Abstract
Background & objectives:
The comparative prognostic value of C-reactive protein (CRP) and fibrinogen for cardiovascular events has been inconclusively investigated. This study was carried out to compare the prognostic value of CRP versus fibrinogen in patients with coronary artery disease (CAD).
Methods:
The study included 13,100 patients with coronary angiography-confirmed CAD. Plasma CRP and fibrinogen levels were measured before angiography in all patients. The levels of CRP>3 mg/l and fibrinogen>350 mg/dl were considered as elevated. The primary outcome was 1-year all-cause mortality.
Results:
Patients were divided into four groups: patients with CRP≤3 mg/l and fibrinogen ≤350 mg/dl (n=4206); patients with CRP≤3 mg/l and fibrinogen >350 mg/dl (n=3132); patients with CRP>3 mg/l and fibrinogen ≤ 350 mg/dl (n=1273) and CRP >3 mg/l and patients with fibrinogen >350 mg/dl (n=4489). There were 634 deaths: 75 deaths in patients with CRP ≤3 mg/l and fibrinogen ≤350 mg/dl, 91 deaths in patients with CRP ≤3 mg/l and fibrinogen >350 mg/dl, 87 deaths in patients with CRP >3 mg/l and fibrinogen ≤350 mg/dl and 381 deaths in patients with CRP >3 mg/l and fibrinogen >350 mg/dl (Kaplan-Meier estimates of all-cause mortality, 1.8, 3.0, 7.0 and 8.7 %, log-rank test P<0.001). The multivariate analysis showed that CRP [adjusted hazard ratio (HR)=1.31, 95% confidence interval (CI) 1.18-1.45, for each standard deviation increase in the logarithmic scale] but not fibrinogen [adjusted HR=0.99 (0.90-1.09), for each standard deviation increase in the logarithmic scale] was an independent correlate of mortality.
Interpretation & conclusions:
The findings indicated that in patients with CAD, CRP was a better predictor of mortality than fibrinogen and offered prognostic information beyond that provided by the conventional cardiovascular risk factors.
Keywords: Cardiovascular risk factor, coronary artery disease, C-reactive protein, fibrinogen, mortality, prognostic value
Based on the paradigm that inflammation is involved in the promotion of atherosclerosis and atherosclerotic plaque vulnerability1,2, circulating inflammatory biomarkers have been the focus of intense research. These are believed to reflect the severity of inflammation and correlate with atherosclerotic burden, plaque instability and adverse vascular events including increased risk of mortality3. Over the last decades, fibrinogen and high sensitivity C-reactive protein (CRP) have been singled out among an array of inflammatory markers as valuable indices of systemic inflammation with a close association to atherosclerosis and its clinical manifestations4,5,6,7,8. Only a few studies have directly compared CRP with fibrinogen and the results have been conflicting8,9,10,11,12,13,14,15. Thus, the comparative prognostic value of these biomarkers for cardiovascular events remains poorly investigated. Moreover, since coronary events and myocardial infarction are common and leading cause of death in patients with coronary artery disease (CAD)16, risk stratification of these patients in the setting of secondary prevention studies is of considerable clinical importance.
We undertook this study to compare the prognostic value of CRP versus fibrinogen and investigate whether combined use of these biomarkers could be advantageous compared to individual biomarker for risk prediction of cardiovascular events in patients with CAD.
Material & Methods
Between March 2000 and December 2009, 14,713 consecutive patients underwent coronary angiography and percutaneous coronary intervention (PCI) at the German Heart Center in Munich, Germany, in the setting of clinical trials designed to assess efficacy of antithrombotic regimens in patients undergoing PCI. Blood samples (20 ml) were obtained in all patients with the intention to investigate prognostic values of various biomarkers. For the purposes of this prospective study, plasma was collected to investigate the prognostic value of CRP and fibrinogen. Patients with missing CRP or fibrinogen measurements, acute infections, impaired renal function (serum creatinine ≥2 mg/dl) or known malignant diseases with life expectancy <1 year and patients undergoing coronary angiography in the setting of failed thrombolysis or routine PCI after thrombolysis were excluded from the study. After exclusion of 1613 patients, (11%), 13,100 patients with angiographically proven CAD and CRP and fibrinogen levels available were included in this study. Clinical diagnosis was: stable angina (n=8074; 61.6%), unstable angina (n=2470; 18.9%), non-ST-segment elevation myocardial infarction (n=967; 7.4%) and ST-segment elevation myocardial infarction (n=1589; 12.1%). Written informed consent for angiographic examination, coronary intervention and blood sampling were obtained from all patients. The study protocol was approved by the institutional ethics committee.
Diagnostic criteria: In all patients, the diagnosis of CAD was confirmed by angiographic criteria: angiographic documentation of coronary stenoses with ≥50 per cent lumen obstruction in, at least, one of the three major coronary arteries and/or culprit lesions in patients with acute coronary syndromes. The left ventricular ejection fraction was measured using the area-length method on left ventricular angiograms17. Digital angiograms were analyzed offline with an automated edge detection system (CMS; Medis Medical Imaging Systems, Nuenen, The Netherlands) in the core angiographic laboratory.
The main cardiovascular risk factors were defined using accepted criteria. A total cholesterol of ≥220 mg/dl or prior or ongoing treatment with lipid lowering agents was required for the diagnosis of hypercholesterolaemia. Arterial hypertension was diagnosed if patients were under active treatment with antihypertensive medications or if the systolic blood pressure was ≥140 mmHg or the diastolic blood pressure ≥90 mmHg on, at least, two separate occasions. The diagnosis of diabetes required active treatment with insulin or an oral hypoglycaemic agent; an abnormal fasting blood glucose test (glucose ≥126 mg/dl or ≥7.0 mmol/dl); a blood glucose >200 mg/dl (>11.1 mmol/dl) at any time; or an abnormal 2-h post glucose load test [post-load plasma glucose ≥200 mg/dl (11.1 mmol/l)] according to the World Health Organization criteria18. Current smokers were those who smoked any type or amount (any number of cigarettes per day) of tobacco in the prior six months. Patients’ weight and height were measured and used to calculate the body mass index. The creatinine clearance was estimated using the Cockcroft-Gault formula19.
PCI (mostly coronary stenting) was performed according to standard practice. Antiplatelet therapy included clopidogrel (300 mg or 600 mg as a loading dose followed by 75 mg/day for, at least, one month for bare metal stents and, at least, six months for drug eluting stents) and aspirin (200 mg/day orally for an indefinite period).
Biochemical measurements: Blood samples were obtained before angiography. Plasma concentrations of high-sensitivity CRP were measured using a fully automated latex-enhanced immunoturbidometric assay on a Cobas Integra (Roche Diagnostics, Mannheim, Germany). The C-reactive protein assay had an analytical sensitivity of 0.085 mg/l and a measuring range up to 160 mg/l. The upper limit of the reference range in healthy adults is 5 mg/l. Fibrinogen was determined in citrated plasma by a modification of the Clauss method20 using the BCS analyser (Multifibren U, Siemens Healthcare, Marburg, Germany). The measurement range was between 80 and 1200 mg/dl, and the expected values were between 180 and 350 mg/dl. Plasma heparin concentrations below 2U/ml do not affect the test. Creatinine was measured using a kinetic colorimetric assay based on the compensated Jaffe method21. Personnel involved in the laboratory measurements were unaware of the clinical, angiographic or follow up outcome of the patients. Concentrations of CRP >3 mg/l and fibrinogen >350 mg/dl were considered as elevated.
Outcome definition and follow up: The primary outcome was mortality of any cause at one year. Cardiac mortality at one year, and 1-year occurrences of non-fatal myocardial infarction, stroke and stent thrombosis were defined as secondary outcomes. Information on deaths was obtained from hospital records, death certificates or phone contact with the referring physician(s), relatives of the patient, insurance companies and registration of address office. Cardiac death was defined according to the Academic Research Consortium criteria22. The diagnosis of myocardial infarction required development of new abnormal Q waves in ≥2 contiguous precordial leads or ≥2 adjacent limb leads; or an elevation of creatine kinase – myocardial band (CK-MB) >2 times (or >3 times for the 48 h after PCI) the upper limit of normal. Definite stent thrombosis was defined according to the Academic Research Consortium criteria22. Stroke was diagnosed in the presence of acute neurologic deficits that were confirmed by computed tomography or magnetic resonance imaging of the head. The follow up protocol included a telephone interview at one month, a clinic visit at six months and a telephone interview at 12 months after hospital discharge. In addition, if patients reported cardiac complaints any time during the follow up, they underwent a complete clinical, electrocardiographic, and laboratory evaluation. Follow up, information was obtained and adjudication of events was performed by medical staff unaware of clinical diagnosis or biomarker (CRP or fibrinogen) level.
Statistical analysis: Data are presented as median with 25th-75th percentiles, number of patients/events or proportions (%). The normality of data distribution was tested using the Kolmogorov-Smirnov test. Continuous data were compared with the Wilcoxon rank-sum test. Categorical data were compared with chi-square test. Survival analysis was performed using the Kaplan-Meier method. Differences in survival were compared with the log-rank test. Correlation between CRP and fibrinogen was assessed by calculating the Spearman's correlation coefficient. The Cox proportional hazards model was used to test the independency of association of CRP or fibrinogen with the primary outcome (all-cause mortality). All variables of Table I plus therapy at discharge were entered into the model. CRP or fibrinogen were entered into the model(s) as continuous variables after logarithmic transformation (3 separate models) and the hazard ratios for the association with mortality were calculated per standard deviation increase in the natural logarithmic scale. Bootstrapping (400 samples) was used to validate the stability of the model by ensuring the fidelity of the confidence intervals. Receiver-operating characteristic (ROC) curves (a plot of test sensitivity versus its 1-specificity or false positive rate across a series of cut-off points) and respective areas under the ROC curves were constructed to compare the predictive accuracy of CRP with that of fibrinogen for primary outcome prediction. Comparison of the areas under the ROC curve for CRP versus fibrinogen was performed using the pROC package and bootstrapping method (n=400 samples). The discriminatory power of the Cox models for primary outcome prediction was assessed by calculating the Harrell's c index of the models before and after inclusion of CRP, fibrinogen or both. The 95 % confidence interval of the c index was calculated using the bootstrapping method (400 samples). Comparison of the c indices of the models before and after inclusion of CRP, fibrinogen or both was enabled using the Somer's D package. All analyses were performed using S-plus statistical package (S-PLUS, Insightful Corp, Seattle, Washington, USA) or R package (R2.15.1 version; The R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P <0.05 was considered to indicate statistical significance.
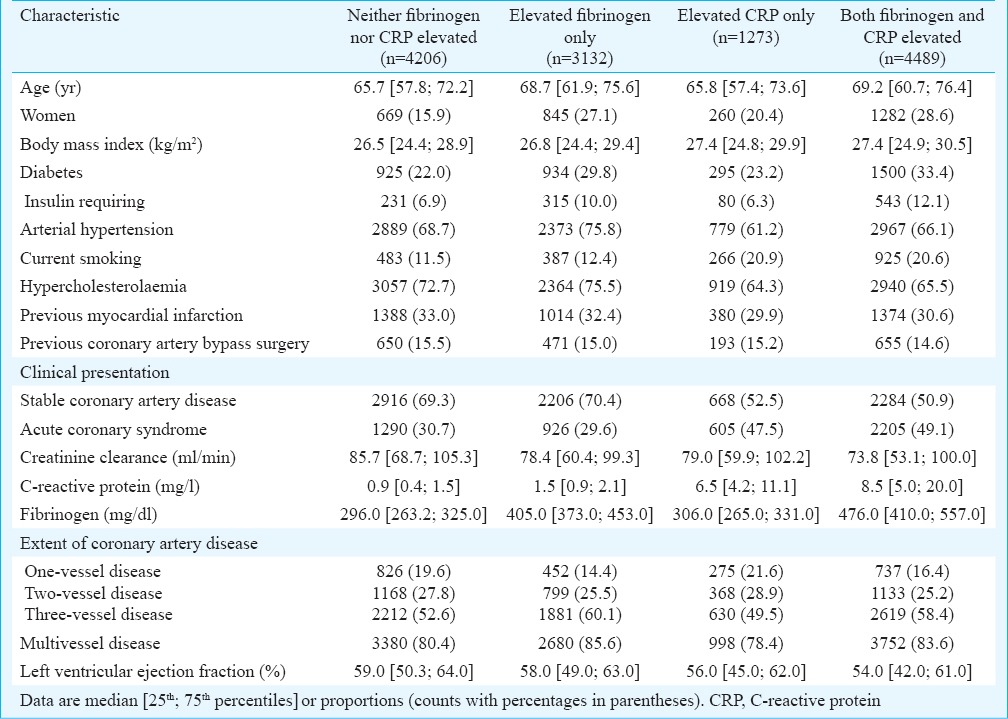
Table I.
Baseline characteristics of the study subjects
Results
The study included 13,100 patients. A cut-off of 3 mg/l for CRP and 350 mg/dl for fibrinogen were used to dichotomize patients into four groups: the group with normal biomarker levels (CRP ≤3 mg/l and fibrinogen ≤350 mg/dl; n=4206 patients; 32.1%); the group with elevated fibrinogen only (CRP ≤3 mg/l and fibrinogen >350 mg/dl; n=3132 patients; 23.9%); the group with elevated CRP only (CRP >3 mg/l and fibrinogen ≤350 mg/dl; n=1273 patients; 9.7%); and the group with elevation of both biomarkers (CRP >3 mg/l and fibrinogen >350 mg/dl; n=4489 patients; 34.3%). Baseline characteristics of patients are shown in Table I. In general, the group with elevated levels of both CRP and fibrinogen showed the worst cardiovascular risk profile as compared with other groups. Main cardiovascular drugs at discharge were: statins (n=12,198; 93.1%), beta-blockers (n=12,312; 94.0%) and angiotensin-converting enzyme inhibitors (n=10,648; 81.0%). Coronary stents were implanted in 11,583 patients (90.5%); drug-eluting stents were implanted in 8986 patients (76.0%).
Baseline characteristics of patients with elevated fibrinogen only and elevated CRP only were compared. In comparison to patients with elevated CRP only, patients with elevated fibrinogen only were older (P<0.001) and had higher proportions of women (P<0.001), diabetes (P<0.001), arterial hypertension (P<0.001), hypercholesterolaemia (P<0.001), multivessel disease (P<0.001) and presented more often with stable CAD (P<0.001). Patients with elevated CRP only were more often current smokers (P<0.001) and presented more often with an acute coronary syndrome (P<0.001; Table I).
There was a weak correlation between CRP and fibrinogen (R2=0.21; P<0.001).
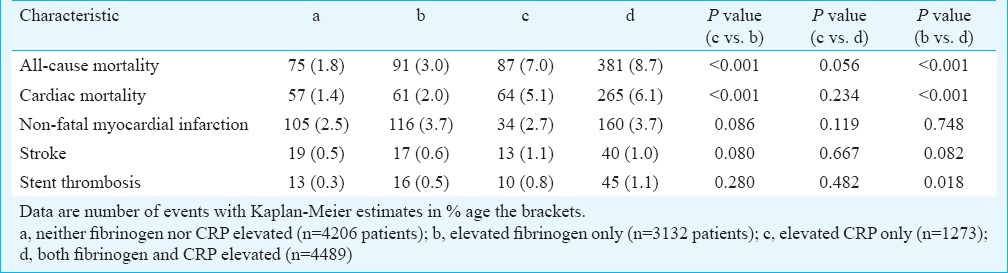
Clinical outcome: All-cause deaths (the primary outcome) occurred in 4.8 per cent of the patients (n=634 deaths). There were 75 deaths in patients with normal biomarker levels, 91 deaths in patients with elevated fibrinogen only, 87 deaths in patients with elevated CRP only and 381 deaths in patients with elevation of both biomarkers (Kaplan-Meier estimates of all-cause mortality, 1.8, 3.0, 7.0 and 8.7 per cent, respectively, log-rank test P<0.001; Fig. 1). As compared with elevated fibrinogen only, the elevated CRP only was associated with an increased risk of all-cause and cardiac deaths. Although those with elevated levels of both biomarkers showed worse clinical outcome compared to those with elevated CRP only, statistical significance was not achieved (Table II).
Fig. 1.
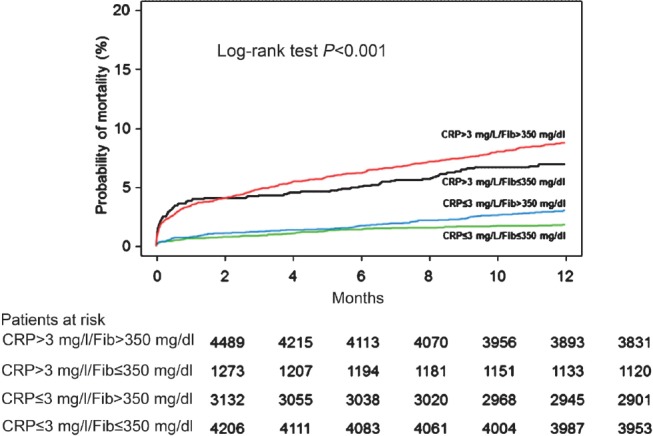
Kaplan-Meier curves of one-year all-cause mortality. CRP, C-reactive protein; Fib, fibrinogen.
Table II.
Clinical outcome of patients in the four groups
Multivariate analysis and accuracy of mortality prediction: The association of CRP and fibrinogen with all-cause mortality was tested in the multivariate Cox proportional hazards model. Fibrinogen and CRP were entered into the model alongside other variables either separately or together. The first model was adjusted for age, sex and smoking status. In this model, fibrinogen [adjusted hazard ratio (HR)=1.50, 95% confidence interval (CI) 1.39-1.63, P<0.001, for each standard deviation increase in the logarithmic scale] and CRP [adjusted HR=2.00 [1.87-2.14], P<0.001, for each standard deviation increase in the logarithmic scale] were independently associated with the increased risk of 1-year all-cause mortality. In the model that adjusted for all baseline variables plus therapy at discharge, fibrinogen was an independent correlate of all-cause mortality [adjusted HR=1.15 (1.05-1.25), P=0.001, for each standard deviation increase in the logarithmic scale]. Likewise, in the model that included CRP plus all baseline variables plus therapy at discharge, CRP was an independent correlate of all-cause mortality [adjusted HR=1.34 (1.19-1.42), P<0.001 for each standard deviation increase in the logarithmic scale]. When both biomarkers were included in the model simultaneously, CRP [adjusted HR=1.31 (1.18-1.45), P<0.001, for each standard deviation increase in the logarithmic scale] but not fibrinogen [adjusted HR=0.99 (0.90-1.09), P=0.812 for each standard deviation increase in the logarithmic scale] remained as a significant correlate of all-cause mortality. The yield of the model with CRP and fibrinogen is shown in Table III. The Cox proportional hazards model with bootstrapping approach confirmed the stability of the models for the association with mortality of fibrinogen [adjusted HR=1.15 (1.04-1.26), P=0.016], CRP [adjusted HR=1.34 (1.21-1.47), P<0.001] or both biomarkers entered simultaneously [HR=0.99 (0.88-1.09) P=0.293 for fibrinogen and HR=1.31 (1.16-1.47), P<0.001 for CRP] with all calculations performed per standard deviation increase in the logarithmic scale.
Table III.
Results of multivariate Cox proportional hazards model for all-cause mortality

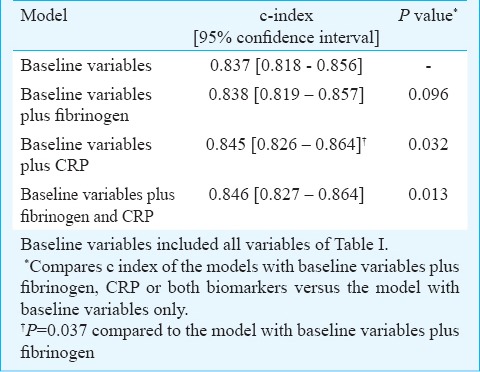
CRP and fibrinogen predicted all-cause mortality with an area under the ROC curve =0.731 [0.701-0.756] and 0.643 [0.613-0.672], respectively (P<0.001; Fig. 2). The best cut-off value, while maximizing sensitivity and specificity was 402 mg/dl for fibrinogen (sensitivity 62% and specificity 62%) and 4.4 mg/l for CRP (sensitivity 67% and specificity 67%). The discriminatory power of the models with fibrinogen, CRP or both biomarkers regarding prediction of 1-year mortality was assessed by calculating the c index in the respective Cox models. The c index [95% confidence interval] of the model with baseline variables only was 0.837 [0.818-0.856]. The c index values of the model with baseline variables plus fibrinogen, plus CRP, and with both fibrinogen and CRP are shown in Table IV. There was a significant difference in the c index of the model with CRP versus c that with fibrinogen (P=0.037) denoting a better discriminatory power of the model with CRP than the model with fibrinogen regarding prediction of 1-year mortality (Table IV).
Fig. 2.
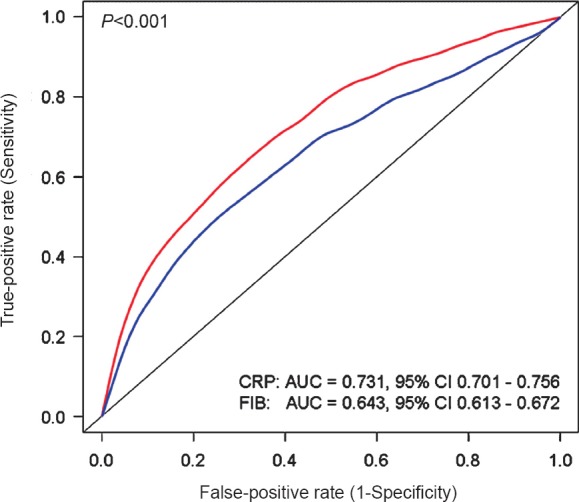
Receiver-operating characteristic (ROC) curves with respective areas under the ROC curve showing the accuracy of CRP and fibrinogen to predict 1-year mortality. Abbreviations: AUC, area under curve; CI, confidence interval; CRP, C-reactive protein; FIB, Fibrinogen.
Table IV.
C-index of the multivariate models before and after inclusion of fibrinogen and C-reactive protein
Discussion
The main findings of the study were (i) CRP and fibrinogen were separately associated with increased risk of 1-year mortality independent of conventional cardiovascular risk factors. However, after simultaneous inclusion of both biomarkers in the multivariate model, CRP, but not fibrinogen retained its independent association with mortality, indicating that CRP was a true independent predictor of mortality and that the joint use of CRP and fibrinogen would not be advantageous for risk prediction compared to CRP alone; (ii) CRP, but not fibrinogen, modestly but significantly improved the discriminatory power of the models for mortality prediction; and (iii) fibrinogen was more closely associated with cardiovascular risk factors than CRP. Consequently, adjustment for cardiovascular risk factors has a greater impact on the association between fibrinogen and mortality than on the association between CRP and mortality.
Previous studies that have compared CRP with fibrinogen as predictors of incident cardiovascular disease or cardiovascular events have reported conflicting findings8,9,10,11,12,13,14,15. Ridker et al9, showed that both fibrinogen and CRP improved predictive value of the models based on lipid markers (total cholesterol or total cholesterol/high-density lipoprotein-cholesterol ratio) with a stronger association for CRP (P<0.001) than fibrinogen (P=0.04). In a study by Mora et al10 which included initially healthy women, fibrinogen and CRP each had a similar predictive value for cardiovascular disease. Moreover, the effect of simultaneous consideration of both markers was greater than the effect of separate biomarkers and both biomarkers provided prognostic information that was incremental to that provided by traditional risk factors in predicting incident cardiovascular disease10. In the Prospective Epidemiological Study of Myocardial infarction (PRIME) that included men aged 50 to 59 yr who were free of CAD at entry, CRP, fibrinogen and interleukin-6 were significantly associated with incident myocardial infarction/coronary death endpoint after adjustment for cardiovascular risk factors; however, only interleukin-6 showed a significant association when all three biomarkers were included in the model11. The National Health and Nutritional Examination Survey III12 data showed that neither CRP nor fibrinogen were significantly associated with angina when entered simultaneously in the model. Both biomarkers were positively associated with myocardial infarction when entered separately into the models and only CRP but not fibrinogen was associated with myocardial infarction when both markers were entered into the model simultaneously12. In patients with ischaemic stroke, CRP but not fibrinogen predicted future combined endpoint of all-cause deaths or non-fatal vascular events13. In the Edinburgh Artery Study14, CRP, fibrinogen and interleukin-6 were associated with incident cardiovascular disease independent of cardiovascular risk factors but they added only modest prognostic information to traditional cardiovascular risk factors and were not specific for cardiovascular disease prediction. In the AtheroGene study15, that included patients with confirmed CAD, CRP and fibrinogen predicted the risk for future cardiovascular events, but did not offer further information on top of that provided by cardiovascular risk factors, thereby emphasizing the clinical importance of traditional risk factors for risk prediction in these patients15.
In the present study CRP showed a stronger association with the risk of 1-year mortality than fibrinogen. The superiority of CRP over fibrinogen as an associate of mortality was evidenced by a significantly greater area under the ROC curve, a greater impact of elevated CRP levels on survival and by the stability of association with mortality after adjustment for conventional cardiovascular risk factors. Moreover, CRP but not fibrinogen added incremental, albeit modest, prognostic information to that provided by the cardiovascular risk factors. The clinical value of such incremental predictive information provided by the CRP remains uncertain. Although, there is no explanation for the difference in the strength of association with mortality between CRP and fibrinogen, two other findings of this study may help to clarify it. First, comparison of baseline characteristics in groups with elevated CRP only or elevated fibrinogen only clearly showed that elevated fibrinogen levels were more closely associated with cardiovascular risk factors (older age and higher proportions of diabetes, arterial hypertension, hypercholesterolaemia and multivessel disease). Thus, CRP appears to be a more stable biomarker of inflammation, whereas fibrinogen may have a greater dependence on the patients’ risk profile. A close association between fibrinogen and cardiovascular risk factors has previously been reported23. This could have two consequences: a greater impact of adjustment for cardiovascular risk factors on the association between fibrinogen and mortality and failure of fibrinogen to provide incremental prognostic information beyond that provided by conventional cardiovascular risk factors. Second, this study and prior studies15 found a weak correlation between CRP and fibrinogen. The possibility that CRP and fibrinogen represent different aspects of inflammation has also been suggested10. It may be hypothesized that because fibrinogen is a major coagulation factor, not every increase in its concentration is tolerable. Conversely, the level of C-reactive protein can increase several hundred-fold within 24-48 h of an inflammatory stimulus24,25 which may make this biomarker a closer correlate of the magnitude of the inflammatory process.
The present study had several limitations. First, although differences in survival at one year were highly significant and survival curves further expanding, a longer follow up was desirable. Second, the prognostic value of CRP or fibrinogen was assessed using single baseline measurements, thus any change in concentration during the follow up remains unaccounted for. However, it has been shown that single CRP measurements provide important information for risk prediction26. Third, this study included patients with confirmed CAD and thus its findings may not be generalized to other categories of patients/subjects.
In conclusion, this comparative study showed that although elevated levels of CRP and fibrinogen were associated with increased risk of 1-year mortality in patients with CAD, CRP was a better predictor of mortality than fibrinogen. CRP, but not fibrinogen, improved the discriminatory power of the multivariate models for prediction of mortality indicating that CRP offers prognostic information that is independent of and supplementary to that provided by the conventional cardiovascular risk factors.
References
- 1.Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–6. [PubMed] [Google Scholar]
- 5.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 7.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 8.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. Emergence Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 10.Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114:381–7. doi: 10.1161/CIRCULATIONAHA.106.634089. [DOI] [PubMed] [Google Scholar]
- 11.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–61. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Giles WH. Serum C-reactive protein and fibrinogen concentrations and self-reported angina pectoris and myocardial infarction: findings from National Health and Nutrition Examination Survey III. J Clin Epidemiol. 2000;53:95–102. doi: 10.1016/s0895-4356(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 13.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–8. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115:2119–27. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 15.Sinning JM, Bickel C, Messow CM, Schnabel R, Lubos E, Rupprecht HJ, et al. Athervo Gene Investigators. Impact of C-reactive protein and fibrinogen on cardiovascular prognosis in patients with stable angina pectoris: the AtheroGene study. Eur Heart J. 2006;27:2962–8. doi: 10.1093/eurheartj/ehl362. [DOI] [PubMed] [Google Scholar]
- 16.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–11. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 17.Sandler H, Dodge HT. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am heart j. 1968;75:325–34. doi: 10.1016/0002-8703(68)90089-6. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 21.Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparion studies for enzymatic and Jaffe creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–5. [PubMed] [Google Scholar]
- 22.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 23.Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost. 2003;89:601–9. [PubMed] [Google Scholar]
- 24.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 25.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23:118–24. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- 26.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]


