, Bacteroides, Erwinia, Acetovibrio, Microbispora, and Streptomyces can produce cellulases. Although many cellulolytic bacteria particularly the cellulolytic anaerobes such as Clostridium thermocellum and Bacteroides cellulosolvens produce cellulase with high specific activity but they do not produce high enzyme titers [5]. Several fungi have been reported to produce cellulases [6]. A non-exhaustive list of cellulolytic microorganisms of aerobic and anaerobic forms isolated from various habitats has been reported [7]. The biggest obstacle in commercial success of enzyme production is the high cost of raw material used as substrate which could be overcome by resorting to microbial fermentation technology using low valued biological substrates including agro- waste, viz., rice straw, wheat straw, rice bran, wheat bran and baggasse etc. and fruit processing waste such as apple pomace, grape pomace, pineapple waste and mango peel [8, 9, 10& 11].
Methodology
Isolation and screening of potent cellulolytic microorganism:
Cellulose rich substrates such as dried leaves and soil samples were collected around sugarcane fields of Lucknow, India and were suspended in sterile saline. One ml. of the aliquots were inoculated on CMC agar plates. Isolated colonies were then purified through a serial streaking method. From these plates, isolated colonies were taken and repeatedly streaked on CMC agar to obtain pure cultures. This isolated culture was screened for their ability to produce enzymes like CMCase, cellulobiase and FPase. The organism showing maximum enzymatic activity was identified and used for further studies. The pure fungal cultures were subsequently maintained on CMC agar slants.
Pretreatment of SCB:
SCB was a kind gift from the Jaggery Unit, IISR, Lucknow. Before inoculating the highest cellulsae producing microorganism in to the SCB rich medium, the substrate was chemically pretreated. Dried SCB was pretreated with alkaline pretreatment (2% NaOH, submerged for overnight; AlSCB) and acid pretreatment (10% H2SO4, submerged for overnight; AcSCB) at room temperature. After treatment the concentrated hydrolysate was detoxified by four staged process: washing with hot dil. water to rinse off the alkali or acid, maintaining the pH to 5.5 (otherwise stated) with calcium oxide or phosphoric acid followed by incubation for 1h at 200 rpm and 30°C. After each stage, the hydrolysate was filtered under vacuum and finally it was autoclaved at 15 psi and 121°C for 15 min and was used in the fermentation reactions.
Optimization of fermentation condition for cellulase production:
Different variables of fermentation conditions viz., substrate concentration (2-15% w/v; Sterilized AlSCB), pH (3.0-9.0), incubation period (3-7 days) and temperature (20 – 60°C) were studied in different set of experiments. Every experiment was performed in duplicate or triplicate. After incubation, culture filtrate was filtered through whattman filter paper no.4. Culture filtrate was obtained and assay for the cellulase complex were performed. The fermentation condition, at which maximum enzyme production was observed, was taken as optimum for enzyme production. The amount of released reducing sugar was quantified by using Miller, G.L. 1959 method using glucose as standard for determining enzyme activity and expressed in terms of U/g of substrate utilized [12]. One unit of enzyme activity is defined as the amount of enzyme required to release 1 µm equivalent of glucose/min/g of substrate utilized. Protein concentration was estimated by method of Lowry et al. (1951) [13].
Purification of CMCase:
The culture filtrate was precipitated using ammonium sulphate at 20-70% saturation and was dialyzed twice against the 50 mM of acetate buffer (pH 5.0). Affinity chromatography of the concentrated enzyme was performed using preequilibrated agarose matrix 100mM acetate buffer (pH 5) as bed. The flow rate of 1 ml/min was maintained and 1 ml protein fraction was collected up to 45 ml.
Characterization of CMCase:
The fraction showed maximum enzyme activity were pooled and electrophoressed to determine the molecular weight of the obtained enzyme. Using carboxy methyl cellulose as substrate, Michaelis-Menten equation constant (Km and Vmax) of the obtained enzyme was studied. Reaction was carried out at 30oC in 100 mM acetate buffer pH 5.0 at various concentrations of substrate from 0-3.0%.
Results & Discussion
Primary screening of microorganisms were done according to plate assay method. Among thirty two microorganisms, six fungal strains and four bacterial strains were selected for secondary screening for cellulase production. A fungal isolate, Trichoderma spp., isolated from degrading sugarcane leaf, was exhibiting the maximum cellulase activity (0.45 U for CMCase; 3.187 U for Cellulobiase and 0.091 U for FPase, respectively). The isolate was subjected to produce the enzyme on synthetic carbon and various agro-waste before selecting it for further studies (Results are not shown). The isolate exhibit maximum CMCase activity with carboxymethyl cellulose (1.84 U, among the synthetic sources) and with SCB (0.49 U, among agro-waste tried). The bagasse after treatment with 2% NaOH exhibited maximum release of reducing (10.81 mg/g) and non-reducing (28.07 mg/g) sugar in the medium. The pretreatment process is cost effective and reliable way to disrupt the cellulose– hemicelluloses–lignin complex, and to achieve the increased digestibility of polysaccharides [14]. Alkali pretreatment method was reported to cause less sugar degradation than acid pretreatment, and exhibits lesser hemicellulose and cellulose loss than acid or hydrothermal processes [15]. After the addition of NaOH, SCB swells in order to increase the internal surface of cellulose, decreases the degree of polymerization and disruption of lignin structure. In a report, it has been established that 33%, 25.5% and 35.5% hydrolysis using wheat straw, rice straw and SCB pretreated with 2% NaOH.
Fermentation medium supplied with 5% SCB as sole carbon source was found suitable for maximum CMCase (0.582 U), Cellulobiase (3.008 U) and FPase (0.129 U) production. A decline in enzyme production was observed with the increase or decrease in substrate concentration. This might be due to the catabolite repression and or the accumulation of the phenolic compounds in the fermentation medium. The liquid fraction from the hydrolysis of lignocellulosic materials contains both hexose and pentose sugars. The hydrolysate typically consists of sugars, organic acids, furans and phenolics [16]. It was observed that CMCase (0.534 U) and cellulobiase (3.227 U) production was highest in alkali treated medium, while after an increase in production after 5 days, there was no significant increase in enzyme production. While in case of FPase production, it was higher in acid treated medium (0.154 U) and there was sharp decline in enzyme production after 5 days of incubation (0.084 U). Fermentation medium comprising of AlSCB (5% w/v) at pH 5.0 was found suitable for the production of cellulase complex. However, it was noticed that CMCase (0.622 U) and Cellulobaise (3.388 U) production was maximum at 5th day of incubation at 40 and 30°C temperature, respectively. Maximum production of FPase was observed at 2nd day of incubation at 30°C. A similar pattern was noticed by Abubakar and Oloyede, while experimenting with orange peel as carbon source [17]. They found production of cellulase complex (14.30µmol/min) at 48 hours. This is in line with findings of that enzyme could be harvested at about 72 hours of fermentation.
The CMCase produced (0.715 µg/ml/min) from Trichoderma cells was extracted, precipitated by ammonium sulphate upto 70% saturation and dialyzed twice in acetate buffer (50mM, pH 5.0). It was further purified to the extent of 59.06 fold by affinity chromatography on agarose column with 49.09% recovery. Purification to extent of 24-26 folds had been reported earlier in cellulase and pectinase Table 1 (see supplementary material).
The elution profile of protein fractions revealed that fractions numbering 14 to 22 showed peak CMCase activity. These fractions were pooled and electrophoresed on 12% SDS-PAGE and 10% Native PAGE. A single band corresponding to 33 kDa was observed in 12% SDS-PAGE (Figure 1). There are many reports of various cellulases of various molecular weights. From Bacillus sp 29 kDa alkaline cellulose, from Bacillus pumilus, 30-65 kDa cellulose, from Paenibacillus polymyxa, 72 kDa, from Sinorhizobium fredii, 94 kDa and from Aspergillus niger 83 and 50 kDa CMCase have been already reported [11]. The effect of substrate on CMCase activity shows that maximum activity was obtained at lower concentration of carboxy methyl cellulose. Increase in the concentration of substrate, lowers the activity of the enzyme. It indicates that the active sites of the enzyme are not available to form substrate-enzyme complex. The Km and Vmax for CMCase from Trichoderma was found 507.04 mg/ml and 65.32 mM/min, respectively. Earlier, Km values for CMCase have reported from Paenibacillus polymyxa as 8.73 mg/ml and 17.805 mM/min and Candida peltala as 66 mg/ml [11].
Figure 1.
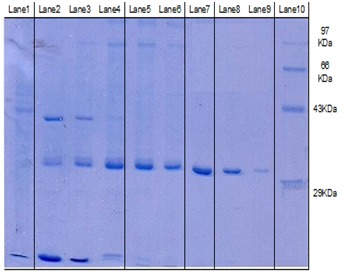
Lane 1-3: Sample of crude culture filtrate, Lane 4-6: Sample of Ammonium sulphate precipitate, Lane 7-9: Sample of purified CMCase pooled after agarose chromatography, Lane 10: Marker
CMCase of Trichoderma exhibited maximum activity at 30°C at pH 5.0 (0.363 U). The purified enzyme was found to be stable over range of 20-60°C and pH 5.0-7.5. CMCase activity declined by 81.5% at 60°C temperature and only 5% activity was retained at pH 7.5. Increase or decrease in the temperature or pH from optimum condition, significantly decreases the CMCase activity.
Conclusion
For majority of commercial enzymes including cellulase complex (CMCase and cellulobias) used by the industries, India is dependent on imports. There is an opportunity to exploit sugarcane bagasse as a substrate for the microbial production of cellulase complex using Trichoderma. The present study revealed that the alkaline pretreatment (2% NaOH, overnight) of SCB could enhance the cellulase production. Fermentation medium containing 5% substrate concentration at pH 5.0 after 5 days of incubation period at 40°C (CMCase) and 30°C (cellubiase resulted in maximum production of enzyme. Single band corresponding to 33 kDa (purified CMCase; to the extent of 59.06 fold by affinity chromatography with 49.09% recovery) was achieved on 12% SDS-PAGE. The CMCase from Trichoderma was thermo-stable and was found stable over range of 20-60°C and pH 5.0-7.5.
Supplementary material
Footnotes
Citation:Ashfaque et al, Bioinformation 10(10): 606-610 (2014)
References
- 1.Vijayalaxmi, et al. Appl Biochem Biotechnol. 2013;171:246. doi: 10.1007/s12010-013-0366-0. [DOI] [PubMed] [Google Scholar]
- 2.Lynd LR, et al. Micro Mol Biol Rev. 2002;66:506. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukumaran, et al. J Sci Ind Res. 2005;64:832. [Google Scholar]
- 4.Wu ZW, Lee YY. Appl Biochem Biotechnol. 1997;63:21. doi: 10.1007/BF02920409. [DOI] [PubMed] [Google Scholar]
- 5.Duff SJB, Murray WD. Bioresour Technol. 1996;55:1. [Google Scholar]
- 6.Sun Y, Cheng JY. Bioresour Technol. 2002;83:1. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 7.Tamaru, et al. Environ Technol. 2010;31:889. doi: 10.1080/09593330.2010.490856. [DOI] [PubMed] [Google Scholar]
- 8.Krishna C, et al. Bioresource Technol. 1999;69:231. [Google Scholar]
- 9.Omojasola , et al. Nature and Science. 2008;6:64. [Google Scholar]
- 10.Sun , et al. Afr J Biotechnol. 2010;9:163. [Google Scholar]
- 11.Kumar, et al. J Environ Biol. 2012;33:81. [PubMed] [Google Scholar]
- 12.Miller GL. Anal Chem. 1959;31:426. [Google Scholar]
- 13.Lowry, et al. J Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- 14.Soderstrom, et al. Biomass Bioenergy. 2003;24:475. [Google Scholar]
- 15.Carvalheiro, et al. J Sci Ind Res. 2008;67:849. [Google Scholar]
- 16.Keshwani DR, Cheng JJ. Bioresour Technol. 2009;100:1515. doi: 10.1016/j.biortech.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Abubakar FA, Oloyede OB. Internat J Sci Res Mgmt. 2013;1:285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


