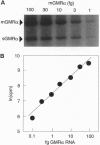
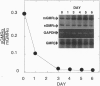
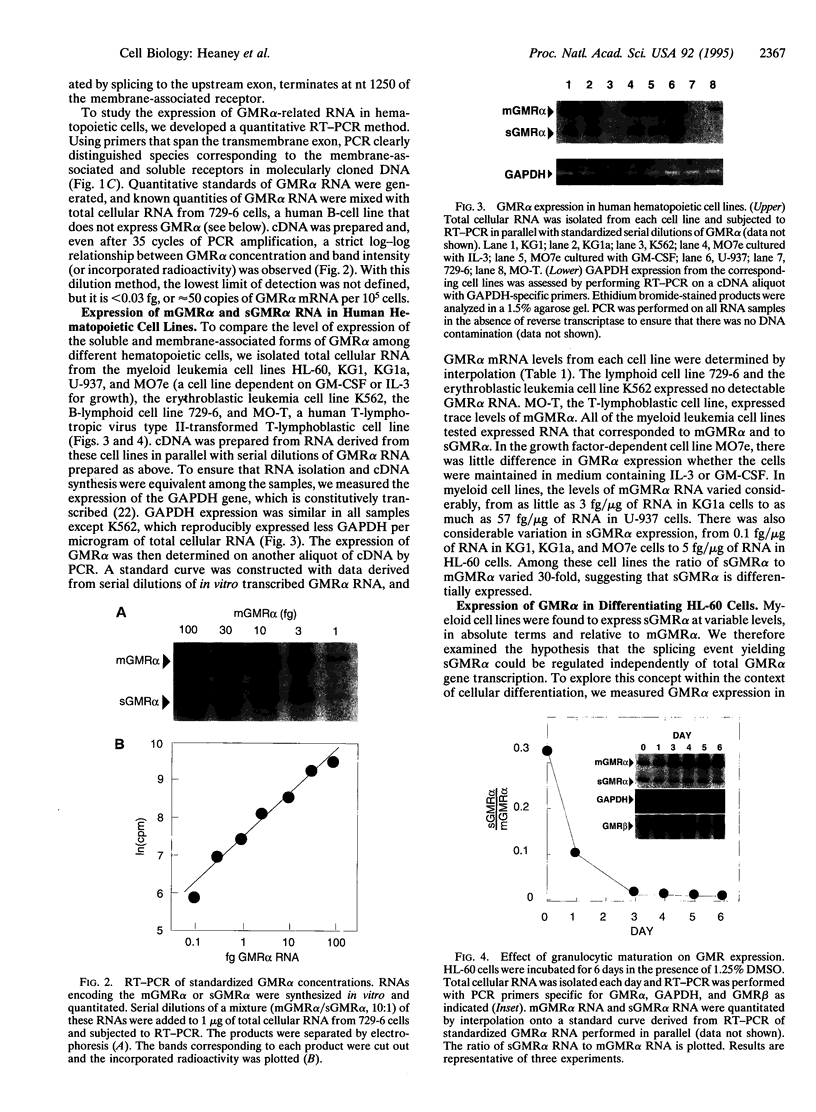
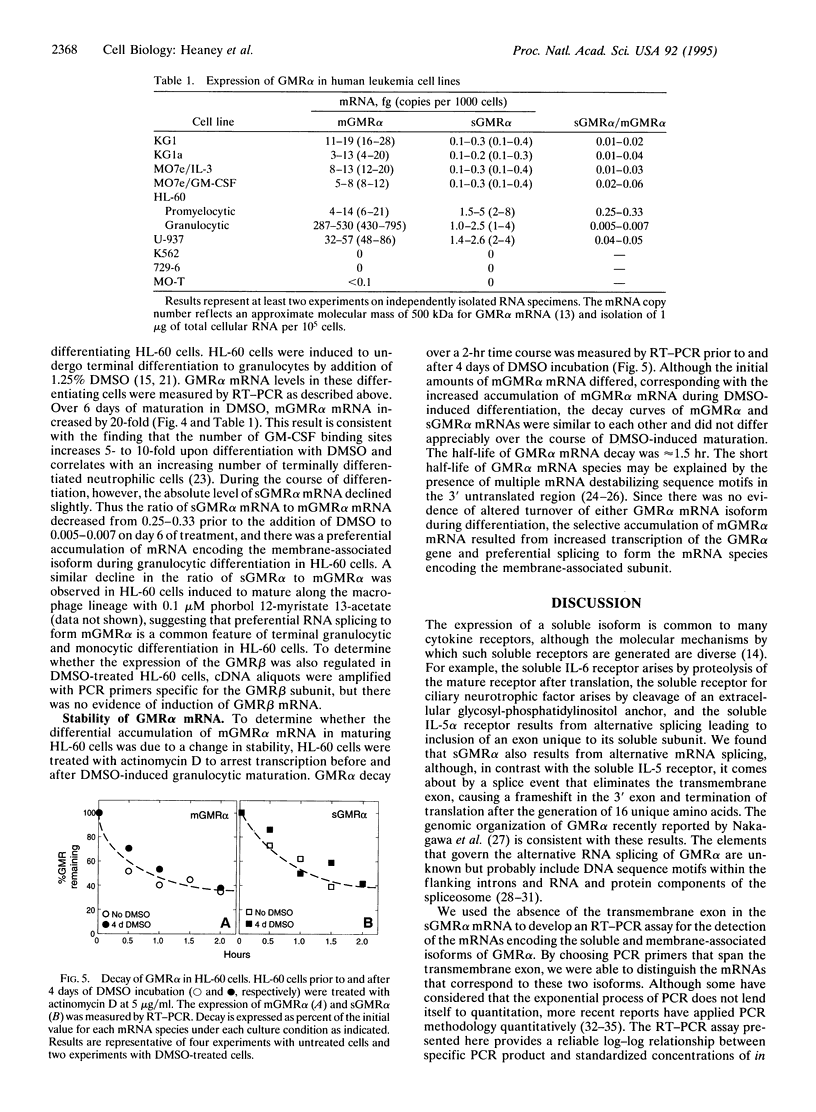
Abstract
The effects of granulocyte/macrophage-colony-stimulating factor (GM-CSF) are mediated by interaction with its composite receptor (GMR), which consists of a unique alpha subunit (GMR alpha) and a beta subunit (GMR beta) that is common to the receptors for GM-CSF, interleukin 3, and interleukin 5. GMR beta is required for high-affinity binding, cell proliferation, and protein phosphorylation but has no intrinsic GM-CSF-binding activity. GMR alpha in isolation binds to GM-CSF with low affinity and can signal for increased glucose uptake. In addition to the membrane-bound receptor (mGMR alpha), there is a naturally occurring soluble isoform (sGMR alpha) that is released free into the pericellular milieu. Analysis of genomic sequences reveals that the soluble GMR alpha isoform comes about by alternative mRNA splicing. To examine GMR alpha expression, we developed a quantitative reverse transcription-polymerase chain reaction assay based on serial dilutions of in vitro transcribed GMR alpha RNA. This assay provides a strict log-log measure of GMR alpha RNA expression, distinguishes transcripts related to the soluble and membrane-associated isoforms, and quantitatively detects 0.1 fg of GMR alpha-related mRNA. There was little or no GMR alpha expression in two human lymphoid cell lines and in the erythroblastic leukemia cell line K562, but all myeloid cell lines tested expressed both the membrane-associated and soluble isoforms of GMR alpha. Baseline level of expression of both isoforms varied > 20-fold among the myeloid cell lines studied. Differentiation of HL-60 cells to neutrophils with dimethyl sulfoxide led to a 2-fold downregulation of sGMR alpha and a 20-fold upregulation of mGMR alpha. These differentiation-induced transcriptional changes were unrelated to changes in mRNA stability. These findings indicate that sGMR alpha is differentially expressed from mGMR alpha in human hematopoietic cells and that programmed downregulation of sGMR alpha may be important in myeloid maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharon T., Schneider R. J. Selective destabilization of short-lived mRNAs with the granulocyte-macrophage colony-stimulating factor AU-rich 3' noncoding region is mediated by a cotranslational mechanism. Mol Cell Biol. 1993 Mar;13(3):1971–1980. doi: 10.1128/mcb.13.3.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S. J., Weitsman S., Rosenblatt J. D., Chen I. S. Analysis of rev gene function on human immunodeficiency virus type 1 replication in lymphoid cells by using a quantitative polymerase chain reaction method. J Virol. 1989 Nov;63(11):4875–4881. doi: 10.1128/jvi.63.11.4875-4881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A., Kraft A. Cloning of a potentially soluble receptor for human GM-CSF. Nucleic Acids Res. 1990 Dec 11;18(23):7178–7178. doi: 10.1093/nar/18.23.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzi G. C., Brizzi M. F., Giannotti J., Ciarletta A., Yang Y. C., Pegoraro L., Clark S. C. M-07e human leukemic factor-dependent cell line provides a rapid and sensitive bioassay for the human cytokines GM-CSF and IL-3. J Cell Physiol. 1990 Dec;145(3):458–464. doi: 10.1002/jcp.1041450310. [DOI] [PubMed] [Google Scholar]
- Baldwin G. C., Golde D. W., Widhopf G. F., Economou J., Gasson J. C. Identification and characterization of a low-affinity granulocyte-macrophage colony-stimulating factor receptor on primary and cultured human melanoma cells. Blood. 1991 Aug 1;78(3):609–615. [PubMed] [Google Scholar]
- Ballou L. R., Chao C. P., Holness M. A., Barker S. C., Raghow R. Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem. 1992 Oct 5;267(28):20044–20050. [PubMed] [Google Scholar]
- Bouaboula M., Legoux P., Pességué B., Delpech B., Dumont X., Piechaczyk M., Casellas P., Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992 Oct 25;267(30):21830–21838. [PubMed] [Google Scholar]
- Cannistra S. A., Groshek P., Garlick R., Miller J., Griffin J. D. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):93–97. doi: 10.1073/pnas.87.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Shibuya K., Piao Y. F., Tojo A., Sasaki N., Matsuki S., Miyagawa K., Miyazono K., Takaku F. Identification and cellular distribution of distinct proteins forming human GM-CSF receptor. Cell Regul. 1990 Mar;1(4):327–335. doi: 10.1091/mbc.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. F., Hedvat C., Ford C. F., Golde D. W., Gasson J. C. Characterization of the soluble human granulocyte-macrophage colony-stimulating factor receptor complex. J Biol Chem. 1991 Jan 5;266(1):279–286. [PubMed] [Google Scholar]
- DiPersio J., Billing P., Kaufman S., Eghtesady P., Williams R. E., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1988 Feb 5;263(4):1834–1841. [PubMed] [Google Scholar]
- Ding D. X., Rivas C. I., Heaney M. L., Raines M. A., Vera J. C., Golde D. W. The alpha subunit of the human granulocyte-macrophage colony-stimulating factor receptor signals for glucose transport via a phosphorylation-independent pathway. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2537–2541. doi: 10.1073/pnas.91.7.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassy M. C., Handley H. H., Hagiwara H., Royston I. UC 729-6, a human lymphoblastoid B-cell line useful for generating antibody-secreting human-human hybridomas. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6327–6331. doi: 10.1073/pnas.80.20.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney M. L., Golde D. W. Soluble hormone receptors. Blood. 1993 Oct 1;82(7):1945–1948. [PubMed] [Google Scholar]
- Hodges D., Bernstein S. I. Genetic and biochemical analysis of alternative RNA splicing. Adv Genet. 1994;31:207–281. doi: 10.1016/s0065-2660(08)60399-5. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Manninen T., Peltonen L., Syvänen A. C. Quantitative determination of rare mRNA species by PCR and solid-phase minisequencing. PCR Methods Appl. 1992 May;1(4):234–240. doi: 10.1101/gr.1.4.234. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Lu J., Chen H., Werner S., Williams L. T. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991 Sep;11(9):4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Human myeloid leukemia cell lines: a review. Blood. 1980 Sep;56(3):344–350. [PubMed] [Google Scholar]
- Koeffler H. P. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983 Oct;62(4):709–721. [PubMed] [Google Scholar]
- McKeown M. Alternative mRNA splicing. Annu Rev Cell Biol. 1992;8:133–155. doi: 10.1146/annurev.cb.08.110192.001025. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Bottaro D. P., Fleming T. P., Smith C. L., Burgess W. H., Chan A. M., Aaronson S. A. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D. E., Yokota A., Caro J. F., Flier J. S. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989 Aug;3(8):1263–1269. doi: 10.1210/mend-3-8-1263. [DOI] [PubMed] [Google Scholar]
- Mosthaf L., Grako K., Dull T. J., Coussens L., Ullrich A., McClain D. A. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990 Aug;9(8):2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Kosugi H., Miyajima A., Arai K., Yokota T. Structure of the gene encoding the alpha subunit of the human granulocyte-macrophage colony stimulating factor receptor. Implications for the evolution of the cytokine receptor superfamily. J Biol Chem. 1994 Apr 8;269(14):10905–10912. [PubMed] [Google Scholar]
- Norton P. A. Alternative pre-mRNA splicing: factors involved in splice site selection. J Cell Sci. 1994 Jan;107(Pt 1):1–7. doi: 10.1242/jcs.107.1.1. [DOI] [PubMed] [Google Scholar]
- Park L. S., Martin U., Sorensen R., Luhr S., Morrissey P. J., Cosman D., Larsen A. Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4295–4299. doi: 10.1073/pnas.89.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M., Jr, Luk K. C., Williams B., Lifson J. D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. Biotechniques. 1993 Jan;14(1):70–81. [PubMed] [Google Scholar]
- Raines M. A., Liu L., Quan S. G., Joe V., DiPersio J. F., Golde D. W. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8203–8207. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Chiba S., Mano H., Yazaki Y., Hirai H. Identification of a soluble GM-CSF binding protein in the supernatant of a human choriocarcinoma cell line. Biochem Biophys Res Commun. 1992 Feb 28;183(1):252–257. doi: 10.1016/0006-291x(92)91636-5. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978 Mar;88(3):323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- Seino S., Bell G. I. Alternative splicing of human insulin receptor messenger RNA. Biochem Biophys Res Commun. 1989 Feb 28;159(1):312–316. doi: 10.1016/0006-291x(89)92439-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Split genes and RNA splicing. Cell. 1994 Jun 17;77(6):805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]