Abstract
Background & objectives:
The development of alloantibodies can significantly complicate transfusion therapy and results in difficulties in cross-matching of blood. Most literature on alloimmunization is limited to multitransfused individuals, with very few studies on the general hospital patients. This study was aimed at assessing the frequency and type of unexpected red cell antibodies in the general patient population at a multispecialty tertiary care centre in New Delhi, India.
Methods:
The results of 49,077 antibody screening tests carried out on patients, from January 2009 to December 2012 were analyzed. The clinical and transfusion records were reviewed. The data were compiled and statistically analysed.
Results:
A total of 49,077 (29,917; 60.96% males and 19,160; 39.04% females) patient samples were screened for the presence of unexpected antibodies. Antibody screening was positive in 403 patients (0.82%). In the serum samples of 164 patients only autoantibodies were identified, 27 revealed autoantibodies with one or more underlying alloantibodies, while 212 patients had only alloantibody/ies in their serum. The overall alloimmunization rate was 0.49 per cent. Antibodies against the Rh system were the most frequent (64.1%), the most common alloantibody identified being anti E (37.2%), followed by anti D (19.2%).
Interpretation & conclusions:
Since clinically significant antibodies are frequently detected in our patient population, antibody screening and if required, identification is the need of the hour. Since antibodies against the common Rh and Kell blood group antigens are the most frequent, provision of Rh and Kell matched red cells may be of protective value.
Keywords: Alloimmunization, autoantibodies, general patient population, multitransfused, unexpected red cell antibodies
Provision of safe blood for transfusion does not only imply thorough testing for infectious markers, but also protection from haemolytic transfusion reactions resulting from alloimmunization against red cell antigens. Ever-increasing efforts at improving blood safety have led to incorporation of regular screening protocols for detection of unexpected immune antibodies at the various transfusion centres across the globe. The ultimate goal is to determine the exact specificity of the antibody and to provide blood that lacks the corresponding antigen to the patient1. Alloimmunization occurs when a foreign antigen introduced in an immuno-competent host evokes an immune response. This commonly occurs following transfusion of blood or in pregnancy, when red cells that bear antigens absent from the individual's own blood enter the circulation2.
The most important unexpected red blood cell alloantibodies in daily transfusion practice, in terms of frequency of occurrence, are directed towards the Rh (D, C, E, c and e) and Kell (K) antigens, followed by other blood group antigens of the Duffy, Kidd, MNS and other minor blood group systems3. These antibodies can cause acute and delayed haemolytic transfusion reactions as well as haemolytic disease of foetus and newborn3,4. Pre-transfusion antibody screening of patients’ samples prior to cross-matching is an essential component of compatibility testing in many countries, However, it still needs to be initiated in most centres in India.
Most literature on alloimmunization is limited to multitransfused individuals3,5,6,7,8, with a few studies on the general hospital patients9,10,11,12,13. This study was carried out to assess the frequency and type of unexpected red cell antibodies in the general patient population at a multispecialty tertiary care centre in New Delhi, India.
Material & Methods
To determine the frequency of unexpected red cell antibodies, the results of 49, 077 antibody screening tests carried out at the Department of Transfusion Medicine, Indraprastha Apollo hospitals, New Delhi, India over a period of four years from January 2009 to December 2012 were analysed. Since all patients irrespective of their clinical diagnoses undergo “Group and Screen” at our hospital, all consecutive sample received during the study period were included. Antibody screening was performed on the fully automated Immunohaematology analyzer (Galileo: Immucor Inc. Norcross GA, USA), using a commercial cell panel (Capture R Ready Screen) (Immucor Inc. Norcross GA, USA), with Solid Phase Red Cell Adherence technology. The screening cell panels covered most antigens against which clinically significant antibodies are formed, with homozygous expression of the most important ones. In case of a positive antibody screen, further testing was performed to precisely characterize the unexpected antibody(ies) and to determine their specificities in case of alloantibodies. Antibody identification was performed using different cell panels from (Immucor Inc. Norcross GA, USA) by Capture technique. Adsorption and elution techniques were applied whereever applicable, especially in cases of autoantibodies to look for any underlying alloantibodies. An attempt to determine the thermal amplitudes of the autoantibodies was made by testing at various temperatures to assess their clinical significance.
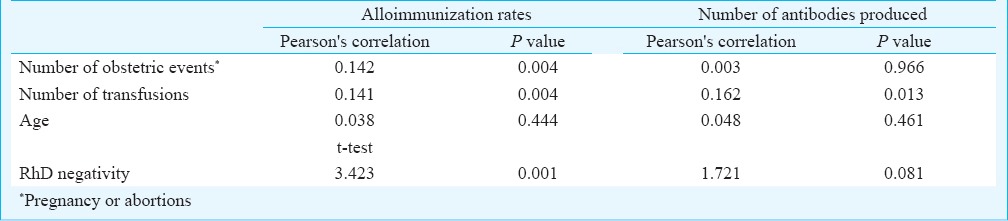
Statistical analysis: Descriptive analysis and Pearson's correlation were performed for continuous variables while t test was used for non continuous variables. Correlations were considered statistically significant at P ≤0.05. (Table I).
Table I.
Relationship between various factors and alloimmunization status
Results
A total of 49,077 patient samples were screened for the presence of unexpected antibodies. This included 29,917 (60.96%) males and 19,160 (39.04%) females. Antibody screening was positive in 403 patients (0.82%). In 164 patients (0.33%) only autoantibodies were identified, 27 (0.05%) revealed autoantibodies with one or more underlying alloantibody/ies, while 212 patients (0.43%) had only alloantibody/ies. The overall alloimmunization rate was 0.49 per cent, being 0.74 per cent (142/19,160) among females and 0.32 per cent (97/29,917) in males, the difference between the two being statistically significant (P<0.001). A single alloantibody was identified in 179 patients, while 54 patients had developed more than one alloantibodies. In six samples the alloantibody/ies could not be accurately characterized. Among the alloimmunized cases, 166 had received one or more transfusions of blood and blood components in the past. Of the 142 alloimmunized women, 75 had a history including one or more pregnancies.
In the alloimmunized group, a significant increase in the rate of alloimmunization was observed with an increase in number of transfusions and pregnancies (P=0.004 for both). A significantly higher number of alloantibodies were produced per patient with increasing number of transfusions (P=0.013), however, no such association was observed between number of alloantibodies and pregnancy.
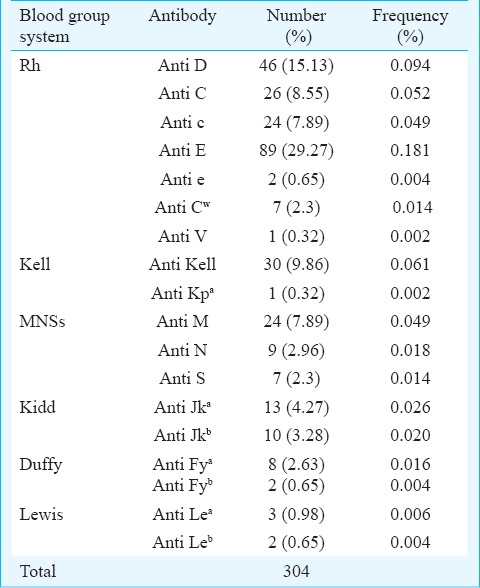
Antibodies against the Rh system were the most frequent (195 of 304 alloantibodies, 64.1%). The most common alloantibody identified was anti E (89/239 cases, 37.2%), closely followed by anti D (46/239 cases, 19.2%). The exact specificity of the antibodies could not be determined in the serum of six patients. One of these cases was reported as antibody against a high incidence antigen, since most of the panel cells were reacting with it. The others were either antibodies against a low incidence antigen or an antigen not typed in the cell panel. The frequency and specificities of the various alloantibodies identified are provided in Table II. Though rate of alloimmunization was significantly higher in RhD negative individuals (P=0.001) compared to RhD positive individuals, the number of alloantibodies identified per patient did not show any significant correlation with the RhD status.
Table II.
Frequency and specificities of the alloantibodies identified
Of the 191 autoantibodies identified, 145 were warm reacting (at 37°C) and 46 were cold autoantibodies reacting best at 4°C but showing variable thermal amplitudes and clinical significance. Clinical conditions associated with the presence of autoantibodies included haematological diseases including neoplasms, thalassaemia and aplastic anaemia (42%), renal (24%) and liver disease (11%), cardiac disease (9%) and solid organ tumours (3%).
Age of the immunized patients ranged from 1 to 91 yr with a mean age of 46.3 yr. No correlation was observed between the age of the patient and rate of alloimmunization or the number of antibodies identified.
Alloimmunization rates were higher in women with more obstetric events (P=0.004) and in individuals with higher number of transfusions (P=0.004). An increase in the number of transfusions significantly raised the level of alloantibodies developed in a patient (0.013) (Table I).
Discussion
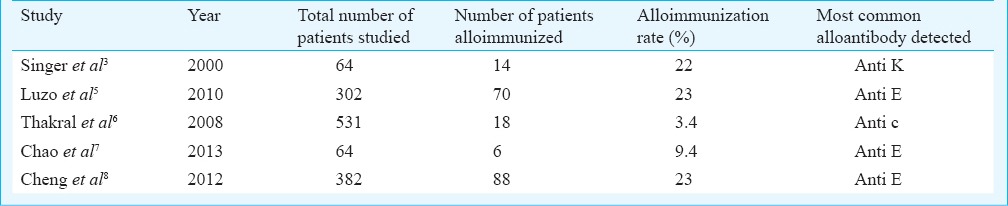
Red cell alloimmunization results from the genetic disparity between red blood cell antigens of donor and recipient or from mother and foetus2. The information available on alloimmunization especially in India is limited to select patient populations like multitransfused6, or pregnant women14 and very limited data are available for general patients. Various observational studies in general patients have estimated the presence of alloantibodies against red cell antigens between 0.46 to 2.4 per cent (Table III), while studies in multitransfused patients have reported higher alloimmunization rates (Table IV). The rate of alloimmunization in our general patient population was 0.49 per cent. The reasons for this could be that our study population comprised general hospital patients and not the high risk groups like the multitransfused. Also the antibody detection method used at our centre is specific for IgG antibodies. Various studies have used different methods for antibody screening and identification that may also detect IgM antibodies, indicating detection of a significant proportion of antibodies which are not clinically significant yet detected.
Table III.
Studies on alloimmunization in general hospital patients
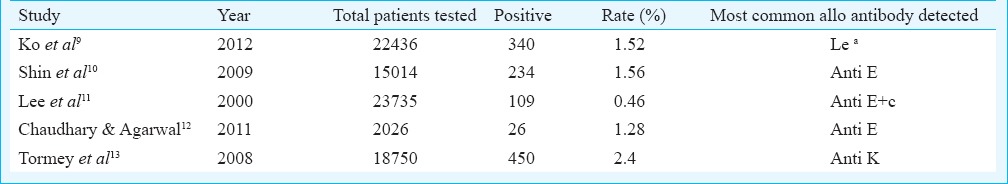
Table IV.
Studies on alloimmunization in multitransfused patients
Rh and Kell antibodies have been reported to be the most common clinically significant alloantibodies3,4. In our study anti E was identified as the most common antibody followed by anti D. In five patients serum samples showed reaction with occasional cells in the panel and the specificity of the antibody could not be ascertained. These could either represent antibodies against low incidence antigens or antigens not typed in the cell panels that was used. Most commercial cell panels currently in use in India, including the one used in this study are prepared in the West, with their own donors; thus, genetic and demographic differences in between these donors and Indian patients are inevitable.
A higher rate of alloimmunization was observed in females than in males. A similar observation was made by Hoeltge et al15 as they found higher percentage of immunized females than males. Higher rates of alloimmunization in females may be attributed to antigenic exposure during pregnancy in addition to transfusions which is the only source of exposure in men. Earlier transfusion and the number of transfusions have been identified as a significant determinant of alloimmunization2,16,17, although several studies have not been confirmed this association18,19. In our study the number of transfusions and pregnancies was a significant factor affecting alloimmunization rates. The development of alloantibodies can significantly complicate transfusion therapy and result in difficulties in cross-matching of blood. Clinically significant antibodies are capable of causing mild or severe adverse events following transfusion, such as haemolytic transfusion reactions or haemolytic disease of the foetus and newborn20. Thus, knowledge of such alloantibodies is essential not only in the multitransfused patients but in all hospital patients who require or may require transfusion. This not only helps in selecting appropriate RBC products for transfusion but also avoids unnecessary delays in provision of blood in case of emergencies or surgical complications.
As is evident from our results and existing literature3,4 the most common alloantibodies found were against the common Rh and Kell antigens (D, C, c, E, e, K), comprising nearly 71 per cent of the total alloantibodies identified. These findings emphasize the role of extended antigen typing for recipients as well as donors, and the importance of being able to provide Rh and Kell matched blood. Besides, Rh and Kell typing of blood donors makes provision of blood for patients who have developed antibodies against these antigens easier and less time consuming. In our study, an antibody was found in the serum sample of five patients reacting with a few cells in the panel. The specificity of the antibody could not be determined and it probably represented an antibody against certain low incidence antigens or an antigen not typed in the cell panel. This indicates towards a need to study and understand frequencies of a broader spectrum of antigens in the Indian population, besides the ones currently typed in the commercial cell panels, to determine which additional antibodies may need identification.
In many centres in India, the current practice for providing compatible blood to patients in cases of alloimmunization is still reliant upon random cross-matching of available units in the inventory. Since clinically significant antibodies are frequently detected in our patient population and cross-matching is not fully effective as a procedure of ensuring absence of the antigen to which the patient is immunized,12 this practice is neither safe nor cost-effective. Antibody screening and if required identification, combined with phenotyping donors to provide a supply of antigen-negative blood is necessary for safe transfusion.
References
- 1.Chow EYD. The impact of the type and screen test policy on hospital transfusion practice. Hong Kong Med J. 1999;5:275–9. [PubMed] [Google Scholar]
- 2.Schonewille H. Leiden: University Press; 2008. Red blood cell alloantibodies after transfusion. [Google Scholar]
- 3.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 4.Pahuja S, Pujani M, Gupta SK, Chandra J, Jain M. Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–7. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 5.Luzo ACM, Pereira FB, de Oliveira RC, Azevedo PR, Cunha Rd, Leonardi MI, et al. Red blood cell antigen alloimmunization in liver transplant recipients. Transplant P. 2010;42:494–5. doi: 10.1016/j.transproceed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Thakral B, Saluja K, Sharma RR, Marwaha N. Red cell alloimmunization in a transfused patient population: a study from a tertiary care hospital in north India. Hematology. 2008;13:313–8. doi: 10.1179/102453308X343419. [DOI] [PubMed] [Google Scholar]
- 7.Chao YH, Wu KH, Lu JJ, Shih MC, Peng CT, Chang CW. Red blood cell alloimmunisation among Chinese patients with β-thalassaemia major in Taiwan. Blood Transfus. 2013;11:71–4. doi: 10.2450/2012.0153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CK, Lee CK, Lin CK. Clinically significant red blood cell antibodies in chronically transfused patients: a survey of Chinese thalassemia major patients and literature review. Transfusion. 2012;52:2220–4. doi: 10.1111/j.1537-2995.2012.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Ko KH, Yoo BH, Kim KM, Lee WY, Yon JH, Hong KH, et al. Frequency of unexpected antibody and consideration during transfusion. Korean J Anesthesiol. 2012;62:412–7. doi: 10.4097/kjae.2012.62.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JH, Lee JY, Kim JH, Kim HR, Lee JN. Screening and identification of unexpected red cell antibodies by simultaneous LISS/coombs and NaCl/enzyme gel methods. J Korean Med Sci. 2009;24:632–5. doi: 10.3346/jkms.2009.24.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WH, Kim SY, Kim HO. The incidence of unexpected antibodies in transfusion candidates. Korean J Blood Transfus. 2000;11:99–103. [Google Scholar]
- 12.Chaudhary R, Agarwal N. Safety of type and screen method compared to conventional antiglobulin crossmatch procedures for compatibility testing in Indian setting. Asian J Transfus Sci. 2011;5:157–9. doi: 10.4103/0973-6247.83243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–76. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 14.Pahuja S, Gupta SK, Pujani M, Jain M. The prevalence of irregular erythrocyte antibodies among antenatal women in Delhi. Blood Transfus. 2011;9:388–93. doi: 10.2450/2011.0050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–5. [PubMed] [Google Scholar]
- 16.Olujohungbe A, Hambleton I, Stephens L, Serjeant B, Serjeant G. Red cell antibodies in patients with homozygous sickle cell disease: a comparison of patients in Jamaica and the United Kingdom. Brit J Haematol. 2001;113:661–5. doi: 10.1046/j.1365-2141.2001.02819.x. [DOI] [PubMed] [Google Scholar]
- 17.Murao M, Viana MB. Risk factors for alloimmunization by patients with sickle cell disease. Braz J Med Bio Res. 2005;38:675–82. doi: 10.1590/s0100-879x2005000500004. [DOI] [PubMed] [Google Scholar]
- 18.Bhatti FA, Salamat N, Nadeem A, Shabbir N. Red cell alloimmunization in beta thalassemia major. J Coll Physicians Surg Pak. 2004;14:657–60. [PubMed] [Google Scholar]
- 19.Domen RE, Ramirez G. Red cell alloimmunization in chronic renal failure patients undergoing hemodialysis. Nephron. 1988;48:284–5. doi: 10.1159/000184943. [DOI] [PubMed] [Google Scholar]
- 20.Poole J, Daniels G. Blood group antibodies and their significance in transfusion medicine. Transfus Med Rev. 2007;21:58–71. doi: 10.1016/j.tmrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Makroo RN, Bhatia A, Gupta R, Phillip J. Prevalence of Rh, Duffy, Kell, Kidd and MNSs blood group antigens in the Indian blood donor population. Indian J Med Res. 2013;137:521–6. [PMC free article] [PubMed] [Google Scholar]


