Abstract
Background & objectives:
Tumour infiltrating lymphocytes (TILs) represent the host immune response against cancer cells associated with good or bad prognosis in different tumour types. This study was undertaken to evaluate the significance of CD3+, CD4+ and CD8+ TILs in breast cancer tissues in relation to clinico-pathological variables and survival outcome.
Methods:
Immunohistochemistry (IHC) was performed with antibodies against CD3, CD4 and CD8 antigens on formalin-fixed paraffin-embedded tissue sections of 150 breast cancer patients. Intratumoural and stromal TIL counting was performed semiquantitatively.
Results:
The higher CD3+, CD4+ and CD8+ intratumoural and stromal counts showed independent and direct association with good prognosis. The prognostic predictor value of intratumoural counts was higher than stromal counts. The independent associations of intratumoural and stromal counts became more prominent when adjusted with stage and grade, respectively. Among intratumoural counts, the high (++/+++) CD4+ count (OR=3.85, 95% CI=3.28-16.71, P<0.001) showed the highest survival followed by CD3+ (OR=2.70, 95% CI=1.76-8.30, P=0.001) and CD8+ (OR=2.58, 95% CI=1.55-5.86, P=0.001) the least when compared to respective low (+) counts. In contrast, among stromal counts, the high CD8+ count (OR=3.13, 95% CI=2.20-9.57, P<0.001) showed the highest survival followed by CD4+ (OR=3.02, 95% CI=2.07-8.89, P<0.001) and CD3+ (OR=2.45, 95% CI=1.53-6.73, P=0.002) the least.
Interpretation & conclusions:
Our results suggest that intratumoural CD4+ and stromal CD8+ counts by immunohistochemistry may serve as an independent prognosticator for favourable outcome in breast cancer.
Keywords: Breast cancer, CD3, CD4, CD8, ductal carcinoma, tumour infiltrating lymphocytes
Breast cancer is a leading cause of mortality in women worldwide1. In India, it is the second most important cancer among females after cervical cancer2. Breast cancer pathogenesis is multifactorial. The traditional prognostic markers for breast cancer include tumour grade, clinical stage, lymph node with estrogen (ER), progesterone (PR) and human epidermal growth factor receptor (HER2/neu or c-erb B2) status3.
Human cancer tissue is infiltrated by lymphocytes called tumour infiltrating lymphocytes (TILs) representing the local immune response directed against tumour growth and metastasis. These TILs have been considered as independent prognostic indicator in a number of malignant tumours4,5,6,7,8,9,10,11,12. Breast cancer tissue is invaded by a mixed population of immune cells, including T cells, B cells, natural killer (NK) cells and macrophages. Despite the existence of this immune response, many breast cancers progress and spread, questioning the function of TILs in the tumour microenvironment13. There is no conclusive data about the link between amount and kind of lymphocyte infiltration and the tumour growth in different kinds of breast carcinomas. These interrelationships play an important role in the pathogenesis and growth of breast cancer.
CD3 antigen is a receptor glycoprotein present on mature T lymphocytes. While high CD3+ cell density has been reported to correlate with favourable outcome in oropharyngeal cancer14, a low CD3+ count has been shown to predict a shorter disease free survival in colon and cervical cancer5,15. CD4 antigen is a glycoprotein found on the surface of helper T cells, regulatory T cells, monocytes and macrophages. CD4+ T lymphocytes are an essential part of adaptive immunity. CD8 antigen is also a T cell receptor glycoprotein. Generally both CD4+ and CD8+ TILs are necessary for effective tumour elimination9, however, there are studies which indicate that CD4+ TILs are adequate to remove cancer cells in the absence of the CD8+ TILs16,17. Higher CD8+ and other TILs have been shown to be associated with good prognosis in colon and ovarian carcinoma18. It has been also suggested that CD8+ infiltration may inhibit tumour growth6,18. In particular, higher number of CD8+ TILs have been linked with disease free survival and overall survival7,18,19.
The present study was aimed to evaluate the prognostic relevance of CD3+, CD4+ and CD8+ TILs in breast cancer tissue compared to the conventional prognostic markers viz. tumour staging, grading, lymph node and hormone receptor status.
Material & Methods
A total of 150 histologically proven consecutive cases of breast cancer recruited between December, 2008 to October, 2011 from the Department of General Surgery, King George's Medical University, Lucknow, UP, India were included in the study after obtaining informed written consent. The study protocol was approved by the Ethics Committee of King George's Medical University, Lucknow. Demographic details, clinical history, complete general/ local examination and epidemiological risk factors including family history, clinical stage, tumour grade, lymph node status, ER, PR, and HER-2/neu were recorded on a detailed proforma especially designed for the study.
All patients had a pre-operative tissue diagnosis of breast cancer either by fine needle aspiration cytology and/or core biopsy of the breast (Fig. 1a). All patients underwent surgery with axillary lymph node dissection, and none of these patients had received pre-operative antitumour therapy. Histological grading was done by Bloom and Richardson scoring20. No patient had evidence of active infection or inflammatory disease. Detailed histopathological examination was done in the Department of Pathology, King George's Medical University, Lucknow, India.
Fig. 1.
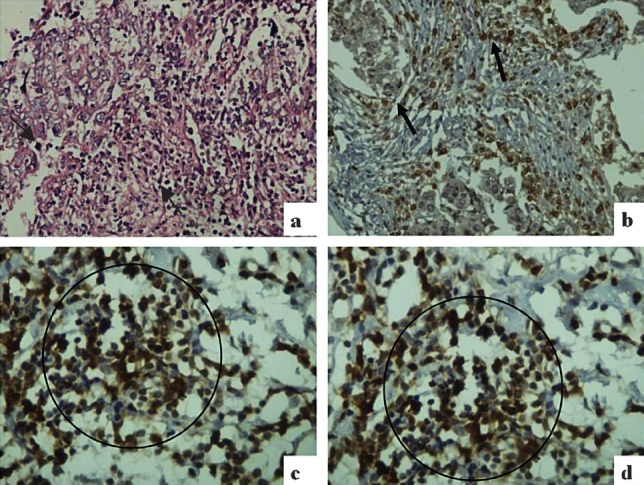
(a) Histological section from infiltrating ductal carcinoma (IDC) breast showing intratumoural and stromal TILs (black arrows) (Hematoxylin & Eosin (HE) × 200); (b) Intratumoural and stromal CD3+ TILs in IDC (black arrows) (Immunostain ×200); (c) Intratumoural CD4+ TILs in IDC (black circle) (Immunostain × 400); (d) Stromal CD8+ TILs in IDC (black circle) (Immunostain ×400).
Immunohistochemistry: Immunohistochemistry was performed using primary antibodies against CD3+, CD4+, CD8+, ER, PR and HER-2/neu using Novolink Min Polymer Detection system (Novacastra, Leica Biosystem Newcastle Ltd, UK). Formalin-fixed, paraffin-embedded tissues sections (3-4μm thick) were taken on 3-aminopropyltriethoxysilane (APTS) coated glass slides. Sections were deparaffinised in xylene followed by hydration in graded ethanol. Antigen retrieval was performed by heating specimens at 100°C for 20 min in 0.01M citrate buffer (pH 6.0) using an EZ antigen retriever system (Biogenex, USA). Endogenous peroxidase was blocked by incubating sections with 0.3 per cent hydrogen peroxide for 5 min and the non specific binding sites were blocked with a protein block for 5 min. Sections were covered with primary antibody and the slides were incubated in moist chamber overnight at 4 °C. Slides were then washed with Tris buffer saline (TBS, pH 7.4), followed by a 30 min incubation with post primary block at room temperature. Sections were washed twice in TBS followed by incubation with Novolink polymer for 30 min at room temperature. After three washes in TBS, sections were treated with DAB chromogen (3, 3’--diaminobenzidine tetrahydrochloride) for 5-10 min in the dark. Sections were counterstained with hematoxylin, dehydrated with ethanol and xylene, and mounted permanently with Di-n-butylPhthalate in Xylene (DPX). Negative control slides omitting the primary antibody were included in all batches. Section from tonsillar tissue served as positive control for CD3+, CD4+, CD8+.
Microscopic evaluation of CD3+, CD4+, and CD8+ TILs: Scoring of immune stained positive TILs was done independently by two pathologists. CD3+, CD4+, and CD8+ TILs were counted in five randomly selected high power fields at 40X magnification and the counts were averaged. Initially TIL count was recorded as: + (1-25 cells), ++ (26-50 cells), +++ (≥51 cells) in the tumour and the stroma separately21. Positive TILs upto 25 cells were considered as low TIL count and more than 25 cells (i.e. ++, +++) were considered as high TIL count (Fig. 1b-d).
Microscopic evaluation of ER, PR and HER-2/neu was done as per American Society of Clinical Oncology/College of American Pathologists Guidelines (ASCO/CAP guidelines)22.
ER/PR scoring was done as follows: 0 - negative, no nuclear staining; 1+ - <10 per cent nuclear staining (Borderline); 2+ - 10-75 per cent cells show nuclear staining; and 3+ >75 per cent cells show nuclear staining. At least 1 per cent of tumour cells showing positive nuclear staining of any intensity in tumour cells with antibodies to ER and PR was taken as positive.
HER-2/neu criteria for scoring was as follows: 0 - no staining or membrane staining in <10 per cent tumour cells; 1+ - faint staining in 10 per cent of cells, partial membrane staining; 2+ - a weak to moderate positive staining in >10 per cent of tumour cells; and 3+ - a strong complete membrane staining is observed in >10 per cent of tumour cells for interpretation, 0 and 1+ were considered as negative, 2 + as weak positive staining, and 3+ was considered as positive staining.
IHC result of 3+ cell surface protein expression (defined as uniform intense membrane staining of > 30 per cent of invasive tumour cells) was considered as a positive HER-2neu test. Details of clinical progress and survival of patients was obtained from the hospital records. The follow up period was 43 months.
Statistical analysis: Continuous data were summarized as mean ± SD while discrete (categorical) data were expressed in percentage. The continuous variables were compared by Student's t test and the discrete variables by Fisher's exact test or Chi-square (χ2) test. Significance of each TIL marker with study end outcome was evaluated by binary univariate and multivariate logistic regression analysis, considering end outcome (not well=0 and well=1) as the dependent variable and TIL marker as the independent variable. Each model was adjusted with demographic confounder variables viz. age, menstrual status and family history. Kaplan-Meier method was used to calculate overall survival proportion and the difference of survival between the two groups was performed by log-rank test. The overall survival (OS) time was calculated for each patient to the nearest month, taken from the time of presentation to the time of death or last recorded follow up. The Kendall τb correlation analysis was used to assess association between the TIL markers. A two-sided (α=2) P<0.05 was considered significant. Analyses were performed using GraphPad Prism (version 5.0) (Graph Pad Software, Inc. LaJolla, CA, USA) and MINITAB (version 13.0) (Mini Tab Ltd. Conventry, UK) softwares.
Results
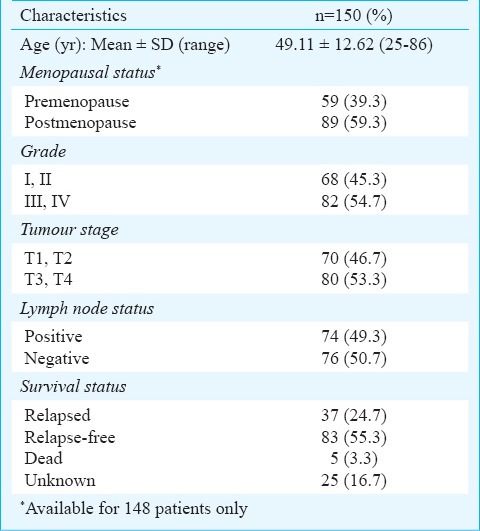
The basic, clinico-pathological characteristics and survival status of all patients (n=150) at admission are summarized in Table I. Age of these patients ranged from 25-86 yr with an average of 49.11 ± 12.62 yr. The menstrual cycle history was available only for 148 patients as two women had undergone hysterectomy. Majority (n=89, 59.3%) of the subjects were postmenopausal. All patients had infiltrating ductal carcinomas with 54.7 per cent showing a higher grade (III, IV), 53.3 per cent with higher stage (T3, T4) and 50.7 per cent with negative lymph node status. The survival status of only 125 (83.3%) patients was available. Of these, 83 (55.3%) were relapse free survivors, 37 (24.7%) had metastases, and 5 did not survive (Table I).
Table I.
Clinico-pathological characteristics and survival status of the breast cancer patients
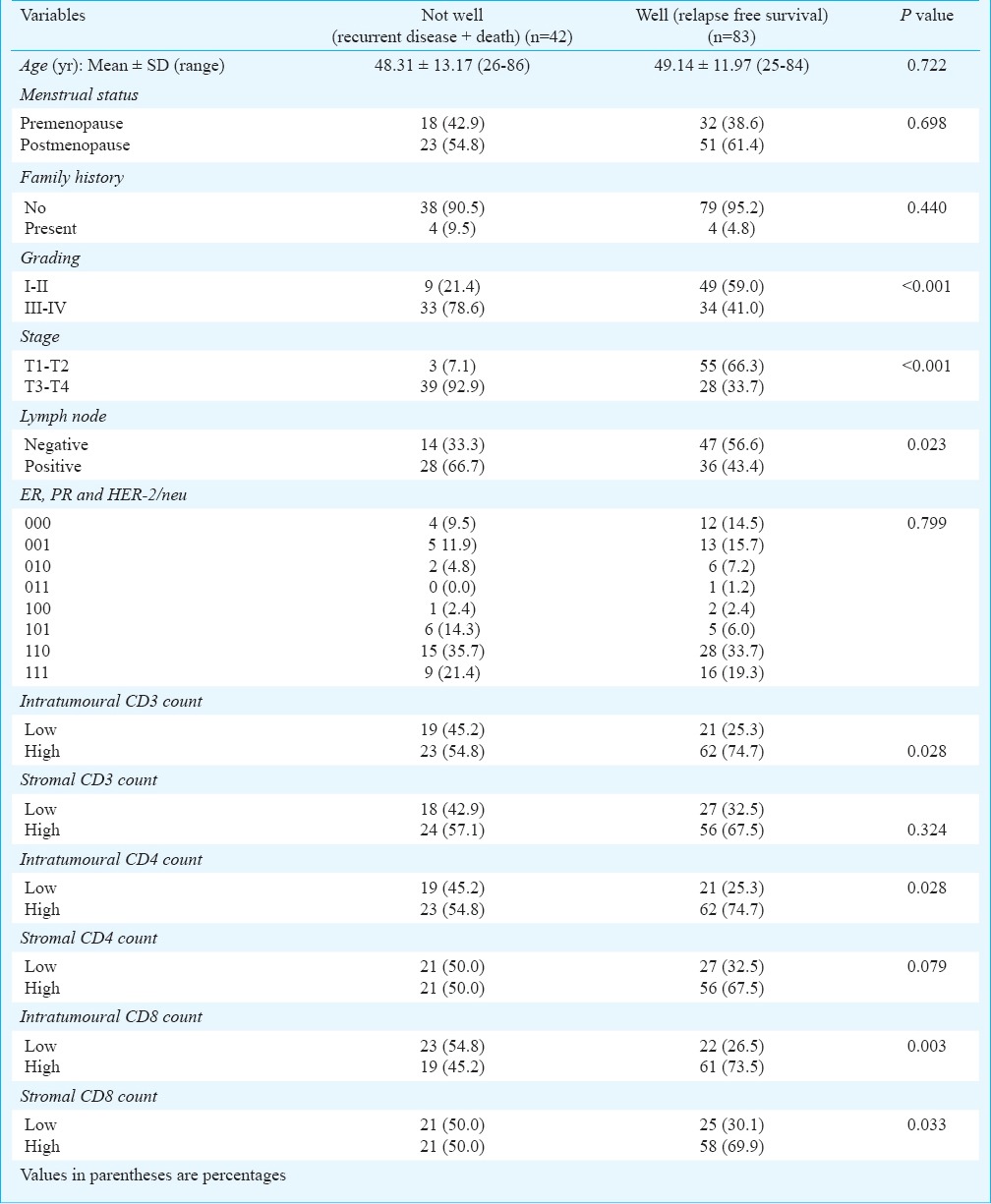
The association of each independent variable (basic, clinico-pathological characteristics and survival status) with end outcome [not well (death + recurrent disease: n=42) vs. well (relapse free survival: n=83)] are summarized in Table II. The mean age and the frequency (% age) of menstrual cycle and family history were found similar between the two groups, indicating insignificant association of these with the end outcome. Similarly, ER, PR and HER-2/neu, and stromal CD3+ and CD4+ counts also showed insignificant association with the end outcome. However, grading, stage and lymph node status showed significant (P<0.05 or P<0.001) association with the end outcome. Further, high CD3+, CD4+ and CD8+ intratumoural counts showed a significant (P<0.05 or P<0.01) and direct association with relapse free survival. In contrast, high count of only CD8+ stromal showed a significant (P<0.05) and direct association with relapse free survival.
Table II.
Association of clinico-pathological characteristics and TIL counts with end outcomes
To assess the independent significance of TILs with end outcome, the association of each marker was further evaluated by univariate (unadjusted or crude) and multiple (adjusted with clinico-pathological characteristics) logistic regression (LR) analysis considering the end outcome (not well=0 and well=1) as dependent variable and TILs as the independent variable. Univariate LR found all three intratumoural CD3+ (OR=2.31, 95% CI=1.02-5.26; P=0.046), CD4+ (OR=2.96, 95% CI=1.31-6.73; P=0.009) and CD8+ (OR=3.69, 95% CI=1.63-8.35; P=0.002) counts as significant (P<0.05 or P<0.01) and independent predictors of end outcome. In contrast, among stromal, CD4+ (OR=2.51, 95% CI=1.13-5.60; P=0.024) and CD8+ (OR=3.10, 95% CI=1.32-7.29; P=0.009) counts were found significant (P<0.05 or P<0.01) and independent predictors of end outcome (Table III).
Table III.
Unadjusted and adjusted association of TIL markers with end outcomes
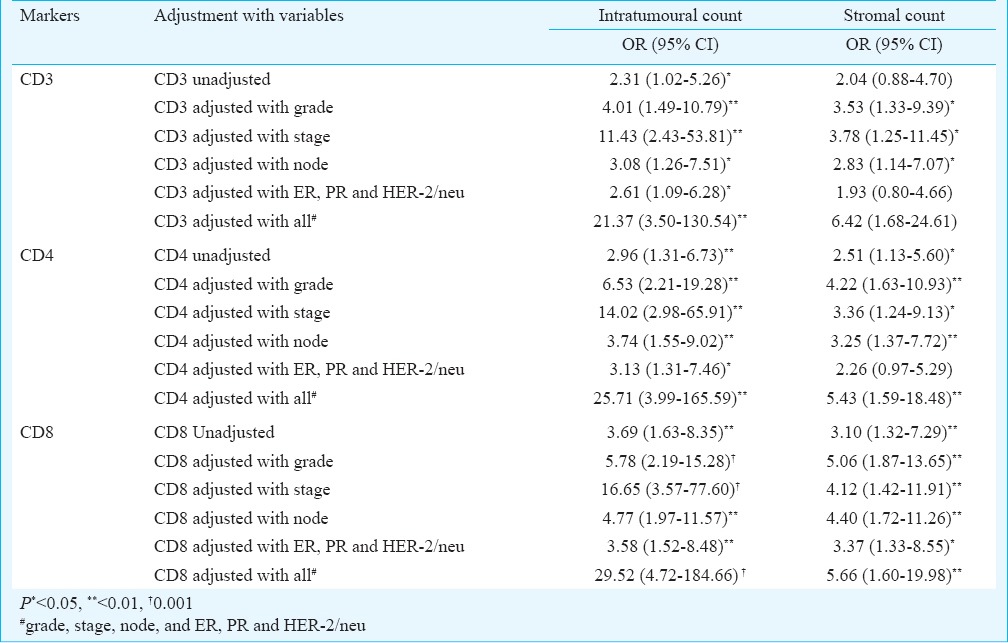
The multiple LR showed that the independent associations of TILs became more significant when adjusted with risk factors (viz., grade, stage, node; and combination of ER, PR and HER-2/neu). Further, the insignificant stromal CD3+ count also became significant when adjusted with grade, (OR=3.53, 95% CI=1.33-9.39; P=0.011), stage (OR=3.78, 95% CI=1.25-11.45; P=0.019) and node (OR=2.83, 95% CI=1.14-7.07; P=0.026). The association of both intratumoural and stromal counts were significant when adjusted with stage and grade, respectively. On an average, with end outcome, the association of intratumoural counts was higher than the stromal counts.
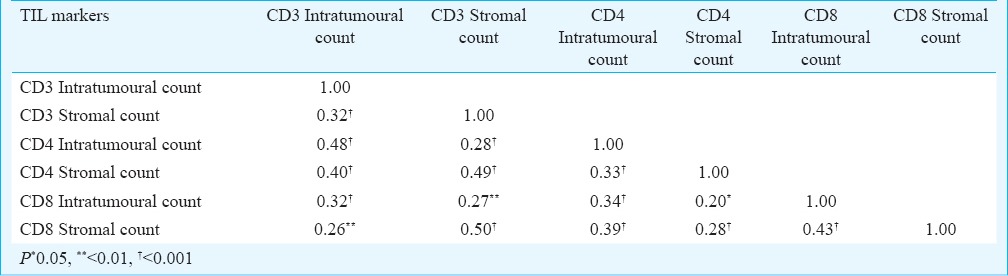
The association between TIL markers is summarized in Table IV. All markers showed significant (P<0.05 or P<0.01 or P<0.001) and positive correlation with each other, with highest association seen between CD3+ stromal count and CD8+ stromal count (r=0.50, P<0.001) and least between CD4+ stromal count and CD8+ intratumoural count (r=0.20, P<0.05).
Table IV.
Association between TIL markers (n=150)
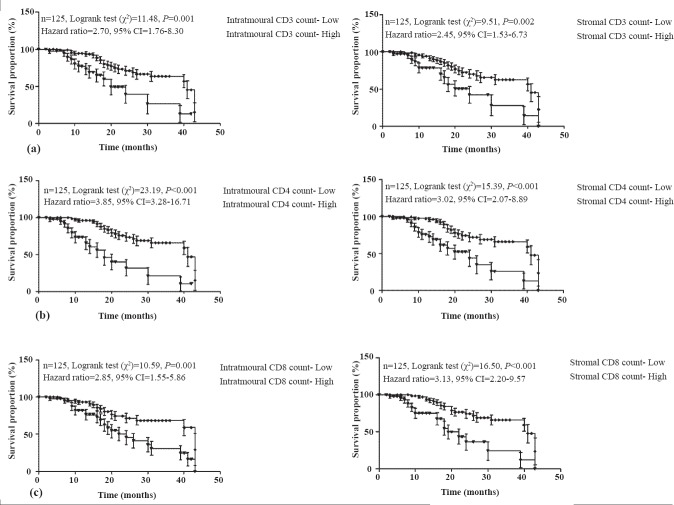
The overall survival proportion of each TIL markers according to low (+) and high (++, +++) intratumoural and stromal counts are summarized in Fig. 2. The high CD3+, CD4+ and CD8+ intratumoural counts showed significantly (P<0.01 or P<0.001) different and 2.70 (95% CI=1.76-8.30), 3.85 (95% CI=3.28-16.71) and 2.58 (95% CI=1.55-5.86) fold higher survival, respectively as compared to low intratumoural counts. Further, high CD3+, CD4+ and CD8+ stromal counts also showed significantly (P<0.01 or P<0.001) different and 2.45 (95% CI=1.53-6.73), 3.02 (95% CI=2.07-8.89) and 3.13 (95% CI=2.20-9.57) fold higher survival, respectively as compared to low stromal counts.
Fig. 2.
Overall survival proportions according to CD3+ (a), CD4+ (b) and CD8+ (c) intratumoural and stromal TIL counts, respectively.
Discussion
The prognostic significance of tumour infiltrating lymphocytes has been a long standing topic of debate. In cases where TILs have improved patient outcome, these are recognized as the main effectors of antitumour immune responses23. In our study, infiltration of CD3+, CD4+ and CD8+ TILs was significantly associated with good prognosis. Importantly, the higher densities of CD3+, CD4+ and CD8+ TILs decreased the risk of relapse of the disease. Intratumoural CD4+ TILs were observed as better prognostic marker than CD3+ and CD8+ TILs. The observation that tumours with high TILs will have good prognosis, may not be true for all types of cancers. This may vary even within the tumour types of the same organ. Medullary carcinoma having both intratumoural and stromal CD4 or CD8 positive TILs are associated with good prognosis, but not the papillary carcinoma breast (that has only stromal)21. In his study on invasive micropapillary carcinoma of breast21, TILs were found to be associated with increased lymph node metastasis and a poorer prognosis. The results suggest that effective immunity provided by TILs varies in different tumours and the relative lack of tumour-killing cytotoxic TILs in invasive micropapillary carcinoma may explain, in part, the adverse association of TILs with the biological behavior of invasive micropapillary carcinoma of breast. Matkowski et al24, observed that patients with high expression of CD 4+ and CD8+ TILs had distinctly worse cancer specific overall survival. La Rocca et al25 observed that the number of CD4 and CD8 expressing cells was higher in node negative than in node positive invasive ductal breast lesions. Kim et al26 reported that decreased number of CD8+ TILs in breast tumours were significantly associated with lymph node metastasis, higher stage and high proliferative index.
The location of TILs, whether intratumoural or stromal is also important. In our study, higher counts of TILs were observed in both intratumoural and stromal areas of breast cancer, which were uniformly significantly associated with favourable prognosis. The densities of intratumoural TILs were found to be stronger predictor for survival than stromal TILs. A systematic review and meta analysis of 33 studies was carried out by Gooden et al27 to analyse the prognostic influence of tumour-infiltrating lymphocytes in cancer. The authors however, included studies only with intratumoural TILs. In three of these studies, the CD3+ /CD8 + ratio was used, but each of them used a different interpretation of this ratio. Han et al28 found an independent positive effect of either CD3+ or CD8+ compared with no CD3+ or CD8+ in ovarian cancer. In gastric cancer, it was observed that high numbers of both CD3+ and CD8+ were favourable compared with low numbers of both cell types29. Kobayashi et al30 found that high numbers of CD8+ compared with CD3+ were not a prognostic factor in hepatocellular cancer. It is worth mentioning that while considering the CD3+ /CD8 + ratio it is important to keep in mind that most CD8+ cytotoxic lymphocytes are also CD3 positive. CD8+ /CD4+ ratio was analyzed in three studies18,19,31, and found to be a positive prognostic predictor in only one of these18.
Although CD4+ TILs have been shown to be sufficient to eliminate tumour cells in the absence of CD8+ T cells in some tumour models, more often both CD4+ and CD8+ are required for effective tumour rejection16,17. The role of CD4+ TILs in antitumour responses is often to activate the CD8+ TILs, leading to the destruction of the tumour by CD8+ cytotoxic T lymphocytes (CTL). The CD4+ T-cells help the CD8+ CTLs in tumour immunity through three phases, viz. by early induction, effector maintenance, and memory of CD 8+ CTL responses.
In the present study, CD3+, CD4+ and CD8+ TIL counts were not significantly associated with age, menopausal status and family history. A significant association of higher tumour grade and clinical stage with the severity of disease was found. Lymph node positivity was also correlated with the relapse of breast cancer. As all the assessed cases in the present study were of infiltrating ductal carcinomas (NOS), the prognostication cannot be generalized for all subtypes of breast cancer. An increase in TILs may not always be associated with a better prognosis. There is evidence in the literature to indicate that tumour specific CD4+ TILs may change their phenotype from effectors to suppressors during cancer progression. Conversion from effector cells may coincide with a substantial reduction in the antigen expression level, resulting in tumour persistence that ultimately leads to tumour tolerance. This negative regulatory role of CD4+ TILs needs to be distinguished from the conventional role of activated CD4+ TILs23.
To conclude, CD3+, CD4+ and CD8+ TILs were found to be good prognosticators of overall survival of infiltrating ductal carcinoma of breast. Although quantifying TILs by histopathology and immunohistochemistry is a frequently used approach, the methodological factors may confound the exact magnitude of TILs on prognosis. Moreover, just quantifying TILs may not take into account the dynamics and functionality of the tumour microenvironment. The study needs to be done in various histological subtypes of breast cancer, in a larger sample size, including the cytokines or cytotoxicity assay along with molecular signatures for a better understanding of role of TILs27.
References
- 1.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 2.Ray A, Mitra AB. Estrogen and breast cancer. ICMR Bull. 2003;33:13–24. [Google Scholar]
- 3.Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23(Suppl 2):S60–4. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 5.Ancuta E, Ancuþa C, Zugun-Eloae F, Iordache C, Chirieac R, Carasevici E. Predictive value of cellular immune response in cervical cancer. Rom J Morphol Embryol. 2009;50:651–5. [PubMed] [Google Scholar]
- 6.Strater J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, et al. Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma: clinical evidence for immunoselection and CD95L mediated control of minimal residual disease. Gut. 2005;54:661–5. doi: 10.1136/gut.2004.052696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoural CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–6. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–6. [PubMed] [Google Scholar]
- 9.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumour-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–72. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–97. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 11.Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, et al. Melanoma-specific tumour-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006;55:451–8. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97:1135–8. doi: 10.1038/sj.bjc.6604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139:33–41. doi: 10.1016/s0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 14.Rajjoub S, Basha SR, Einhorn E, Cohen MC, Marvel DM, Sewell DA. Prognostic significance of tumour-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J. 2007;86:506–11. [PubMed] [Google Scholar]
- 15.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–9. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumour angiogenesis by tumour-infiltrating CD4+ T cells requires tumour responsiveness to IFN-gamma. J Immunol. 2001;166:2276–82. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara H, Fukuzawa M, Yoshioka T, Nakajima H, Hamaoka T. The role of tumour- specific Lyt-1+2-T cells in eradicating tumour cells in vivo. I. Lyt-1+2-T cells do not necessarily require recruitment of host's cytotoxic T cell precursors for implementation of in vivo immunity. J Immunol. 1984;133:1671–6. [PubMed] [Google Scholar]
- 18.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumour infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–35. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 20.Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359–77. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Fan Y, Lang R, Gu F, Chen L, Cui L, et al. Tumour infiltrating lymphocytes differ in invasive micropapillary carcinoma and medullary carcinoma of breast. Mod Pathol. 2008;21:1101–7. doi: 10.1038/modpathol.2008.72. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Pathologists Practice Guidelines update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Fu YX. Tumour-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–45. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 24.Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, et al. The prognostic role of tumour-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–51. [PubMed] [Google Scholar]
- 25.La Rocca G, Anzalone R, Corrao S, Magno F, Rappa F, Marasa’ S, et al. CD1a down-regulation in primary invasive ductal breast carcinoma may predict regional lymph node invasion and patient outcome. Histopathology. 2008;52:203–12. doi: 10.1111/j.1365-2559.2007.02919.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, et al. Tumour-infiltrating lymphocytes, tumour characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–31. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 27.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, et al. HLA class I antigen processing machinery component expression and intratumoural T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372–9. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–11. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 31.Zingg U, Montani M, Frey DM, Dirnhofer S, Esterman AJ, Went P, et al. Tumour-infiltrating lymphocytes and survival in patients with adenocarcinoma of the oesophagus. Eur J Surg Oncol. 2010;36:670–7. doi: 10.1016/j.ejso.2010.05.012. [DOI] [PubMed] [Google Scholar]