Abstract
Purpose of review
Aldosterone and the mineralocorticoid receptor contribute to resistant hypertension and cardiovascular mortality, and mineralocorticoid receptor antagonists effectively reduce these complications. Their use is limited in certain populations with a higher risk of hyperkalemia or renal dysfunction. This review will highlight recent developments in extra-renal mineralocorticoid receptor research and the development of novel mineralocorticoid receptor antagonists.
Recent findings
Tissue-specific knockout-out models provide definitive evidence that vascular mineralocorticoid receptor directly contributes to hypertension and vascular remodeling, independent of renal effects. Several non-steroidal mineralocorticoid receptor antagonists are in pre-clinical development or early-stage clinical trials. Several non-steroidal MR antagonists have demonstrated preserved cardiovascular benefit with a reduced incidence of hyperkalemia in pre-clinical studies.
Summary
Novel, potent non-steroidal MR antagonists are in development, although their effect on cardiovascular and adverse drug events requires further investigation.
Keywords: Aldosterone, Mineralocorticoid receptor, hyperkalemia
Introduction
Syndromes of mineralocorticoid excess led to the discovery of aldosterone in the 1950’s, and the genetic characterization of monogenic hypertension has repeatedly demonstrated the importance of aldosterone and renal sodium reabsorption in the genesis of hypertension. Recent studies have changed our view of traditional MR activation to include actions of aldosterone and the mineralocorticoid receptor in extra-renal tissues such as vascular smooth muscle and cardiomyocytes. Traditional MR antagonists are effective treatment for congestive heart failure and resistant hypertension [1–4], but the risk posed by hyperkalemia and renal dysfunction limits broad application in the clinical setting. Because MRAs are beneficial even in high risk individuals [5], withholding this therapy could deprive patients with the greatest need of effective treatment. It remains to be seen if next-generation MRAs can minimize these risks while maintaining their cardiovascular benefits, but recent drug developments show promise towards achieving this goal [6, 7].
Pathologic consequences of extra-renal MR activation
Aldosterone is critical for maximal renal sodium reabsorption during volume depletion and for urinary potassium excretion during potassium excess. These effects are mediated primarily by MR activation in the distal nephron segments, which increases epithelial sodium channel (ENaC) expression and activity. In the cardiovascular system, the mineralocorticoid receptor is expressed in cardiomyocytes, vascular endothelium, and vascular smooth muscle where it contributes to injury independent of renal effects. Administration of aldosterone plus high salt diet induces cardiac hypertrophy with perivascular inflammation and fibrosis via MR activation, suggesting a direct effect of aldosterone and MR activation in extra-renal tissues independent of blood pressure.
In the Randomized Aldactone Evaluation Study (RALES), spironolactone reduced cardiovascular mortality and rehospitalization rates by ~30% in patients with severe congestive heart failure [2]. The selective MR antagonist eplerenone also reduced cardiovascular mortality by 17% in patients with acute myocardial infarction complicated by heart failure [1]. These benefits occurred in the absence of blood pressure reduction, stimulating further research to determine the mechanism of extra-renal MR- induced injury. Using Cre recombinase technology, investigators have demonstrated that MR activation within vascular smooth muscle, cardiomyocytes, and monocytes/macrophages directly contributes to adverse cardiovascular consequences [8–11]. Tissue-specific MR deletion in smooth muscle cells (SMC) prevents age-related blood pressure elevation and vascular remodeling [10] and prevents injury-induced vascular SMC hypertrophy [8]. After experimental myocardial infarction, cardiac-specific MR inactivation reduced cardiac hypertrophy, diastolic dysfunction, and pulmonary edema [12].
Traditional MR antagonists
Spironolactone is the prototypical steroidal MRA, but has non-selective progesterone receptor agonistic and androgen receptor antagonistic activity (Table 1). This non-selectivity contributes to the unwelcome side effects of gynecomastia, menstrual irregularities, and decreased libido, respectively. Spironolactone causes breast pain or gynecomastia in males in a dose- and time-dependent manner, with an incidence of ~10% after 25 mg daily, and as high as 52% after 150 mg daily over two years [2, 20]. Clinically approved MRAs are structurally similar spirolactones, possessing a γ-lactone ring at the C17 position and various substituents attached to the C7 steroid carbon [21]. Eplerenone is the most selective MR antagonist without significant progesterone or androgen activity, but at expense of potency compared to spironolactone (Table 1).
Table 1.
Functional in vitro nuclear hormone antagonist potency
| IC50 (µM) |
|||||||
|---|---|---|---|---|---|---|---|
| Drug | MR | PR (EC50) | AR | GR | ER | Manufacturer | Reference |
| Cd-(R)14c | 0.005 | 2.5 | >10 | >10 | >10a | Pfizer | [13] |
| Progesterone | 0.010 | 0.0002 | NR | none | NR | [14] | |
| Spironolactone | 0.013 | 2.6 | 0.52 | 6.9 | 5.7 | Generic | [15] |
| BAY-94-8862 | 0.018 | >10 | >10 | >10 | >10 | Bayer | [16] |
| PF-03882845 | 0.021 | >100 | >10 | >10 | NR | Pfizer | [13,15] |
| BR-4628 | 0.028 | 9.0 | 4.4 | 5.5 | NR | Bayer | [17] |
| Cd 13i | 0.038 | NR | >5 | >5 | >5 | Merck | [18] |
| Eplerenone | 0.122 | >10 | >10 | NR | >10 | Novartis | [13,15] |
| SM-368229 | 0.130 | NR | 5.0 | 30 | NR | Dainippon Sumitomo | [19] |
NR, either not reported or done using comparable methodology
IC50 for receptor binding
Although the off-target side effects are reduced during eplerenone versus spironolactone treatment [22], side effects related to renal MR inhibition limit their use in patients at high risk for hyperkalemia. Those with heart failure with the highest risk of mortality also carry the highest risk for hyperkalemia, namely those with diabetes and renal dysfunction. The incidence of hyperkalemia during MR antagonism is relatively low in highly controlled clinical trial settings [1, 2, 5, 23, 24], but application to broader populations without close monitoring has been associated with increased hospitalization rates for hyperkalemia [25]. In general practice, spironolactone was discontinued in 33% of patients with heart failure, primarily due to hyperkalemia (10%) and worsening renal function (10%) [26]. Although a decline in renal function is relatively common in patients with ischemic events complicated by heart failure, the cardiovascular benefits of MR antagonism persist [5, 27]. Therefore, MR antagonists are needed which would allow administration to patients with the highest risk of hyperkalemia, such as those with chronic kidney disease, diabetes, and elevated baseline potassium or the elderly. Non-steroidal MR antagonists are being developed with these goals in mind.
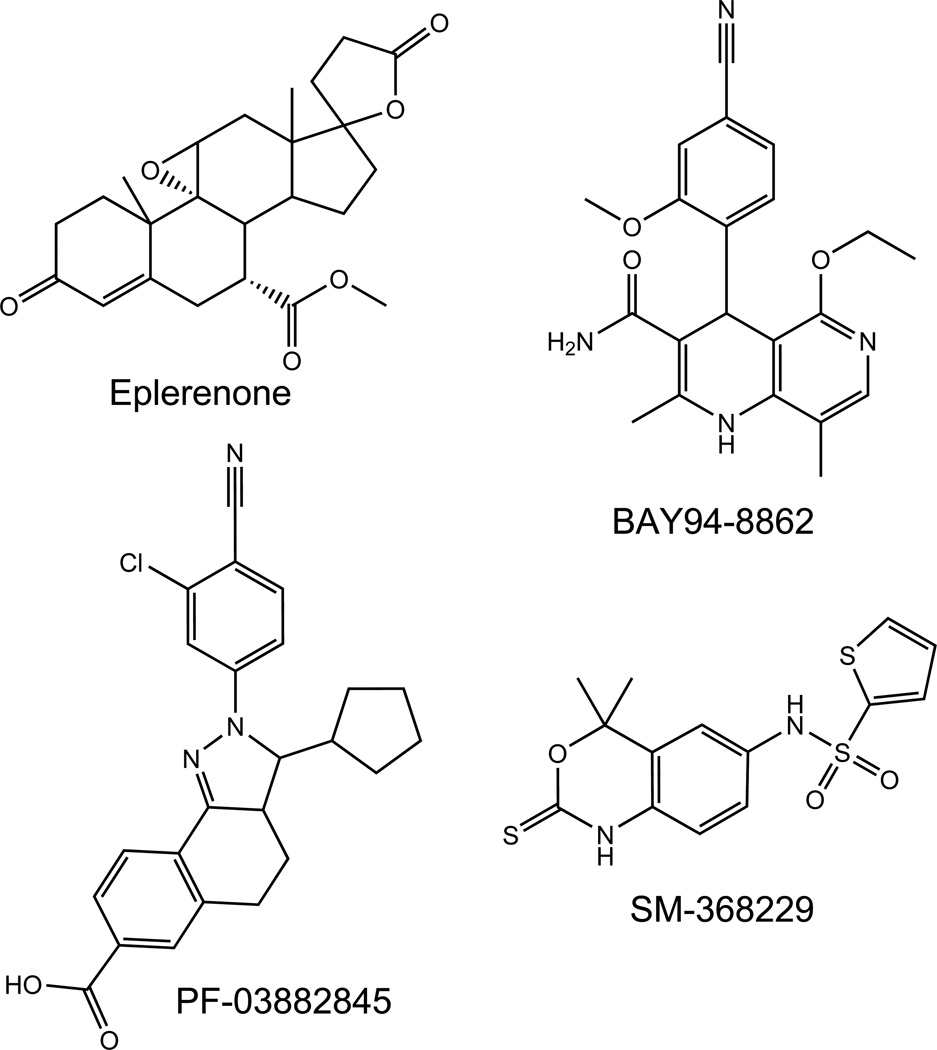
Over the past decade, pharmaceutical companies launched drug-discovery programs for non-steroidal MRAs using various high-throughput screening methods, which has led to the identification of several drug classes which possess MRA activity [13, 16–19, 28, 29]. Review of published patent applications demonstrates that additional compounds have been identified but not yet published in the scientific literature. Compounds with detailed pharmacologic potency and demonstrated in vivo effectiveness will be discussed further (Figure 1).
Figure 1.
Heading: Non-steroidal Mineralocorticoid receptor antagonists
Legend: The chemical structures of selected non-steroidal MRAs are representative of dihydropyridine (BAY-94-8862), Pyrazoline (PF-03882845), and Benzoxazinethione (SM-368229) derivatives.
Source: Original
Dihydropyridine-derived MR antagonists
Dietz et al. demonstrated that most clinically approved dihydropyridine calcium channel blockers possess the ability to block aldosterone-induced MR activation in vitro using luciferase reporter assays [30]. The effect of nimodipine, felodipine, and nitrendipine were particularly effective, achieving complete inhibition, although at suprapharmacologic concentrations. The concentration at which nimodipine achieve 50% inhibition (i.e., the IC50) was within the range achieved in humans using standard clinical dosing. Nifedipine was somewhat less effective, while amlodipine possessed the lowest potency. The IC50 for nifedipine was 0.7 nM, although a maximum concentration of only 0.3 nM is typically achieved after chronic oral administration of 90 mg of sustained-release nifedipine (package insert). The predominant clinical effect of these agents is produced by their calcium channel blocking effect, rather than MRA activity. Other studies have confirmed the MRA activity of calcium channel blockers at micromolar concentrations, but also suggest that they non-selectively inhibit multiple nuclear receptors [17].
Modification of these existing compounds has aided the development of novel non-steroidal MRAs, some of which have proven effective in preclinical and clinical trials. Using the dihydropyridine structure, compounds have been modified to remove calcium channel inhibitory activity and to increase MR selectivity, pharmacokinetic suitability, bioavailability [31]. One compound, (R)-7d met these requirements and was used in further in vivo studies. When given to Dahl salt-sensitive rats at 60mg/kg/day, compound (R)-7d attenuated salt-induced hypertension to the same extent as eplerenone.
Fagart et al. similarly identified dihydropyridines as MRAs using a high-throughput screening method, and characterized BR-4628 as a selective non-steroidal MR antagonist, although with still some PR agonist activity. The independent discovery and optimization of multiple dihydropyridine compounds provides further proof of concept that dihydropyridine derivatives can function as MRAs. BR-4628 was also active in vivo when given orally to rats, as measured by the urinary sodium to potassium ratio [17]. In pre-clinical studies, BR4628 prevented DOCA/salt-induced kidney injury independent of hypertension in vivo [32], and prevented fibrotic cardiomyocyte remodeling in vitro [33].
Further drug optimization has led to the development of BAY94-8862, which is more potent (IC50 18 nM) and has no measurable activity at the PR, AR, GR, or ER in vitro [16]. This compound increased the urinary sodium/ratio and demonstrated evidence of cardiovascular benefit in preclinical studies. BAY94-8862 has become the first non-steroidal to advance in clinical trials (the minerAlocorticoid Receptor antagonist Tolerability Study (ARTS) to investigate whether it can be safely used in high-risk subjects with heart failure, reduced ejection fraction <40% (HFREF), and renal dysfunction [34, 35].
The initial phase I study enrolled subjects in part A with mild renal insufficiency (eGFR 60–90 mL/min/1.73m2 by the MDRD equation) in a randomized, placebo-controlled parallel trial (n≥16 each arm) to BAY94-8862 (either 2.5, 5.0, or 10 mg daily) or placebo for 4 weeks to assess safety. The drug was well tolerated in this initial phase study, with mildly elevated potassium in 1 subject that did not require treatment or discontinuation. In part B, the study enrolled 393 subjects with moderate renal insufficiency (eGFR 30–60 mL/min/1.73m2) randomized to either BAY94-8862 (either 2.5, 5.0, or 10 mg daily), placebo, or open-label spironolactone for 4 weeks. Spironolactone was given 25mg daily for 14 days, and then increased to 50mg daily if serum potassium remained <4.8 mEq/L. BAY94-8862 increased serum potassium at 4 weeks in a dose-dependent manner, but to a lesser degree than spironolactone and produced no more renal dysfunction than placebo. It also did not alter blood pressure or plasma aldosterone to the same extent as spironolactone. At the same time, urine albumin/creatinine and circulating N-terminal B-type natriuretic peptide appeared to improve to a similar degree during BAY94-8862 10mg daily. Based on these results, this drug has entered phase II studies for the treatment of diabetic nephropathy.
Pyrazoline-derived compounds
Using a high-throughput screening method, a pyrazoline compound has been discovered to have MR antagonist activity [13, 15]. Pyrazoline-related compounds had previously been described to bind to other steroid nuclear receptors, and chemical optimization led to the identification of PF-03882845. This compound is highly selective for the MR without significant effects on the AR, PR, or GR in vitro. PF-03882845 effectively reduced blood pressure in Dahl salt-sensitive rats, preventing renal injury assessed by histology and albuminuria to the same or greater extent at 40 mg/kg/day compared to eplerenone [15]. There was a trend for serum potassium to increase to a lesser extent during PF-03882845 treatment. Although this compound reached clinical testing for diabetic nephropathy, early studies were terminated due to “poor recruitment and a shift in business strategy” (ClinicalTrials.gov NCT01488877). Further refinement of this compound also led to the identification of compound (R)14c, which has improved MR potency, selectivity over PR, and increased solubility compared to PF-03882845 [13]. Compound (R)14c increases the urinary sodium/potassium ratio in rats, suggesting effective MR antagonism in vivo, although its effect on cardiovascular and renal injury has not yet been proven.
Sulfonamide-derived Benzoxazinethiones
Further evidence that non-steroidal MRAs can prevent cardiovascular injury is provided by the sulfonamide SM-368229 [19, 36, 37]. SM-368229 reduced blood pressure, ventricular hypertrophy, and renal injury in spontaneously hypertensive rats and in aldosterone treated rats without increasing serum potassium [36, 37]. Further studies in potassium-loaded rats demonstrated no increase in serum potassium during chronic SM-368229 administration [36].
Can the risk of hyperkalemia be separated from cardiovascular benefit of MRAs?
Clinical applicability of MRAs to patients with renal dysfunction is limited due to hyperkalemia, and if new agents can achieve similar cardiovascular benefits with a reduced hyperkalemia risk, this would significantly expand the target population. It is well-known that aldosterone increases urinary potassium excretion which causes hypokalemia in the setting of inappropriate aldosterone excess.
A number of mechanisms have been proposed by which non-steroidal MRAs could avoid the risk of hyperkalemia [6]. Spironolactone and eplerenone accumulate in renal to a greater extent than cardiovascular tissue, and several publications have suggested that the novel drug has a more balanced tissue distribution. However, this alone may not prevent the risk of hyperkalemia. Aldosterone also affects potassium handling via extra-renal effects in skeletal muscle and/or other tissues. Aldosterone and the MR directly affect skeletal muscle potassium transport in chronic adaptation to high potassium diet [38–40]. Other mineralocorticoids such as deoxycorticosterone (DOCA), but not glucocorticoids, also stimulate skeletal muscle potassium uptake which is prevented by MR antagonism with spironolactone [40].
Another mechanistic possibility is differential effects on co-activators in different tissues. Steroidal MRAs and BR-4628 bind in the ligand binding domain of the MR, although BR-4628 has a bulky side chain which induces conformational changes. These changes can alter recruitment of co-activators and co-repressors, thus altering the stability, nuclear translocation, and activation. Similar molecular mechanisms are responsible for selective estrogen receptor modulators. More research is needed to identify tissue-specific MR partners which contribute to cardiovascular injury, along with further drug modification. However, these findings indicate the future possibility of selectively modifying cardiovascular MR responses while minimizing risk of renal complications.
Conclusions
The critical challenge for non-steroidal MRAs will be to maintain cardiovascular effectiveness of non-steroidal MRAs, namely for heart failure and resistant hypertension, while reducing side effects. If successful, the decreased risk of hyperkalemia could expand the use of these agents into populations at high risk of complications, such as those with renal insufficiency. This could further expand a role for these agents in diabetic nephropathy. Although spironolactone in addition to standard care effectively reduces proteinuria in diabetic nephrophathy [41], its effectiveness in reducing progression to death, dialysis, or need for transplantion has not, and likely will not be determined. A significant proportion of patients with diabetic nephropathy progress to renal failure or die of cardiovascular complications. Furthermore, diabetic renal disease is the leading cause of renal failure, which is among the costliest diseases in the United States health care system. Therefore, reduction in the number of patients requiring dialysis would have significant clinical and cost benefits. The initial studies of non-steroidal MRAs are being conducted in patients with diabetic nephropathy, a population with markedly elevated risk of cardiovascular complications.
Key points.
Although the renal mineralocorticoid receptor stimulates sodium reabsorption and potassium excretion and contributes to hypertension, activation of the MR in extra-renal tissues also contributes to the pathogenesis of cardiovascular injury.
Tissue-specific MR knockout animals have demonstrated an important role for extra-renal MR in learning, vascular injury, and potassium homeostasis.
Selective non-steroidal MR antagonists have been recently developed and demonstrate effectiveness in early clinical trials
Acknowledgements
Funding:supported in part by NIH grants DK081662 and DK096994
Abbreviations
- MR
Mineralocorticoid receptor
- AR
Androgen receptor
- PR
Progesterone receptor
- GR
Glucocorticoid receptor
- ER
Estrogen receptor
- SMC
Smooth muscle cell
Footnotes
Conflict of Interest: no conflict of interest declared
Reference and recommended reading
- 1.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 3.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 4.Ouzan J, Perault C, Lincoff AM, et al. The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens. 2002;15:333–339. doi: 10.1016/s0895-7061(01)02342-1. [DOI] [PubMed] [Google Scholar]
- 5.Eschalier R, McMurray JJ, Swedberg K, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: Analyses of the emphasis-hf study subgroups (eplerenone in mild patients hospitalization and survival study in heart failure) J Am Coll Cardiol. 2013;62:1585–1593. doi: 10.1016/j.jacc.2013.04.086. [DOI] [PubMed] [Google Scholar]
- 6. Kolkhof P, Borden SA. Molecular pharmacology of the mineralocorticoid receptor: Prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350:310–317. doi: 10.1016/j.mce.2011.06.025. *This review provides an excellent overview of early non-steroidal development and rationale from an industry perspective
- 7.Funder JW. Mineralocorticoid-receptor blockade, hypertension and heart failure. Nat Clin Pract Endocrinol Metab. 2005;1:4–5. doi: 10.1038/ncpendmet0016. [DOI] [PubMed] [Google Scholar]
- 8.Pruthi D, McCurley A, Aronovitz M, et al. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galmiche G, Pizard A, Gueret A, et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCurley A, Pires PW, Bender SB, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. * This publication definitively establishes that vascular smooth muscle mineralocorticoid receptors contribute to vascular function and blood pressure regulation, independent of any renal action.
- 11.Dupont JJ, Hill MA, Bender SB, et al. Aldosterone and vascular mineralocorticoid receptors: Regulators of ion channels beyond the kidney. Hypertension. 2014;63:632–637. doi: 10.1161/HYPERTENSIONAHA.113.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraccarollo D, Berger S, Galuppo P, et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–408. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 13.Casimiro-Garcia A, Piotrowski DW, Ambler C, et al. Identification of (r)-6-(1-(4-cyano-3-methylphenyl)-5-cyclopentyl-4,5-dihydro-1h-pyrazol-3-yl)-2-me thoxynicotinic acid, a highly potent and selective nonsteroidal mineralocorticoid receptor antagonist. J Med Chem. 2014 doi: 10.1021/jm500206r. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Souque A, Fagart J, Couette B, et al. The mineralocorticoid activity of progesterone derivatives depends on the nature of the c18 substituent. Endocrinology. 1995;136:5651–5658. doi: 10.1210/endo.136.12.7588320. [DOI] [PubMed] [Google Scholar]
- 15.Meyers MJ, Arhancet GB, Hockerman SL, et al. Discovery of (3s,3ar)-2-(3-chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2h-benzo[g] indazole-7-carboxylic acid (pf-3882845), an orally efficacious mineralocorticoid receptor (mr) antagonist for hypertension and nephropathy. J Med Chem. 2010;53:5979–6002. doi: 10.1021/jm100505n. [DOI] [PubMed] [Google Scholar]
- 16.Barfacker L, Kuhl A, Hillisch A, et al. Discovery of bay 94-8862: A nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7:1385–1403. doi: 10.1002/cmdc.201200081. [DOI] [PubMed] [Google Scholar]
- 17.Fagart J, Hillisch A, Huyet J, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285:29932–29940. doi: 10.1074/jbc.M110.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox JM, Chu HD, Yang C, et al. Mineralocorticoid receptor antagonists: Identification of heterocyclic amide replacements in the oxazolidinedione series. Bioorg Med Chem Lett. 2014;24:1681–1684. doi: 10.1016/j.bmcl.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 19.Nariai T, Fujita K, Mori M, et al. Sm-368229, a novel selective and potent non-steroidal mineralocorticoid receptor antagonist with strong urinary na+ excretion activity. J Pharmacol Sci. 2011;115:346–353. doi: 10.1254/jphs.10285fp. [DOI] [PubMed] [Google Scholar]
- 20.de Gasparo M, Whitebread SE, Preiswerk G, et al. Antialdosterones: Incidence and prevention of sexual side effects. J Steroid Biochem. 1989;32:223–227. doi: 10.1016/0022-4731(89)90169-6. [DOI] [PubMed] [Google Scholar]
- 21.Fagart J, Seguin C, Pinon GM, et al. The met852 residue is a key organizer of the ligand-binding cavity of the human mineralocorticoid receptor. Mol Pharmacol. 2005;67:1714–1722. doi: 10.1124/mol.104.010710. [DOI] [PubMed] [Google Scholar]
- 22.Menard J. The 45-year story of the development of an anti-aldosterone more specific than spironolactone. Mol Cell Endocrinol. 2004;217:45–52. doi: 10.1016/j.mce.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 24. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. ** This publication is the first to demonstrate effectiveness of non-steroidal MR antagonists in humans.
- 25.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 26.Witham MD, Gillespie ND, Struthers AD. Tolerability of spironolactone in patients with chronic heart failure -- a cautionary message. Br J Clin Pharmacol. 2004;58:554–557. doi: 10.1111/j.1365-2125.2004.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossignol P, Cleland JG, Bhandari S, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: Insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study. Circulation. 2012;125:271–279. doi: 10.1161/CIRCULATIONAHA.111.028282. [DOI] [PubMed] [Google Scholar]
- 28.Neel DA, Brown ML, Lander PA, et al. 3,3-bisaryloxindoles as mineralocorticoid receptor antagonists. Bioorg Med Chem Lett. 2005;15:2553–2557. doi: 10.1016/j.bmcl.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 29.Bell MG, Gernert DL, Grese TA, et al. (s)-n-{3-[1-cyclopropyl-1-(2,4-difluoro-phenyl)-ethyl]-1h-indol-7-yl}-methanesulf onamide: A potent, nonsteroidal, functional antagonist of the mineralocorticoid receptor. J Med Chem. 2007;50:6443–6445. doi: 10.1021/jm701186z. [DOI] [PubMed] [Google Scholar]
- 30.Dietz JD, Du S, Bolten CW, et al. A number of marketed dihydropyridine calcium channel blockers have mineralocorticoid receptor antagonist activity. Hypertension. 2008;51:742–748. doi: 10.1161/HYPERTENSIONAHA.107.103580. [DOI] [PubMed] [Google Scholar]
- 31.Arhancet GB, Woodard SS, Iyanar K, et al. Discovery of novel cyanodihydropyridines as potent mineralocorticoid receptor antagonists. J Med Chem. 2010;53:5970–5978. doi: 10.1021/jm100506y. [DOI] [PubMed] [Google Scholar]
- 32.Schupp N, Kolkhof P, Queisser N, et al. Mineralocorticoid receptor-mediated DNA damage in kidneys of doca-salt hypertensive rats. FASEB J. 2011;25:968–978. doi: 10.1096/fj.10-173286. [DOI] [PubMed] [Google Scholar]
- 33.Lavall D, Selzer C, Schuster P, et al. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014 doi: 10.1074/jbc.M113.519256. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist bay 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt B, Filippatos G, Gheorghiade M, et al. Rationale and design of arts: A randomized, double-blind study of bay 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail. 2012;14:668–675. doi: 10.1093/eurjhf/hfs061. [DOI] [PubMed] [Google Scholar]
- 36.Nariai T, Fujita K, Mori M, et al. Sm-368229, a novel promising mineralocorticoid receptor antagonist, shows antihypertensive efficacy with minimal effect on serum potassium level in rats. J Cardiovasc Pharmacol. 2012;59:458–464. doi: 10.1097/FJC.0b013e3182495543. [DOI] [PubMed] [Google Scholar]
- 37.Nariai T, Fujita K, Mori M, et al. Antihypertensive and cardiorenal protective effects of sm-368229, a novel mineralocorticoid receptor antagonist, in aldosterone/salt-treated rats. Pharmacology. 2012;89:44–52. doi: 10.1159/000335559. [DOI] [PubMed] [Google Scholar]
- 38.Alexander EA, Levinsky NG. An extrarenal mechanism of potassium adaptation. J Clin Invest. 1968;47:740–748. doi: 10.1172/JCI105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler S. An extrarenal action of aldosterone on mammalian skeletal muscle. Am J Physiol. 1970;218:616–621. doi: 10.1152/ajplegacy.1970.218.3.616. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb S, Cox M, Singer I, et al. Acute hyperkalemia induced by hyperglycemia: Hormonal mechanisms. Ann Intern Med. 1976;84:426–432. doi: 10.7326/0003-4819-84-4-426. [DOI] [PubMed] [Google Scholar]
- 41.Mehdi UF, Adams-Huet B, Raskin P, et al. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]