Abstract
Obstructive sleep apnoea (OSA) and obstructive sleep apnoea syndrome (OSAS) are subsets of sleep-disordered breathing. Awareness about OSA and its consequences amongst the general public as well as the majority of primary care physcians across India is poor. This necessiated the development of the INdian initiative on Obstructive sleep apnoea (INOSA) guidelines under the auspices of Department of Health Research, Ministry of Health & Family Welfare, Government of India. OSA is the occurrence of an average five or more episodes of obstructive respiratory events per hour of sleep with either sleep related symptoms or co-morbidities or ≥ 15 such episodes without any sleep related symptoms or co-morbidities. OSAS is defined as OSA associated with daytime symptoms, most often excessive sleepiness. Patients undergoing routine health check-up with snoring, daytime sleepiness, obesity, hypertension, motor vehicular accidents and high risk cases should undergo a comprehensive sleep evaluation. Medical examiners evaluating drivers, air pilots, railway drivers and heavy machinery workers should be educated about OSA and should comprehensively evaluate applicants for OSA. Those suspected to have OSA on comprehensive sleep evaluation should be referred for a sleep study. Supervised overnight polysomnography (PSG) is the “gold standard” for evaluation of OSA. Positive airway pressure (PAP) therapy is the mainstay of treatment of OSA. Oral appliances are indicated for use in patients with mild to moderate OSA who prefer oral appliances to PAP, or who do not respond to PAP or who fail treatment attempts with PAP or behavioural measures. Surgical treatment is recommended in patients who have failed or are intolerant to PAP therapy.
Keywords: Bariatric surgery, CPAP, Indian guidelines, OSA, OSAS, polysomnography, sleep apnoea, sleep study, Syndrome Z
Introduction
In obstructive sleep apnoea (OSA), repetitive collapse of the upper airway occurs, that leads to snoring, frequent episodes of sleep interruption, hypoxemia, hypercapnia, swings in intrathoracic pressure and increased sympathetic activity. Management of OSA needs a long-term multi-disciplinary approach. Once diagnosed, patients should be properly counselled to manage their illness including co-morbidities through their active participation.
OSA is being increasingly recognized as an emerging important public health problem worldwide, including India. Awareness among lay public and even among primary care physicians is dismally low in India. This disorder is common among obese individuals, children and post-menopausal women. It is usually associated with several co-morbidities such as insulin resistance, metabolic syndrome, diabetes mellitus, hypertension, stroke, coronary artery disease, increased risk of vehicular accidents and various psychiatric disorders. Though there are guidelines regarding the diagnosis and management of this condition by various bodies in the western world, these recommendations may not be entirely applicable to the developing countries like India. There was a need to develop comprehensive guidelines on OSA in the Indian context. Thus, the consensus and evidence-based INdian initiative on Obstructive Sleep Apnoea Guidelines (INOSA Guidelines) were developed under the auspices of Department of Health Research, Ministry of Health & Family Welfare, Government of India following a series of meetings and discussions under the convenership of the Department of Medicine, All India Institute of Medical Sciences (AIIMS), New Delhi, with the support of Indian Council of Medical Resaerch (ICMR). During this first Indian initiative, in light of the available evidence, consensus statements were developed and finalized by the various national experts in the field of sleep medicine including internists, pulmonologists, neurologists, otorhinolaryngologists, endocrinologists, bariatric surgeons and dental surgeons.
In order to make the guidelines evidence based, the expert group reviewed the available evidence and graded the recommendations according to the quality of evidence as mentioned in Fig. 11.
Fig. 1.
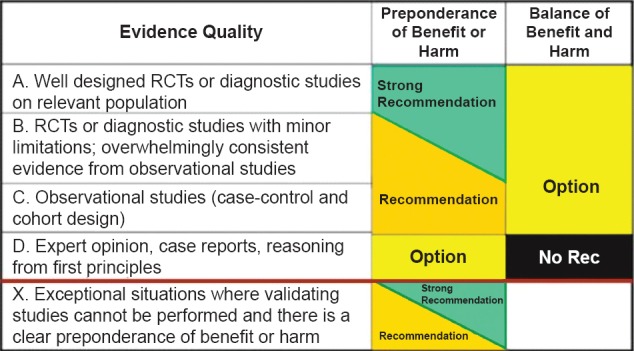
Evidence quality. Reproduced with permission from: American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines.Pediatrics 2004; 114: 874-71.
1 Epidemiology and risk factors of OSA
1.1 Epidemiology
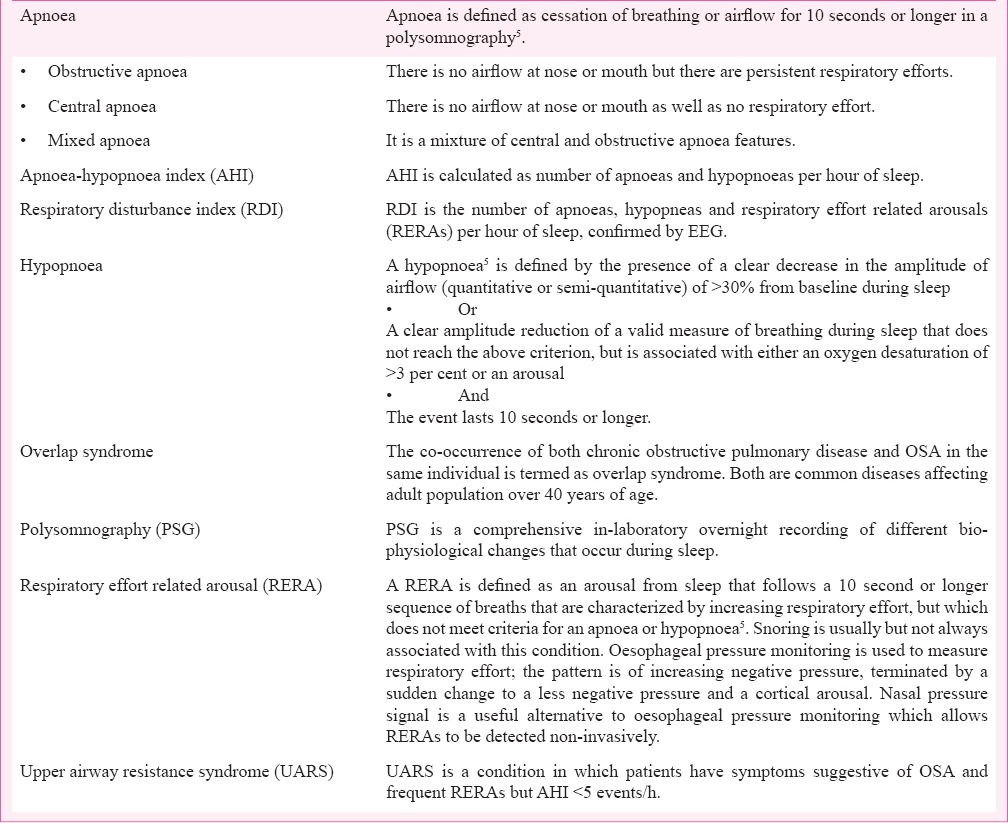
Obstructive sleep apnoea is a major public health problem. The International Classification of Sleep Disorders, Third Edition classifies sleep-disordered breathing into three basic categories: central sleep apnoea syndrome, obstructive sleep apnoea syndrome, and sleep-related hypoventilation/hypoxia syndrome2,3. Community-based epidemiological studies from several parts of India have estimated that the prevalence of OSAS is 2.4 to 4.96 per cent in men and 1 to 2 per cent in women4. Table I summarizes some of the important definitions5.
Table I.
Definitions5
1.2 Pathogenesis
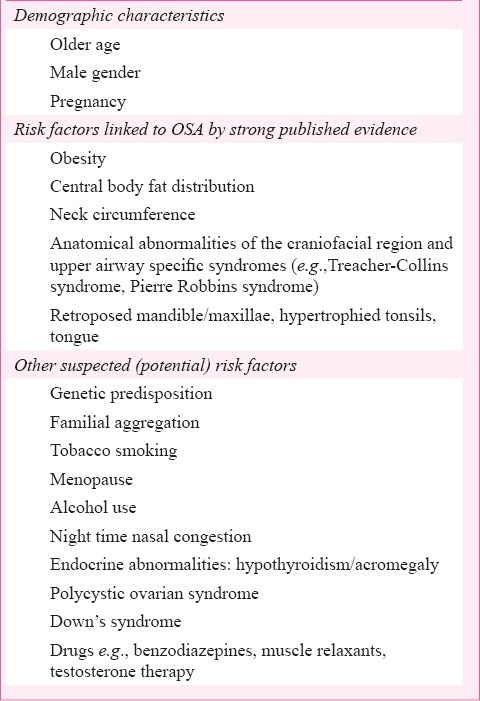
Multiple factors (Table II)6,7 are responsible for pathogenesis of OSA with inter-individual variation. OSA patients have repeated narrowing or obstruction of pharyngeal airway during sleep. It has been suggested that pathophysiological mechanisms, such as anatomic compromise, pharyngeal dilator muscle dysfunction, lowered arousal threshold, ventilatory control instability, and/or reduced lung volume tethering are the pathophysiological mechanisms leading to OSA8.
Table II.
2 Consequences of OSA
2.1 OSA and Mortality
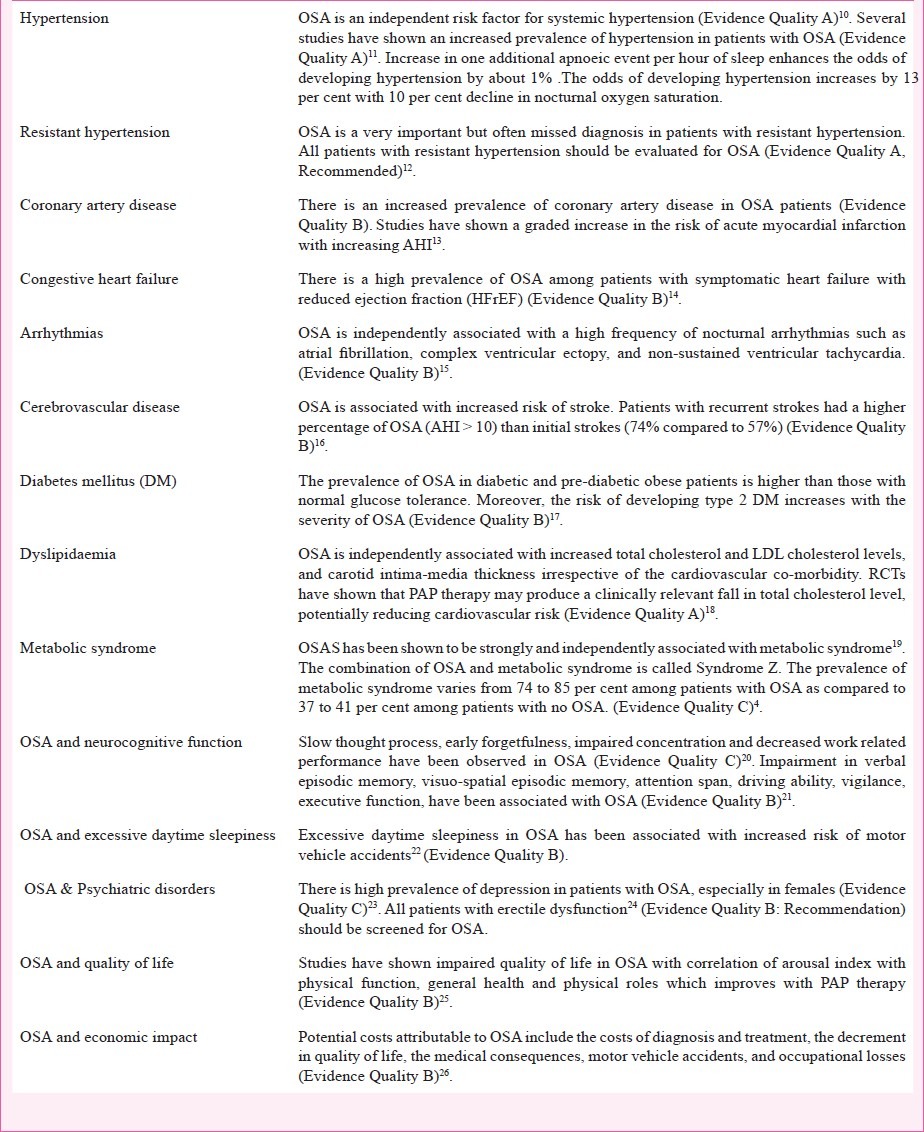
It has been demonstrated that OSA is associated with increased mortality. Severe sleep disordered breathing (SDB) has a 3.8 fold greater risk for all-cause mortality and 5.2-fold greater risk for cardiovascular mortality than those without SDB (Evidence Quality B)9. The consequences of OSA are described in Table III4,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26.
Table III.
3 Diagnosis of OSA
3.1 History and Physical Examination
The diagnosis of OSA requires a high index of suspicion. OSA may be suspected during routine health check-up or while evaluating high-risk patients27. The chances of underdiagnosis are minimized if individuals with risk factors are subjected to a comprehensive sleep evaluation during routine health check-up. Similarly, high-risk patients like those with congestive heart failure, extreme obesity, diabetes mellitus, coronary artery disease, stroke, nocturnal dysrhythmias including atrial fibrillation, pulmonary hypertension, preoperative patients should have comprehensive sleep evaluation (Boxes 1 and 2)27. Additionally, medical examiners evaluating drivers, pilots, railway drivers and heavy machinery workers should be educated about OSA and should refer them for evaluation if snoring, daytime sleepiness or obesity is noted (Evidence Quality B, Strong Recommendation).
Box 1.
Symptoms of OSAS

Box 2.
Clinical examination finding suggestive of OSAS

In a patient suspected to have OSA, secondary causes such as hypothyroidism, facial abnormality, tonsil/adenoid hypertrophy and musculoskeletal abnormalities should be ruled out and the patient should be evaluated for consequences of OSA like metabolic syndrome, diabetes mellitus, hypertension, CAD, stroke and gastroesophageal reflux27,28. Patient should also be investigated for associated co-morbid illnesses like allergic rhinosinusitis, nasal polyps, asthma, chronic obstructive pulmonary disease (COPD), obesity hypoventilation syndrome and kyphoscoliosis27,28. The clinical examination should include detailed anthropometry including measurement of neck circumference, BMI, modified Mallampati score and a comprehensive upper airway assessment27.
3.2 Other Diagnostic Investigations
Anthropometric measurements, nasal and upper airway examination, orthodontic assessments and radiological measurements have low sensitivity and specificity when used alone for diagnosis of OSA28. Patients suspected to have OSA should be referred for an appropriate type of sleep study after detailed history, examination and basic investigations. Various questionnaires for the prediction of OSA are available and can be used prior to sleep study, but the same is not mandatory.
3.3 Epworth Sleepiness Scale (ESS)
Epworth Sleepiness Scale is a simple, self-administered measurement of sleep propensity during daytime in adults that requires the subject to rate the probability of dozing off in eight different situations that are met in day-to-day life on a scale of 0-3. Thus, the sum of the score can vary from 0 to 24. ESS score >10 is defined as excessive daytime sleepiness and has a sensitivity of 49 per cent and specificity of 80 per cent for predicting OSA29. (Evidence Quality C, Recommended).
3.4 Clinical prediction rules for OSA
Various algorithms have been devised for screening and risk stratification of patients suspected to have OSA. The utility of these tools to estimate the clinical severity of OSA and to suggest the likelihood of OSA related consequences have not been studied systematically30.
Berlin questionnaire has three categories of questions. Category 1 questions are about snoring with five questions and 2 to 5 multiple choice answers. Category 2 includes excessive daytime sleepiness with four or more multiple choice answers. Category 3 has body mass index (BMI) and blood pressure. With Berlin questionnaire, OSA was considered probable if two of the categories are positive. The Berlin questionnaire was modified at AIIMS, New Delhi, in 2006 for application in the setting of developing countries31. Both Berlin questionnaire and modified Berlin questionnaire are moderately accurate (sensitivity and specificity generally <90%) in screening for OSA30,31 (Evidence Quality C; Recommended). Although, these questionnaires have not been adequately studied, these can be used to screen the patients for OSA. The snoring, daytime tiredness, observed apnoea, high blood pressure, body mass index, age, neck circumference, and gender (STOP-BANG) questionnaire (Evidence Quality C, Recommended) is the most appropriate questionnaire for the screening in preoperative cases.
Patients who have both symptoms and physical findings suggestive of OSA on comprehensive sleep evaluation along with Epworth's sleepiness score greater than or equal to 10 have a high risk of OSA and the diagnosis is confirmed and severity determined with objective testing in an expedited manner in order to initiate treatment. Patients who have neither are at low probability and the rest have moderate probability for OSA. Fig. 2 shows the algorithm for the diagnosis of OSA.
Fig. 2.
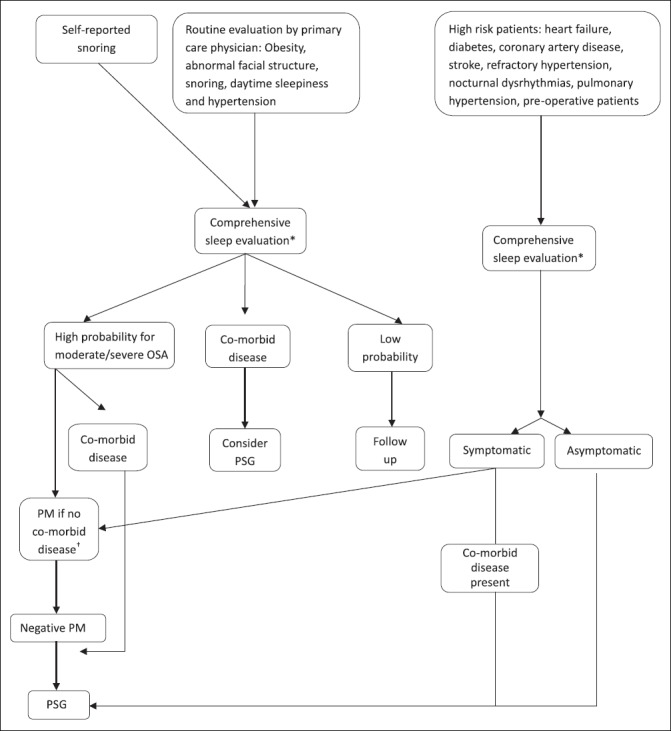
Algorithm for diagnosis of OSA. *Boxes 1 and 2 †Pulmonary disease, neuromuscular disease, or congestive heart failure. PM, portable monitoring; PSG, polysomnography.
3.5 Types of Sleep Study
The diagnosis and severity of OSA must be ascertained before initiating the treatment of OSA. The standard diagnostic test for OSA is an attended in-laboratory polysomnography (PSG) or portable monitoring (PM)27. PSG is supervised by a trained technician with at least seven channels whereas PM is performed without a technician and has fewer channels. Various types of sleep studies are described in Fig. 3. In laboratory PSG with electroencephalography (EEG) based sleep staging, the “gold standard” for the diagnosis of OSA is not necessary in all patients suspected to have OSA28. Portable monitoring with type 3 and 4 devices in conjunction with comprehensive sleep evaluation is adequate for diagnosis of OSA in patients with high pre-test probability of moderate to severe OSA without co-morbid sleep or medical disorders such as neuromuscular disease, pulmonary disease, or congestive heart failure (Evidence Quality A, Strong Recommendation)32. PSG is mainly useful for patients with symptoms of excessive daytime sleepiness but no objective evidence of obstructive sleep apnoea on PM (Box 3).
Fig. 3.
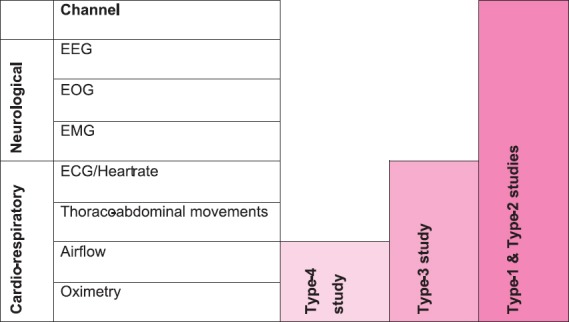
Types of sleep studies. Type 1: Fully attended polysomnography (≥ 7 channels) in a laboratory setting, Type 2: Fully unattended polysomnography (≥ 7 channels), Type 3: Limited channel study (usually using 4-7 channels), Type 4: 1 or 2 channels usually using oximetry as one of the parameters, EEG, electroencephalography; EOG, electro-oculography; EMG, electromyography; ECG, electrocardiography.
Box 3.
Indications of portable monitoring (PM) and polysomnography (PSG)

3.5.1 Attended in-laboratory polysomnography - Type 1 Sleep Study: Type 1 study or in-laboratory, technician-attended, overnight PSG is the present reference or “gold” standard for evaluation of sleep and sleep-disordered breathing (Evidence Quality A, Strong Recommendation)33. The recommended parameters to be evaluated in PSG include sleep state and stages, ventilatory parameters, cardiac function and limb movements34 by recording EEG, electro-oculography (EOG), electrocardiography (ECG), chin and leg electromyography (EMG), nasal and oral airflow, chest and abdominal efforts and pulse oximetry33. The study requires the constant presence of a trained individual with appropriate sleep-related training who can monitor patient compliance, technical adequacy and relevant patient behaviour (Evidence Quality B, Recommended)27. It is advisable to take prior informed consent for PSG. Social acceptability of full-night PSG for women is an issue in the Indian context. Full-night PSG is recommended for the diagnosis of OSA (Evidence Quality A, Strong Recommendation).
3.5.2 Unattended polysomnography-Type 2 Sleep Study: Type 2 devices can record the same variables as type 1 study in absence of a technician. Thus, type 2 sleep monitoring can be practically used as portable monitors, but is not used frequently in the outpatient setting. Type 2 study may identify apnoea hypopnoea index (AHI) suggestive of OSA with high positive likelihood ratio and low negative likelihood ratio, though differences in AHI have been encountered between type 2 study and PSG30,35 (Evidence Quality B, Recommended).
3.5.3 Portable monitoring/Out-of-centre Sleep Testing (OCST)/Home Sleep Testing (HST)/Unattended Limited Channel Testing (ULCT) (Type 3 & 4 Sleep Study): Portable monitoring or OCST as a diagnostic test for OSA has evolved as an alternative to PSG27 due to convenience and lower cost. The disadvantage of PM or OCST, however, is that AHI may be falsely low30,36. This is because in the absence of EEG recording in these tests, actual sleep time cannot be determined and the the denominator is the total recording time instead of the total sleep time. Comprehensive sleep evaluation should always be done prior to PM studies27.
The diagnosis and severity assessment should be performed using the same definitions as used for PSG. PM should be performed only in conjunction with comprehensive sleep evaluation and in the presence of a practitioner eligible for conducting sleep studies (Evidence Quality B, Recommended)32. Overall, PM (type 3 and 4) may be useful, cost-effective, convenient and speedy method of diagnosis if the patient is selected carefully. Hospital-based PSG is the investigation of choice for patients who cannot be investigated adequately at home or whose home study result does not match with the clinical suspicion of the investigating physician28.
3.6 Preoperative Evaluation of OSA
The incidence of post-operative desaturation, respiratory failure, post-operative cardiac events and intensive care unit transfers is higher in patients with OSA (Evidence Quality A, Strong Recommendation)37. Both PSG and portable monitoring are helpful in diagnosing and categorizing the severity of OSA, but portable monitoring reduces the likelihood of delay in the surgery, inconvenience and high cost of laboratory study. Alternatively, in a case at high risk of OSA, sleep study may be deferred if it is not feasible or causes delay in surgery. Instead, a standby positive airway pressure device with a close monitoring may be advised38. Patients who have previously been diagnosed to have OSA must be asked to use positive airway pressure (PAP) preoperatively and postoperatively.
3.7 Diagnostic criteria for OSA
The diagnostic crieteria for OSA are summarized in Box 4.
Box 4.
Criteria for diagnosis of OSA2

3.8 Optimal continuous positive airway pressure (CPAP) titration
Optimal PAP to treat OSA is the effective pressure that eliminates sleep-disordered breathing events in all sleep positions and stages, particularly REM (rapid eye movement) sleep, improving sleep quality without creating any untoward pressure-related side effects for the patient.
Titration effectiveness has been described by a grading system, detailed below39.
Optimal titration: AHI < 5 per hour and includes supine REM sleep.
Good titration: AHI < 10 per hour or reduced by 50 per cent if the baseline less than 15 per hour and includes supine REM.
Adequate titration: AHI cannot be reduced to less than 10 per hour, but is reduced by 75 per cent from baseline or criterion for optimal or good titration is attained, but without supine REM sleep.
Unacceptable titration: Any one of the above grades is not met, which requires a repeat titration.
3.9 Process of PAP Titration
CPAP titration is done by starting at a minimum pressure of 4 cm water (H2O) which is then increased by 1 cm H2O to a maximum of 20 cm H2O every five minutes or more, with the target of eliminating all the events (Evidence Quality A, Strong Recommendation)40. If this pressure does not allow adequate titration, bilevel positive airway pressure (BPAP) titration is recommended (Evidence Quality C, Recommended)41. Ideally, 15 min of supine REM sleep must be a part of the titration.
3.10 Split Night vs. Single Night Titration
Full-night PSG with attended manual PAP titration is regarded as the gold standard for prescription of PAP therapy (Evidence Quality A, Strong Recommendation). However, split-night study, i.e., initial PSG followed by 3 h of PAP titration may be performed if AHI is >40 events/hour during the first two hours or between 20-40 events/hour with clinical judgment regarding definitiveness of prescribing PAP therapy (Evidence Quality A, Strong Recommendation). It is recommended that the arousals should be abolished with PAP; otherwise, a repeat study with PSG is indicated for PAP titration42. AutoPAP titration using autoPAP devices that monitor snoring, apnoea or hypopnoea by airflow, flow contour, and/or impedance by forced oscillation technique can be tried during attended titration with PSG (Evidence Quality B, Recommended) to determine a fixed PAP level in patients with moderate to severe OSA without significant co-morbid illness such as congestive heart failure (CHF), COPD, central sleep apnoea or hypoventilation syndromes (Evidence Quality B, Recommended).
4 Medical management of OSA
4.1 General measures, including pharmacotherapy43,44,45,46,47
The general measures in the management of OSA are summarized in Box 5.
Box 5.

4.2 Pharmacotherapy in OSA
Several drugs have been tried in OSA in small trials and the data at present are insufficient to recommend primary drug treatment in OSA. Wake promoting agents - modafinil and armodafinil are the only agents approved for excessive daytime sleepiness (EDS) despite adequate PAP therapy in OSA patients48,49. (Evidence Quality A, Strong Recommendation).
4.3 Positive airway pressure therapy
4.3.1 Introduction - The principle of positive airway pressure in OSA is based on providing air under positive pressure through an interface (nasal or face mask), thus creating a pneumatic splint in the upper airway which prevents collapse of the pharyngeal airway, acting at all potential levels of obstruction50. PAP is the most effective and widely used treatment for OSA and is the first-line therapy for moderate to severe OSA. PAP improves quality of life, in terms of clear-cut reductions in daytime sleepiness and quality of life measures. Effective PAP therapy reduces snoring and nocturnal respiratory disturbances and improves nocturnal oxygenation and sleep architecture. Benefits of PAP therapy include reduced daytime sleepiness, improved driving performance, health status and improvement in neuro-cognitive performance. Positive effects on cardiovascular outcomes, such as hypertension, cardiac arrhythmias, nocturnal ischaemia, left ventricular function, and even overall mortality have been reported51,52,53.
4.3.2 Indications for CPAP and BPAP - CPAP is currently the ‘gold standard’ for treatment of moderate to severe OSA (AHI >15 h), and an option for less severe OSA. Treatment of OSA is indicated with the following criteria on PSG: (Evidence Quality A, Strong Recommendation)10,54,55,56.
AHI or (RDI) ≥ 15 events/h
AHI (or RDI) ≥5 but <15 events/h with any of the following symptoms:
-
(i)
Excessive daytime sleepiness (confirmed by either a score of greater than 10 on ESS or inappropriate daytime napping (e.g. during driving, conversation, or eating) or sleepiness that interferes with daily activities on a regular basis.
-
(ii)
Impaired cognition or mood disorders.
-
(iii)
Hypertension.
-
(iv)
Ischaemic heart disease.
-
(v)
History of stroke.
-
(vi)
Cardiac arrhythmias.
-
(vii)
Pulmonary hypertension.
All these factors have to be taken into account while planning treatment of OSA.
Currently, three types of PAP devices are available for treatment of OSA: continuous PAP (CPAP), bi-level PAP (BPAP), and automatic self-adjusting PAP (APAP). CPAP devices generate a fixed continuous pressure during inspiration and expiration. In BPAP, the pressure alternates between a fixed inspiratory and lower expiratory level during the respiratory cycle, which allows differential titration of the inspiratory (IPAP) and expiratory positive airway pressures (EPAP). In APAP, the pressure changes throughout the night in response to changes in airflow, respiratory events, and snoring. There is no evidence base to choose the modality of PAP.
4.3.3 Role of supplemental oxygen - Supplemental oxygen (O2) is used after adequate CPAP/BPAP titration, for residual sleep-related hypoxemia. Specifically, O2 supplementation is done during the PAP titration study, if the SpO2 is less than 88 per cent for five or more minutes in the absence of sleep-disordered breathing events, and oxygen flow rate is increased at a rate of 1 l/min every 15 min to target SpO2 ≥88 per cent. Patients on O2 prior to PAP titration usually need a higher amount of O2 with the PAP device due to flow related dilution of the supplied O2. Supplemental O2 is to be connected to the PAP device outlet and not to the mask. The possibility of a rise in CO2 due to the supplemental O2 is to be kept in mind, and should be monitored with an arterial blood gas next day after disconnecting the PAP device.
4.3.4 Recommendations for APAP57,58 - APAP is a concept based on continuously adjusting positive airway pressure to meet the patient's variable needs to maintain a patent airway, thereby reducing the overall mean airway pressure. This could be done in an unattended setting such as the patient's home, and potentially enhances tolerability and compliance. Figure 4 summarises PAP prescription.
Certain APAP devices may be useful for attended titration with PSG to identify a single pressure for use with standard CPAP [also called fixed CPAP (f-CPAP)] for management of moderate to severe OSA. (Evidence Quality B, Optional Recommendation)
Patients who are being treated with APAP itself, or f-CPAP calculated on the basis of APAP titration must have close clinical follow up to monitor treatment effectiveness. In the event of an inadequate symptomatic or objective response with APAP therapy, a standard attended CPAP titration should be done.
APAP devices are not recommended for split-night titration. (Evidence Quality A, Strong Recommendation)
Patients with CHF, COPD, CSA are not currently considered candidates for APAP titration or treatment. (Evidence Quality A, Not Recommended).
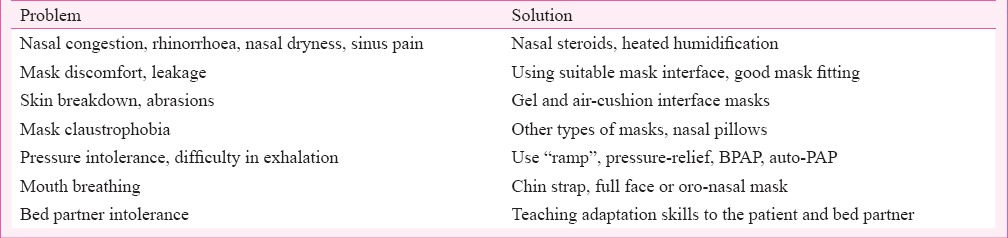
4.3.5 PAP compliance59 - The treatment of sleep apnoea with PAP has inherent problems with initial acceptance and long-term adherence, together called compliance due to discomfort from the mask interface, positive pressure itself, need for daily night use and long-term therapy. Compliance with PAP is a significant problem, and nasal congestion and mask intolerance are the most common complaints that reduce PAP compliance.
Some patients cannot tolerate PAP because of initial discomfort of sudden application of pressure, or discomfort perceived in exhaling against high pressure. Most PAP devices have a pressure “ramp”, where the pressure rise can be slow till it attains the target pressure, over as much as 45 min. An option for reducing expiratory pressure is BPAP, which allows independent adjustment of inspiratory and expiratory pressures, though the comfort benefits of BPAP have not been categorically demonstrated. Pressure-relief CPAP reduces the discomfort of breathing against high pressure during expiration by lowering the pressure at the onset of expiration. Recent Cochrane database review has concluded that pressure-relief CPAP did not improve compliance60. Similarly, APAP, with a lower mean pressure through the night has a minimal impact on improving compliance.
4.3.6 Adverse effects of PAP therapy - Adverse effects of PAP therapy are summarized in Table IV.
Table IV.
Adverse effects of PAP therapy
4.4 Oral Appliances
4.4.1 Background and rationale - Oral appliances (OA) are an established treatment option for snoring and mild to moderate OSA in selected cases and not in severe OSA. OAs are less cumbersome than PAP therapy and should be considered for patients who have failed or refused PAP treatment, for those with snoring or mild to moderate OSA61,62. Dental professionals trained in sleep medicine should prescribe and prepare appropriately fitting OA for the treatment of OSA.
4.4.2 Types of oral appliances
4.4.2.1 Mandibular repositioning appliance: Mandibular repositioning appliance (MRA) works by bringing the mandible forward, thereby increasing the airway volume. It can be either fixed (pre-determined advancement), titratable (adjustable) or either a one-piece or a two-piece appliance. The titratable MRA has an adjustable mechanism that allows progressive advancement of the mandible after initial construction until the optimal mandibular position is achieved. Single-piece or non-adjustable appliances often have to be made again if the initial jaw advancement is insufficient62,63.
4.4.2.2 Tongue retaining appliances: Tongue retaining appliances (TRA) are indicated for patients with large tongue and when use of MRA is limited due to edentulous ridges. Once the patient is using the appliance routinely, overnight PSG is required to assess the clinical response objectively62,63.
4.4.3 Effects of OA therapy - Effects of OA therapy are summarized in Table V61,63,64,65,66,67,68,69,70.
Table V.
Effects of oral appliances (OAs)

4.4.4 Contraindications to OA therapy62,71,72 - Contraindications to OA therapy are summarized in Box 6.
Box 6.

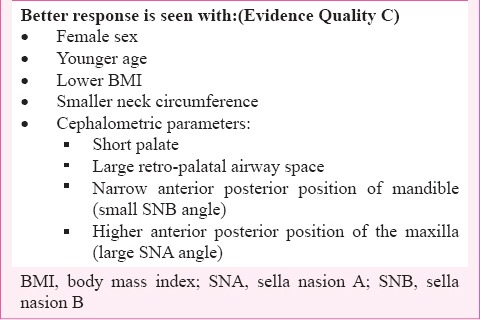
4.4.5 Predictors of response to oral appliances73,74 - Predictors of response to oral appliances are summarized in Box 7.
Box 7.
4.4.6 Adverse effects of OAs75,76,77 - Adverse effects of OAs are summarised in Box 8.
Box 8.

4.4.7 Compliance with OAs - The compliance depends on benefits and discomfort. Various studies reported different level of compliance for different types of OAs in OSA; it ranges from 51 to 88 per cent. Among the various types of oral appliances, compliance is better with mandibular advancement devices (MAD) than any other appliance74,77,78.
(Evidence Quality C)
4.4.8 Other recent advances in the treatment of OSA - Nasal EPAP device is a single-use device applied over the nostrils that functions like an inspiratory valve allowing unimpeded inspiration but offers resistance to expiration, creating an EPAP. This resultant EPAP cannot be titrated. In a large randomized controlled trial (RCT), the nasal EPAP device significantly improved AHI (43 vs. 10%) and ESS at three months as compared to a sham device. However, some patients do not show any improvement in AHI with nasal EPAP, and among the responders not all would achieve an AHI < 10 events/h. The clear-cut indication for nasal EPAP devices is still not well defined79.
5 Surgical treatment of OSA
PAP therapy has been considered to be the first-line of management for patients with OSAS80. However, some patients may prefer alternative treatment options because they are unable to tolerate, and are non-compliant81 or do not benefit from PAP therapy. The lack of randomized controlled trials comparing PAP therapy and surgical treatment make it very difficult to attain a consensus in selecting the appropriate management option82. The decision for surgical management should be strictly individualized after careful assessment of patient with due importance given to the sites of obstruction. The following description provides an insight into the procedures that are currently available and their potential role in routine management.
5.1 Evaluation of level of obstruction: The most significant concern in the assessment of airway is that it can only be performed in an awake patient and the scenario hardly simulates the exact status during sleep. Apart from drug-induced sleep nasoendoscopy (DISE)83,84,85 in patients who are planned for surgery and fiberopticnasopharyngoscopy with Mueller manoeuvre (FNMM)86, other methods like cephalometry, acoustic analysis, somnofluoroscopy, CT and sleep MRI are not recommended for routine use to assess the level of obstruction.
5.2 Surgeries in OSA: Surgical options in OSA are site directed surgeries and bariatric surgery.
5.2.1 Nasal and nasopharyngeal surgery - Patients with OSAS frequently have nasal obstruction which results in snoring and mild sleep apnoea, but nasal blockage per se does not lead to severe OSA.
1. Nasal surgery (correction of anatomical defects) alone is not a useful method of treatment of moderate to severe sleep apnoea87. (Evidence Quality B, Not recommended).
2. It also improves the compliance with PAP and also improves its effectiveness87,88. (Evidence Quality B, Recommended).
5.2.2 Maxillo-mandibular surgeries - Malpositioning of maxilla and mandible contribute to OSAS by reducing the posterior hypopharyngeal space. Role of surgery in the correction of such anatomical abnormalities is summarized in Table VI89,90,91,92,93,94,95,96,97,98.
Table VI.
Maxillo-mandibular surgeries

5.2.3 Role of bariatric surgery for treatment of OSAS - Bariatric surgery (BS) is a surgery done in order to create caloric restriction and/or malabsorption for weight loss. The commonly performed bariatric procedures are adjustable gastric banding (AGB), Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) and bilio-pancreatic diversion (BPD).
5.2.3.1 Impact of BS on OSA: Following BS, there is improvement of post-operative sleep quality, reduction in day time sleepiness, improvement in quality of life, decrease in use of PAP and decrease in use of high PAP pressure requirement. Gastric bypass was the most successful procedure in improving or resolving OSA followed by gastroplasty, BPD and gastric banding being the least effective procedure99. However, in the majority of the patients (62%), the mean residual AHI after surgery was more than 15 events per hour. This indicates that there is a persistent residual disease, even though there has been considerable improvement. As such, all patients should undergo repeat PSG after surgical weight loss and those patients who have residual disease consistent with moderately severe OSA need continued treatment with PAP. The available evidence suggests that that the patients cured of OSA were less obese and younger than those who had residual OSA after bariatric surgery100.
In a recent meta-analysis of 13,900 patients who underwent bariatric surgery, 79 per cent of patients experienced either resolution or improvement of their sleep apnoea. Bariatric surgery is strongly recommended101 for obese OSA patients with BMI ≥35kg/m2 (Evidence Quality B).
Conflicts of interest: None
Acknowledgment
Atul Malhotra, University of California, San Diego, USA
References
- 1.American Academy of Paediatrics Steering Committee on Quality Improvement and Management. Classifying Recommendations for Clinical Practice Guidelines. Pediatrics. 2004;114:874–7. doi: 10.1542/peds.2004-1260. [DOI] [PubMed] [Google Scholar]
- 2.ICSD-3 Online Version - American Academy of Sleep Medicine (AASM) AASM. [accessed on September 27, 2014]. Available from: http://www.aasmnet.org/store/product.aspx?pid=849 .
- 3.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med. 2010;31:179–86. doi: 10.1016/j.ccm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Sharma SK, Reddy EV, Sharma A, Kadhiravan T, Mishra HK, Sreenivas V, et al. Prevalence and risk factors of syndrome Z in urban Indians. Sleep Med. 2010;11:562–8. doi: 10.1016/j.sleep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam JCM, Sharma SK, Lam B. Obstructive sleep apnoea: definitions, epidemiology & natural history. Indian J Med Res. 2010;131:165–70. [PubMed] [Google Scholar]
- 7.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–93. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 12.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 14.Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–5. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirol Carlton Vic. 2013;18:140–6. doi: 10.1111/j.1440-1843.2012.02267.x. [DOI] [PubMed] [Google Scholar]
- 18.Robinson GV, Pepperell JCT, Segal HC, Davies RJO, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59:777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PMA, Wilding JPH. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Sharma H, Sharma SK, Kadhiravan T, Mehta M, Sreenivas V, Gulati V, et al. Pattern & correlates of neurocognitive dysfunction in Asian Indian adults with severe obstructive sleep apnoea. Indian J Med Res. 2010;132:409–14. [PubMed] [Google Scholar]
- 21.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirol Carlton Vic. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 22.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 23.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8:17–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Huang P, Song B, Chen J-M. Effect of continuous positive airway pressure on erectile dysfunction in patients with obstructive sleep apnea syndrome: a meta-analysis. Zhonghua Nan Ke Xue Natl J Androl. 2013;19:77–81. [PubMed] [Google Scholar]
- 25.Baldwin CM, Ervin A-M, Mays MZ, Robbins J, Shafazand S, Walsleben J, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–83. [PMC free article] [PubMed] [Google Scholar]
- 26.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care. 2010;55:1155–67. [PubMed] [Google Scholar]
- 27.Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guidelines for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 28.National Guidelines Clearinghouse (NGC) Management of obstructive sleep apnoea/hypopnoea syndrome in adults. A national clinical guideline. [accessed on September 22, 2014]. Available from: http://www.guideline.gov/content.aspx?id=3878 .
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Balk EM, Moorthy D, Obadan NO, Patel K, Ip S, Chung M, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [accessed on September 27, 2014]. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Available from: http://www.ncbi.nlm.nih.gov/books/NBK63560/ [PubMed] [Google Scholar]
- 31.Sharma SK, Vasudev C, Sinha S, Banga A, Pandey RM, Handa KK. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124:281–90. [PubMed] [Google Scholar]
- 32.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 33.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 34.Vaughn BV, Giallanza P. Technical review of polysomnography. Chest. 2008;134:1310–9. doi: 10.1378/chest.08-0812. [DOI] [PubMed] [Google Scholar]
- 35.Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, et al. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;27:536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 36.Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 38.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;1:CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Clinical Guidelines for the Manual Titration of Positive Airway Pressure in Patients with Obstructive Sleep Apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 40.Lloberes P, Ballester E, Montserrat JM, Botifoll E, Ramirez A, Reolid A, et al. Comparison of manual and automatic CPAP titration in patients with sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1996;154:1755–8. doi: 10.1164/ajrccm.154.6.8970366. [DOI] [PubMed] [Google Scholar]
- 41.Reeves-Hoché MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:443–9. doi: 10.1164/ajrccm.151.2.7842204. [DOI] [PubMed] [Google Scholar]
- 42.Gay P, Weaver T, Loube D, Iber C Positive Airway Pressure Task Force Standards of Practice Committee. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y-N, Li Q-Y, Zhang X-J. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J. 2012;125:3150–6. [PubMed] [Google Scholar]
- 44.Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 45.Dobrosielski DA, Patil S, Schwartz AR, Bandeen-Roche K, Stewart KJ. Effects of exercise and weight loss in older adults with obstructive sleep apnea. Med Sci Sports Exerc. 2014 May 27; doi: 10.1249/MSS.0000000000000387. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson K, Neovius M, Lagerros YT, Harlid R, Rössner S, Granath F, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravesloot MJL, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17:39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue Y, Takasaki Y, Yamashiro Y. Efficacy and safety of adjunctive modafinil treatment on residual excessive daytime sleepiness among nasal continuous positive airway pressure-treated japanese patients with obstructive sleep apnea syndrome: a double-blind placebo-controlled study. J Clin Sleep Med. 2013;9:751–7. doi: 10.5664/jcsm.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kay GG, Feldman N. Effects of armodafinil on simulated driving and self-report measures in obstructive sleep apnea patients prior to treatment with continuous positive airway pressure. J Clin Sleep Med. 2013;9:445–54. doi: 10.5664/jcsm.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 51.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnea syndrome. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1666–70. doi: 10.1152/ajpregu.00401.2007. [DOI] [PubMed] [Google Scholar]
- 52.Denker MG, Cohen DL. Use of continuous positive airway pressure for sleep apnea in the treatment of hypertension. Curr Opin Nephrol Hypertens. 2014;23:462–7. doi: 10.1097/MNH.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 54.Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–75. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 56.Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186:677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meurice JC, Cornette A, Philip-Joet F, Pepin JL, Escourrou P, Ingrand P, et al. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med. 2007;8:695–703. doi: 10.1016/j.sleep.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009;4:CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 59.Yetkin O, Kunter E, Gunen H. CPAP compliance in patients with obstructive sleep apnea syndrome. Sleep Breath. 2008;12:365–7. doi: 10.1007/s11325-008-0188-4. [DOI] [PubMed] [Google Scholar]
- 60.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy: systematic review and meta-analysis. Chest. 2011;139:1322–30. doi: 10.1378/chest.10-2379. [DOI] [PubMed] [Google Scholar]
- 61.Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 62.Ahrens A, McGrath C, Hägg U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur J Orthod. 2011;33:318–24. doi: 10.1093/ejo/cjq079. [DOI] [PubMed] [Google Scholar]
- 63.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 64.Li W, Xiao L, Hu J. The comparison of CPAP and oral appliances in treatment of patients with OSA: a systematic review and meta-analysis. Respir Care. 2013;58:1184–95. doi: 10.4187/respcare.02245. [DOI] [PubMed] [Google Scholar]
- 65.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 66.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 67.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010;14:339–45. doi: 10.1007/s00784-009-0298-9. [DOI] [PubMed] [Google Scholar]
- 68.Gagnadoux F, Fleury B, Vielle B, Pételle B, Meslier N, N’Guyen XL, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34:914–20. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 69.Hoekema A, Stegenga B, Bakker M, Brouwer WH, de Bont LGM, Wijkstra PJ, et al. Simulated driving in obstructive sleep apnoea-hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep Breath Schlaf Atm. 2007;11:129–38. doi: 10.1007/s11325-006-0093-7. [DOI] [PubMed] [Google Scholar]
- 70.Iftikhar IH, Hays ER, Iverson M-A, Magalang UJ, Maas AK. Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2013;9:165–74. doi: 10.5664/jcsm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almeida FR, Lowe AA. Principles of oral appliance therapy for the management of snoring and sleep disordered breathing. Oral Maxillofac Surg Clin N Am. 2009;21:413–20. doi: 10.1016/j.coms.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Petit F-X, Pépin J-L, Bettega G, Sadek H, Raphaël B, Lévy P. Mandibular advancement devices: rate of contraindications in 100 consecutive obstructive sleep apnea patients. Am J Respir Crit Care Med. 2002;166:274–8. doi: 10.1164/rccm.2008167. [DOI] [PubMed] [Google Scholar]
- 73.Ng ATM, Darendeliler MA, Petocz P, Cistulli PA. Cephalometry and prediction of oral appliance treatment outcome. Sleep Breath Schlaf Atm. 2012;16:47–58. doi: 10.1007/s11325-011-0484-2. [DOI] [PubMed] [Google Scholar]
- 74.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 75.Ueda H, Almeida FR, Lowe AA, Ruse ND. Changes in occlusal contact area during oral appliance therapy assessed on study models. Angle Orthod. 2008;78:866–72. doi: 10.2319/100107-470.1. [DOI] [PubMed] [Google Scholar]
- 76.Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofac Orthop. 2007;132:806–14. doi: 10.1016/j.ajodo.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 77.De Almeida FR, Lowe AA, Tsuiki S, Otsuka R, Wong M, Fastlicht S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1:143–52. [PubMed] [Google Scholar]
- 78.Jauhar S, Lyons MF, Banham SW, Cameron DA, Orchardson R. Ten-year follow-up of mandibular advancement devices for the management of snoring and sleep apnea. J Prosthet Dent. 2008;99:314–21. doi: 10.1016/S0022-3913(08)60067-0. [DOI] [PubMed] [Google Scholar]
- 79.Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34:479–85. doi: 10.1093/sleep/34.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 81.Sin DD, Mayers I, Man GCW, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 82.Bridgman SA, Dunn KM. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2000;2:CD001004. doi: 10.1002/14651858.CD001004. [DOI] [PubMed] [Google Scholar]
- 83.Kotecha BT, Hannan SA, Khalil HMB, Georgalas C, Bailey P. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Oto-Rhino-Laryngol. 2007;264:1361–7. doi: 10.1007/s00405-007-0366-1. [DOI] [PubMed] [Google Scholar]
- 84.Soares D, Folbe AJ, Yoo G, Badr MS, Rowley JA, Lin H-S. Drug-induced sleep endoscopy vs awake Müller's maneuver in the diagnosis of severe upper airway obstruction. Otolaryngol-Head Neck Surg. 2013;148:151–6. doi: 10.1177/0194599812460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.George JR, Chung S, Nielsen I, Goldberg AN, Miller A, Kezirian EJ. Comparison of drug-induced sleep endoscopy and lateral cephalometry in obstructive sleep apnea. Laryngoscope. 2012;122:2600–5. doi: 10.1002/lary.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sher AE, Thorpy MJ, Shprintzen RJ, Spielman AJ, Burack B, McGregor PA. Predictive value of Müller maneuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope. 1985;95:1483–7. doi: 10.1288/00005537-198512000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Li H-Y, Wang P-C, Chen Y-P, Lee L-A, Fang T-J, Lin H-C. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25:45–9. doi: 10.2500/ajra.2011.25.3558. [DOI] [PubMed] [Google Scholar]
- 88.Nakata S, Noda A, Yagi H, Yanagi E, Mimura T, Okada T, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43:296–9. [PubMed] [Google Scholar]
- 89.Foltán R, Hoffmannová J, Pretl M, Donev F, Vlk M. Genioglossus advancement and hyoid myotomy in treating obstructive sleep apnoea syndrome - A follow-up study. J Cranio-Maxillo-fac Surg. 2007;35:246–51. doi: 10.1016/j.jcms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 90.Holty J-EC, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287–97. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Li KK, Powell NB, Riley RW, Guilleminault C. Distraction osteogenesis in adult obstructive sleep apnea surgery: a preliminary report. J Oral Maxillofac Surg. 2002;60:6–10. doi: 10.1053/joms.2002.29049. [DOI] [PubMed] [Google Scholar]
- 92.Hicklin LA, Tostevin P, Dasan S. Retrospective survey of long-term results and patient satisfaction with uvulopalatopharyngoplasty for snoring. J Laryngol Otol. 2000;114:675–81. doi: 10.1258/0022215001906697. [DOI] [PubMed] [Google Scholar]
- 93.Boot H, van Wegen R, Poublon RM, Bogaard JM, Schmitz PI, van der Meché FG. Long-term results of uvulopalatopharyngoplasty for obstructive sleep apnea syndrome. Laryngoscope. 2000;110:469–75. doi: 10.1097/00005537-200003000-00027. [DOI] [PubMed] [Google Scholar]
- 94.Golz A, Goldenberg D, Netzer A, Westerman ST, Joachims HZ. Epiglottic carcinoma presenting as obstructive sleep apnea. J Otolaryngol. 2001;30:58–9. doi: 10.2310/7070.2001.20985. [DOI] [PubMed] [Google Scholar]
- 95.Verse T, Kroker BA, Pirsig W, Brosch S. Tonsillectomy as a treatment of obstructive sleep apnea in adults with tonsillar hypertrophy. Laryngoscope. 2000;110:1556–9. doi: 10.1097/00005537-200009000-00029. [DOI] [PubMed] [Google Scholar]
- 96.Lin H-C, Friedman M, Chang H-W, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2008;118:902–8. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 97.Richard W, Kox D, den Herder C, van Tinteren H, de Vries N. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radiofrequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Oto-Rhino-Laryngol. 2007;264:439–44. doi: 10.1007/s00405-006-0182-z. [DOI] [PubMed] [Google Scholar]
- 98.Camacho M, Certal V, Brietzke SE, Holty J-EC, Guilleminault C, Capasso R. Tracheostomy as treatment for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2014;124:803–11. doi: 10.1002/lary.24433. [DOI] [PubMed] [Google Scholar]
- 99.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 100.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 101.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23:414–23. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]


