Abstract
Background
Muscle wasting is a profound side effect of advanced cancer. Cancer-induced cachexia decreases patient quality of life and is associated with poor patient survival. Currently, no clinical therapies exist to treat cancer-induced muscle wasting. Although cancers commonly associated with cachexia occur in older individuals, the standard animal models used to elucidate the causes of cachexia rely on juvenile mice.
Methods
In an effort to better model human cancer cachexia, we determined whether cachectic features seen in young mice could be achieved in adult, pre-sarcopenic mice following colon 26 (C-26) tumor cell inoculation.
Results
Both young and adult mice developed similar-sized tumors and progressed to cachexia with similar kinetics, as evidenced by losses in body mass, and adipose and skeletal muscle tissues. Proteolytic signaling, including proteasome and autophagy genes, was also increased in muscles from both young and adult tumor-bearing animals. Furthermore, tumor-associated muscle damage and activation of Pax7 progenitor cells was induced in both young and adult mice.
Conclusions
Although cancer cachexia generally occurs in older individuals, these data suggest that the phenotype and underlying mechanisms can be effectively modeled using the currently accepted protocol in juvenile mice.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-014-0141-2) contains supplementary material.
Keywords: Muscle wasting, Ubiquitin ligases, Autophagy, Muscle regeneration
Introduction
Cachexia is a multi-factorial syndrome that affects many patients with chronic illness, including patients with advanced cancer. A hallmark symptom of cachexia is significant decreases in body weight; the majority of which are due to loss of both adipose tissue and skeletal muscle [1]. Cancer-induced cachexia is particularly profound in patients with gastrointestinal and pancreatic cancers, in which perhaps one-third of patients lose at least 10 % of their baseline weight [2, 3]. This substantial weight loss affects patient quality of life due to muscle weakness and fatigue and also greatly affects patient treatment tolerance [4]. Cachexia also impacts patient survival, with up to 25 % of cancer deaths being attributed to respiratory insufficiency caused by diaphragm muscle failure [5].
Even after years of research, there remains no clinical therapy to prevent cancer-induced muscle wasting. Because conducting human studies of cancer cachexia is difficult for both practical and ethical reasons, much of what is known about cancer-induced skeletal muscle loss has been determined using xenograft tumors transplanted into rodent models. Among the most commonly used models is the colon 26 (C-26) mouse model, whereby C-26 tumor cells are injected subcutaneously into the flank of mice. This model produces repeatable tumor growth and has become the norm by which to study underlying molecular mechanisms driving muscle wasting in cancer [6].
However, one caveat with using the C-26 model is that it is typically performed with juvenile mice between 2–3 months of age. Since most cancers in humans develop in adults, the question becomes whether administering tumors to juvenile mice accurately reflects the human condition. Thus, we sought to compare the effects of the C-26 cachexia model in young versus adult mice. Standard measures of tumor development and body and tissue mass loss were analyzed in tumor-bearing animals from both age groups. Additionally, as cancer-induced muscle loss is at least partly due to an increase in muscle protein breakdown, activation of many proteolytic genes known to be important in cachexia was assessed to determine whether similar proteolytic pathways are activated in young and adult mice. Finally, indices of muscle damage were assessed, as cancer-induced muscle loss is also at least partially attributable to impaired muscle regeneration [7].
This study reveals that young and adult mice develop C-26 tumors in a similar manner and that these mice experience similar losses of adipose and muscle tissue due to these tumors. Additionally, many of the signaling molecules believed to be responsible for C-26-induced muscle wasting are regulated in the same manner in young and adult mice. Our results support the idea that although juvenile mice do not reflect the age at which most patients present with cancer cachexia, their similarity to adult mice with regard to the activation of common signaling pathways underlying cancer cachexia make their use appropriate as a model of cancer-related muscle loss.
Methods
Mice and cachexia model
Eight-week-old and 12-month-old Balb/c mice were purchased from Charles River (Wilmington, MA, USA). Following a standard incubation period, mice were injected in their right flank with either C-26 tumor cells or phosphate-buffered saline as previously described (n = 9–12/group) [6]. Animals were monitored daily, including measuring body weight, tumor size, and food intake, and were sacrificed 21 days following injection. Muscles, organs, and blood were collected at the time of sacrifice from fed mice. Mice were housed at University Laboratory Animal Services at the Arthur G. James Comprehensive Cancer Center of The Ohio State University. Mice received a standard diet and were housed in conventional conditions with constant temperature and humidity. All experiments were approved by The Ohio State University Institutional Animal Care and Use Committee.
Muscle cross-sectional area
To determine tissue cross-sectional area (CSA), a cryostat (Leica) was used to cut 10-μm sections of muscle. H&E staining was performed on three sections representing the entire length of the muscle. Images were acquired using an Olympus BX51 brightfield microscope, and muscle fiber CSA was determined using the Olympus Microsuite Pathology software. Individual fibers were manually outlined with software assistance and then quantified. Results from all three sections from each animal were averaged prior to statistical analysis. An average of 1,200 ± 329 fibers were counted per muscle.
Serum IL-6
Concentrations of interleukin-6 (IL-6) in serum from control and tumor-bearing mice were determined using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Real-time reverse transcriptase PCR
Real-time RT-PCR was conducted as previously described [8]. GAPDH was used as the housekeeping gene. Primer sequences appear in Supplementary Table 1.
Western blotting
Western blotting against Pax7 was conducted as previously described [8]. α-Tubulin was used as a loading control and was measured on the same membrane as Pax7 after stripping. Images were quantified using Image Studio Lite Analysis Software (LI-COR).
IgG staining
To assess muscle damage, 10-μm sections of gastrocnemius were cut on a cryostat (Leica). Muscle sections were blocked in 5 % goat serum, 2 % BSA, and 0.2 % Triton X-100 in phosphate-buffered saline and incubated with anti-mouse IgG Alexa Fluor 568 (Life Technologies). Muscle sections were visualized with an Axioskop 40 microscope (Zeiss).
Statistics
All data are represented as mean ± SEM. Differences between group means were determined using a two-way ANOVA with factors of age (young, adult) and tumor status (control, C-26) with α set at p < 0.05. Differences in tumor size and tumor burden were assessed using a Student’s t test. Significant differences in the distribution of muscle fiber size were determined using a Χ2 goodness of fit test.
Results
Similar C-26 tumors and weight loss develop in both young and adult mice
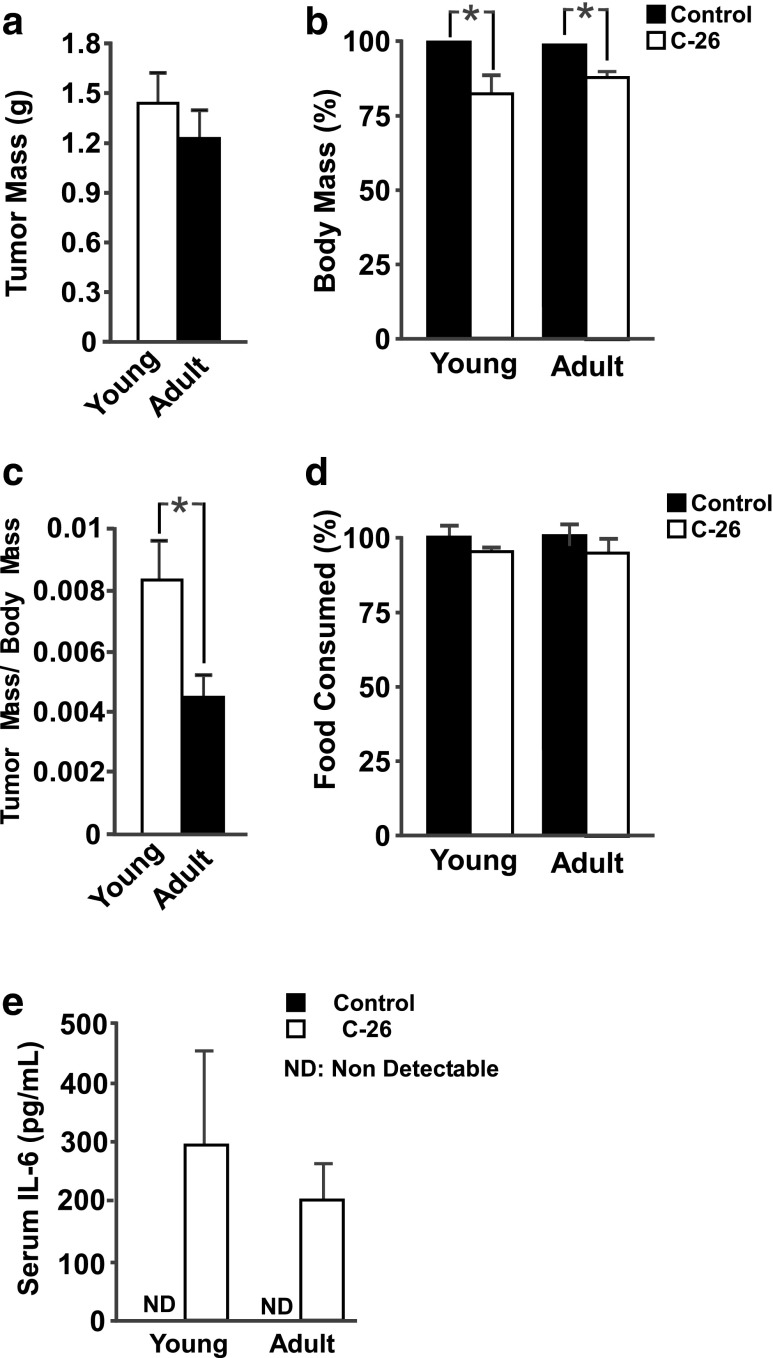
Twenty-one days following C-26 tumor implantation, both young (3 months) and adult (12 months) mice had developed visible, singular tumors. No significant differences in either tumor mass (Fig. 1a) or tumor area (Supplementary Fig. S1A) existed between young and adult mice 21 days after inoculation. The only noticeable difference was in the onset of tumor development: tumors were palpable in young mice at day 7, while tumors in adult mice were not measureable until day 9. Further, young tumors were significantly larger than adult tumors on day 10 through day 12. After this time, there was no statistical difference in tumor progression between young and adult mice.
Fig. 1.
Tumor and animal characteristics 21 days following C-26 cell injection. a Young and adult mice developed similar-sized tumors. b C-26 tumors induced significant body mass loss in both young and adult mice, as determined by a main effect for tumor status. c Young adult mice had greater tumor burden than adult mice. d Neither young nor adult tumor-bearing mice consumed less food, on average, than age-matched control mice. e Significant levels of serum IL-6 were detected in both young and adult C-26 mice, but IL-6 was undetectable in either control group; n = 5–7 per group. *p < 0.05
Importantly, both young and adult mice experienced significant decreases in body mass compared to their age-matched controls (Fig. 1b and Supplementary Fig. S1B, C), with main effects present for both age and tumor status. Young mice had a significantly higher tumor-mass-to-body-mass ratio than adult mice (Fig. 1c). Consistent with previous experiments using the C-26 model [9], neither young nor adult mice consumed significantly less food than their age-matched controls (Fig. 1d and Supplementary Fig. S1D, E, F).
Serum IL-6 increases in both young and adult tumor mice
The C-26 cancer cachexia model is closely associated with an increase in serum IL-6 [10]. Both young and adult tumor-bearing mice had a pronounced elevation in circulating IL-6, while IL-6 levels were undetectable in serum from control animals of both ages (Fig. 1e).
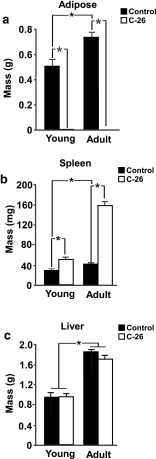
Impact of C-26 tumors on non-muscle tissues in young and adult mice
C-26 cachexia commonly results in the loss of epididymal adipose mass [11] while inducing a large increase in the mass of the spleen [12]. Our study is consistent with previous findings, as tumor-bearing mice experienced significant, near-total losses in epididymal adipose tissue (Fig. 2a) and significant increases in the mass of the spleen (Fig. 2b). As previously reported [12], C-26 tumors did not significantly alter liver mass in either young or adult mice (Fig. 2c).
Fig. 2.
C-26 tumors similarly impact organ mass of young and adult mice. a Epididymal adipose tissue, with an interaction effect demonstrating greater changes in adult mice than young mice, and main effects for both age and tumor status. b Spleen, with an interaction effect demonstrating greater changes in adult mice than young mice, and main effects for both age and tumor status. c Liver, with a main effect for age; n = 5–7 per group. *p < 0.05
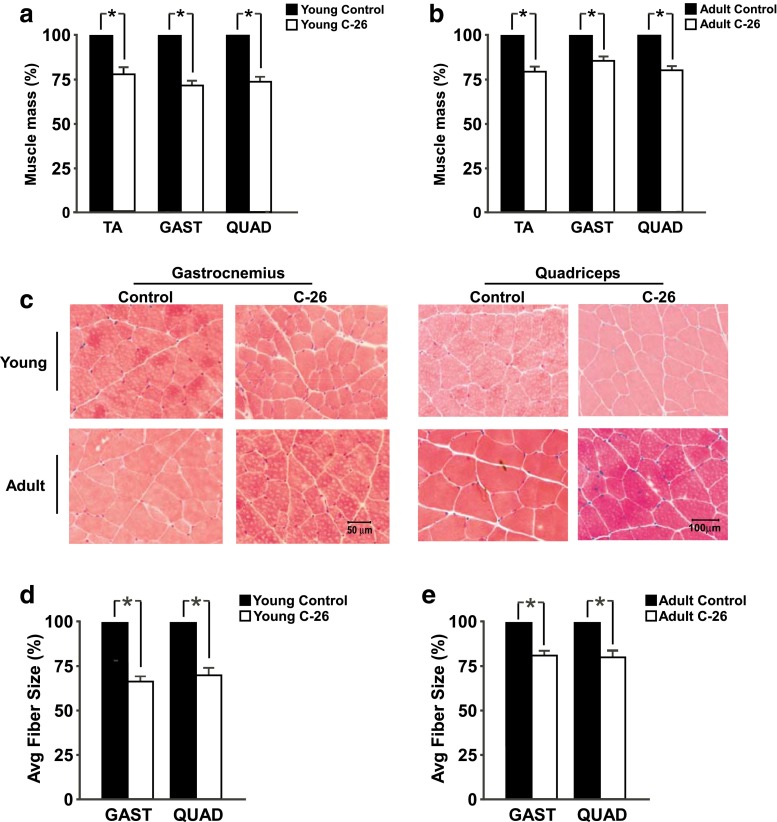
C-26 tumors induce muscle loss in both young and adult mice
C-26 tumors have been reported to induce significant muscle loss [6], and these experiments are consistent with previous results. Twenty-one days post-injection, main effects existed for significant decreases in muscle mass of the tibialis anterior (TA), gastrocnemius (GAST), and quadriceps (QUAD) of tumor-bearing mice compared to control mice (Fig. 3a, b, Supplementary Fig. S2A–C). Representative images of H&E-stained GAST and QUAD appear in Fig. 3c. Consistent with the reduction in muscle mass, both C-26 mice experienced significant decreases in cross-sectional area of both the GAST and QUAD (Fig. 3d, e, Supplementary Fig. S2D, E). Fiber size distributions for GAST and QUAD appear in Supplementary Fig. S2F–I. Χ2 analyses indicate that significant differences exist in the distribution of fiber size between control and tumor-bearing mice of both age groups.
Fig. 3.
C-26 tumors decrease muscle mass and cross-sectional area (CSA). a Masses of tibialis anterior (TA), gastrocnemius (GAST), and quadriceps (QUAD) from young control and tumor-bearing mice, each with main effects for tumor status. b TA, GAST, and QUAD masses from adult control and tumor-bearing mice, each with main effects for tumor status. c Representative images of H&E-stained muscle sections of GAST and QUAD from each group. d Average CSA of young control and tumor-bearing GAST and QUAD, each with main effects for tumor status. e Average CSA of adult control and tumor-bearing GAST and QUAD, each with main effects for tumor status; n = 5–7 per group for muscle weight analysis, n = 4–5 for CSA analysis. *p < 0.05
C-26 tumors increase proteolytic gene expression in muscles from both young and adult mice
Muscle wasting resulting from C-26 tumors occurs, at least in part, due to increases in proteolysis. Two major proteolytic systems in skeletal muscle are the ubiquitin proteasome system and the autophagy system. The ubiquitin proteasome system is made up of a series of ubiquitin ligases, which tag myofilament proteins such as myosin with ubiquitin groups and target these proteins for degradation [13]. Increased expression of the E3 ligases of the ubiquitin proteasome system is characteristic of C-26-induced cancer cachexia [6, 12, 14]. In this study, increases in mRNA expression of the E3 ligases atrogin-1 and muscle ring finger-1 (MuRF1) in the QUAD and TA in response to C-26 tumors were demonstrated by simple main effects (Fig. 4a, b and Supplementary Fig. S3A, B). Additionally, a significant interaction effect was present for MuRF1 in the TA and for atrogin-1 in the QUAD. A main effect for tumor status also existed for the newly described mitochondrial ubiquitin ligase-1 (Mul1) in both muscles [15]. These data suggest that the ubiquitin proteasome system plays a similar role in cancer-induced muscle wasting in both young and adult mice. Why the increases in some ubiquitin proteasome markers were more pronounced in young versus adult tumor-bearing mice is not known, but we suspect this may be related to the delay in tumor development that we observed in adult mice.
Fig. 4.
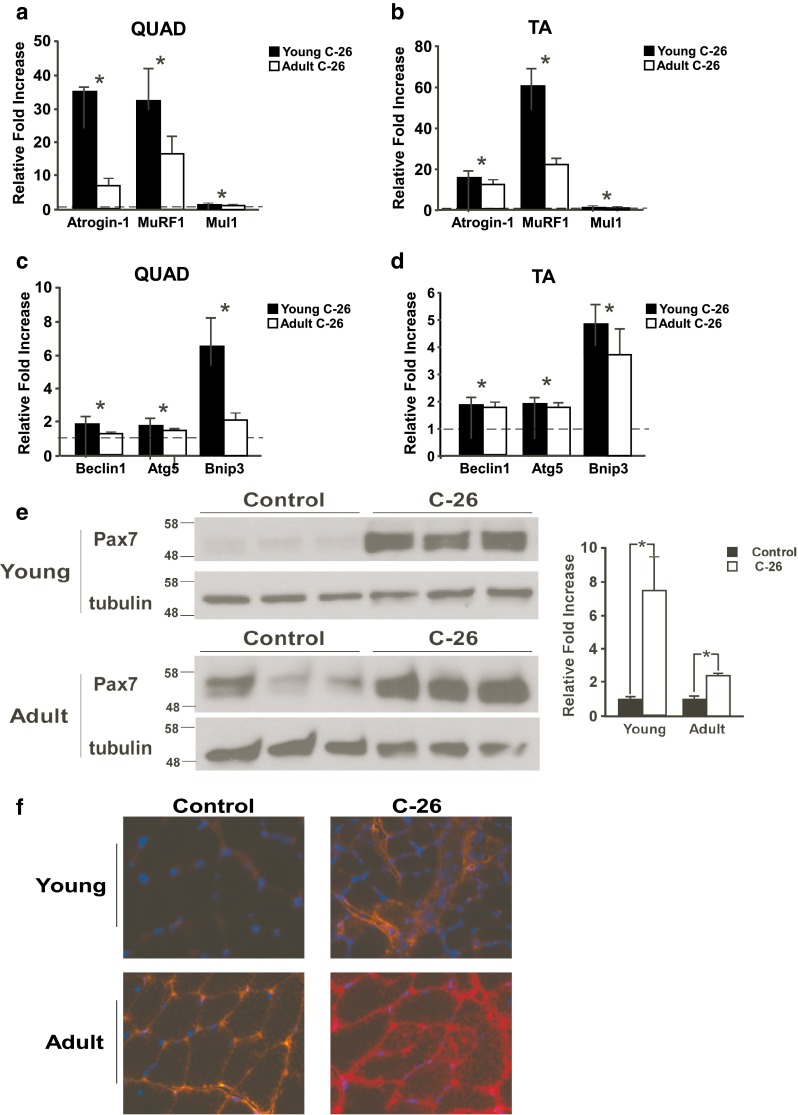
C-26 tumors induce similar proteolytic signaling in young and adult mice. E3 ligase genes are increased in the a QUAD and b TA of both young and adult tumor-bearing mice compared to age-matched controls, with all three genes demonstrating a main effect for tumor status. Autophagy genes are increased in c QUAD and d TA from both young and adult tumor-bearing mice compared to age-matched controls, again with all three genes demonstrating a main effect for tumor status. In a–d, data is expressed as relative fold increase compared to the age-matched controls, which was set at 1 and is represented by the dashed line. e Pax7 abundance is increased in both young and adult tumor-bearing mice compared to age-matched controls, although this increase is greater in young mice. f Muscle sections from tumor-bearing mice demonstrate greater IgG staining than age-matched control sections. Additionally, adult control muscle shows greater IgG staining than young control muscle, indicating that adult muscle is damaged prior to exposure to tumor factors; n = 6–11 per group. *p < 0.05
The autophagy system degrades mitochondria and other cytoskeletal proteins and is also associated with proteolysis induced by C-26 tumors [16]. Main effects for tumor status were present for the autophagy genes Beclin1, Atg5, and Bnip3 in both the QUAD and TA muscles (Fig. 4c, d, Supplementary Fig. S3A–D). Thus, the autophagy system also appears to be similarly regulated in muscle from young and adult tumor-bearing mice.
Recent evidence has demonstrated that expression of the protein Pax7, a key player in muscle regeneration, contributes to muscle wasting during cancer cachexia [7]. Both young and adult tumor-bearing animals demonstrated increased Pax7 protein abundance (Fig. 4e). However, Pax7 expression in tumor-bearing adults was only 2.4-fold higher than adult control animals, compared to a 7.4-fold difference between young control and tumor-bearing animals. Since increases in Pax7 is a direct reflection of muscle damage in tumor-bearing mice, as well as in patients with weight loss diagnosed with pancreatic adenocarcinoma [7], we asked whether the smaller differences seen between control and C-26 tumor-bearing adult mice could be due to a higher basal level of muscle damage in older control animals. Indeed, although the tumor-induced increases in Pax7 in both young and adult mice were associated with an increase in immunoglobulin (IgG) staining (Fig. 4f), which is representative of sarcolemma damage, IgG staining was noticeably higher in adult control mice compared to young control mice. Finally, as we recently described [7], muscle from both young and adult tumor-bearing animals showed reduced expression of extracellular matrix genes (Supplementary Fig. S3e). The increased IgG staining coupled with decreased extracellular matrix gene expression support that cancer cachexia induces changes not only in skeletal muscle, but also in the environment surrounding the muscle fiber.
Discussion
The purpose of this study was to determine if adult mice develop C-26 tumors and cancer-induced muscle wasting in a manner similar to young mice that are commonly used to model human cancer cachexia. Our data support that using young mice in the C-26 model of cancer cachexia is appropriate, even though cancers that induce the greatest cachexia in patients generally occur later in life. A discussion of our findings and their relevance appears below.
Young mice as a model of cachexia
Cancers of the gastrointestinal tract and pancreas typically induce cachexia in a greater proportion of patients than other tumors [17]. Although greater than 85 % of esophageal cancer diagnoses are made in patients over the age of 55 and the median diagnosis age for both stomach and pancreatic cancers is slightly older [19], juvenile mice are often used to study cancer-induced muscle loss. A 3-month-old mouse approximates a human of about 20 years of age [20], far earlier than gastrointestinal and pancreatic cancers generally are diagnosed. Thus, to determine if studying cancer cachexia in young animals is appropriate, we determined if adult mice develop C-26 tumors and skeletal muscle wasting in a similar manner to younger mice.
This study utilized 12-month-old mice, which relate to humans in their forties [20]. We chose this age mouse because it is before significant decreases in survival occur [20] and is before the onset of age-induced muscle loss (sarcopenia) [20], yet these mice are not rapidly growing and have growth factor levels more similar to adult humans than juvenile mice.
C-26 tumors induce similar effects in young and adult mice
Our results demonstrate very few differences between young and adult C-26 tumor-bearing mice 21 days following injection, with young and adult mice demonstrating similar size tumors and similar decreases in body weight. Additionally, young and adult mice experienced changes in spleen and adipose tissue masses due to C-26 tumors, although older mice had more pronounced changes in spleen and adipose tissue masses.
Tumor-bearing young and adult mice both demonstrated decreases in TA, GAST, and QUAD muscle mass compared to non-tumor-bearing control mice. However, young mice lost approximately 30 % of their GAST and QUAD muscle CSA, while adult mice only lost ~20 % of these muscles. This difference is consistent with greater proteolytic gene induction in muscle from younger mice compared to adult mice. As alluded to in the “Results” section, the reason for this more robust proteolytic signaling in young mice is unclear, but one possible reason for the enhanced signaling in the young mice could be related to the greater tumor burden of young mice (tumor-mass-to-body-mass ratio, Fig. 1c) compared to adult mice. The difference in the extent of muscle atrophy and proteolytic signaling also could be related to the differences in the timing of C-26 tumor growth. Tumors are measureable 2 days earlier in young mice than adult mice (day 7 versus day 9, Supplementary Fig. S1A). Additionally, initial tumor growth is much faster in young animals, with tumors in young animals being significantly larger than tumors in adult animals on days 10 through 12 (Supplementary Fig. S1A). Thus, the onset of muscle loss in C-26 cachexia may be slightly delayed in adult mice compared to young mice, and given additional time, it is possible that adult mice would experience the same degree of muscle loss as young mice.
Recent work from our lab has demonstrated that muscle regeneration is defective during cancer cachexia [7]. This defect appears to result from a persistent increase in Pax7 expression, which prevents muscle precursor cells from fusing into and repairing existing muscle fibers. Our experiments demonstrate increases in Pax7 in both young and adult mice, although increases in adult mice were less than those in young mice (Fig. 4e). This lesser increase in Pax7 abundance does not, however, imply that the muscle precursor fusion defect in muscle regeneration is less in adult mice. Indeed, adult control mice demonstrated increased Pax7 expression compared to young mice, suggesting that perhaps muscle precursor cell differentiation into existing muscle fibers is already defective in adult control mice and that tumor factors simply exacerbate this dysfunction. Our suggestion of dysfunction in aged satellite cells is supported by recent evidence demonstrating that satellite cell regeneration is impaired in an aged environment [21] and that satellite cells fail to maintain quiescence as effectively as young satellite cells [22–24]. The idea that muscle precursor fusion is defective in muscle of adult animals is supported by an age-induced increase in muscle damage demonstrated by IgG staining (Fig. 4f).
Experimental limitations
As with all work, this study is not without limitations, which should be acknowledged. First, although C-26 tumor experiments are traditionally conducted in CD2F1 mice [8, 6], due to the availability of adult animals, these experiments were conducted in Balb/c mice. Importantly, other groups have determined that C-26 tumor-bearing Balb/c mice experience significant muscle wasting [25]. Additionally, the experiments detailed here did not test all possible differences between young and adult mice undergoing cancer-induced muscle loss. For example, additional experiments would be required to determine if metabolic responses to cachexia differ between mice of different ages. Furthermore, these experiments were only conducted in the C-26 tumor model of cachexia, and it remains to be tested if these results can be generalized to other cachexia models, including the Lewis lung carcinoma (LLC) model. However, in our experience and those of other laboratories [8, 6, 26, 7, 27, 28], the molecular mechanisms regulating tumor-induced muscle loss are highly comparable between C-26 and LLC models.
Conclusions
Our data support the concept that young and adult mice exhibit similar responses to C-26 tumors including development of cachexia and proteolytic signaling in muscle. Additionally, our data support the concept that both young and adult mice experience tumor-induced defects in muscle regeneration. In total, these results demonstrate that using young mice in experiments seeking to understand the underlying molecular mechanisms of cachexia induced by cancer is acceptable, and currently, there does not seem to be an overwhelming reason to adapt tumor models to older animals to mimic a more equivalent age in which patients develop cancer-induced weight loss. For these same reasons, we also predict that tumor models in young mice would be appropriate to use in pre-clinical trials testing anti-cachectic agents, although further experiments will be required before such conclusions can be drawn.
Electronic supplementary material
Additional animal and tumor characteristics. (A) Daily tumor growth in both young and adult mice. (B) Absolute body mass changes, with main effects present for both age and tumor status. (C) Daily progression of weight loss in both young and adult tumor-bearing mice. (D) Average daily food consumption, with a main effect for age. Daily average food consumption by young (E) and adult (F) mice compared to their age-matched controls; n = 5–7 per group. Asterisks denote p <0.05. (PDF 61 kb)
C-26 tumors induce muscle atrophy. Absolute muscle mass measurements are presented for TA (A), GAST (B), and QUAD (C), with main effects present in each for tumor status, and additional main effects for age in the TA and GAST. Absolute cross-sectional area measurements are presented for GAST (D) and QUAD (E), with main effects for both tumor status and age. Fiber size distributions of (F) young GAST, (G) adult GAST, (H) young QUAD, and (I) adult QUAD muscles from tumor-bearing mice and age-matched controls. Each of the control distributions was determined to be significantly different from the C-26 distribution by Chi squared analysis; n = 4–7 per group. Asterisks denote p <0.05. (PDF 829 kb)
C-26 tumors alter gene expression. Gene expression data from Fig. 4a-d is represented in (A-D), with the young control average set equal to 1. (E) mRNA expression of collagen1a1 (Col1a1) and collagen3a1 (Col3a1) were decreased by C-26 tumors, with interaction effects present in addition to main effects for both tumor status and age. Additionally, fibronectin1 (FN1) demonstrated main effects for both tumor status and age; n = 6–11 per group. Asterisks (*) denote a main effect for tumor status, pound (#) denotes a main effect for age, and plus (+) denotes an interaction effect of age and tumor status, p <0.05. (PDF 2104 kb)
(PDF 53 kb)
Acknowledgments
We thank Katherine Ladner for technical assistance and The Ohio State Comprehensive Cancer Center for support for this project. EET is Weiss Postdoctoral Fellow and is supported by the Ohio State Cancer Genetics NIH T32 training grant, T32 CA106196. Further support was provided by an NIH grant, R01 CA180057 to DCG.
Conflict of interest
Erin Talbert, Gregory Metzger, Wei He, and Denis Guttridge declare that they have no conflicts of interest.
Ethical assurance
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Footnotes
Erin E. Talbert and Gregory A. Metzger contributed equally to this work.
Contributor Information
Erin E. Talbert, Email: erin.talbert@osumc.edu
Gregory A. Metzger, Email: gregory.metzger@osumc.edu
Wei A. He, Email: wei.he@osumc.edu
Denis C. Guttridge, Phone: +1-614-6883137, FAX: +1-614-6884006, Email: denis.guttridge@osumc.edu
References
- 1.Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab. 2013;24:174–83. doi: 10.1016/j.tem.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Glass D, Guttridge D. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Tisdale M. Tumor-host interactions. J Cell Biochem. 2004;93:871–7. doi: 10.1002/jcb.20246. [DOI] [PubMed] [Google Scholar]
- 4.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9. doi: 10.1016/S0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 5.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 6.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–8. doi: 10.1172/JCI200420174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He WA, Berardi E, Cardillo VM, Acharyya S, Aulino P, Thomas-Ahner J et al. NF-kappaB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest. 2013;123:4821–35. [DOI] [PMC free article] [PubMed]
- 8.Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–32. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Murphy K, Chee A, Trieu J, Naim T, Lynch G. Importance of functional and metabolic impairments in the characterization of the C-26 murine model of cancer cachexia. Dis Model Mech. 2012;5:533–45. doi: 10.1242/dmm.008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M, Miyazaki H, Takeda Y, Takeo S. Detection of serum cytokine levels in experimental cancer cachexia of colon 26 adenocarcinoma-bearing mice. Cancer Lett. 1993;72:65–70. doi: 10.1016/0304-3835(93)90012-X. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, et al. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:2290–5. [PubMed] [Google Scholar]
- 12.Aulino P, Berardi E, Cardillo V, Rizzuto E, Perniconi B, Ramina C, et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. doi: 10.1186/1471-2407-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taillandier D, Combaret L, Pouch MN, Samuels SE, Béchet D, Attaix D. The role of ubiquitin-proteasome-dependent proteolysis in the remodelling of skeletal muscle. Proc Nutr Soc. 2004;63:357–61. doi: 10.1079/PAR2004358. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Wang J, Lu J, Song Y, Kwak K, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Lokireddy S, Wijesoma IW, Teng S, Bonala S, Gluckman PD, McFarlane C, et al. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab. 2012;16:613–24. [DOI] [PubMed]
- 16.Roberts B, Ahn B, Smuder A, Al-Rajhi M, Gill L, Beharry A, et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J : Off Publ Fed Am Soc Exp Biol. 2013;27:2600–10. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–7. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013.
- 19.Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, editor. The mouse in biomedical research. 2nd ed. Burlington, MA: American College of Laboratory Medicine (Elsevier); 2007. p. 637–72.
- 20.Sayer AA, Robinson SM, Patel HP, Shavlakadze T, Cooper C, Grounds MD. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42:145–50. doi: 10.1093/ageing/afs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conboy I, Conboy M, Wagers A, Girma E, Weissman I, Rando T. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 22.Chakkalakal J, Jones K, Basson M, Brack A. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–64. [DOI] [PMC free article] [PubMed]
- 24.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–71. [DOI] [PMC free article] [PubMed]
- 25.Di Marco S, Cammas A, Lian X, Kovacs E, Ma J, Hall D, et al. The translation inhibitor pateamine A prevents cachexia-induced muscle wasting in mice. Nature communications. 2012;3:896. doi: 10.1038/ncomms1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penna F, Busquets S, Toledo M, Pin F, Massa D, Lopez-Soriano FJ, et al. Erythropoietin administration partially prevents adipose tissue loss in experimental cancer cachexia models. J Lipid Res. 2013;54:3045–51. doi: 10.1194/jlr.M038406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, Jin B, Li YP. C/EBPbeta mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J. 2011;30:4323–35. doi: 10.1038/emboj.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. AJP: Endocrinol Metab. 2012;303:E410–21. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional animal and tumor characteristics. (A) Daily tumor growth in both young and adult mice. (B) Absolute body mass changes, with main effects present for both age and tumor status. (C) Daily progression of weight loss in both young and adult tumor-bearing mice. (D) Average daily food consumption, with a main effect for age. Daily average food consumption by young (E) and adult (F) mice compared to their age-matched controls; n = 5–7 per group. Asterisks denote p <0.05. (PDF 61 kb)
C-26 tumors induce muscle atrophy. Absolute muscle mass measurements are presented for TA (A), GAST (B), and QUAD (C), with main effects present in each for tumor status, and additional main effects for age in the TA and GAST. Absolute cross-sectional area measurements are presented for GAST (D) and QUAD (E), with main effects for both tumor status and age. Fiber size distributions of (F) young GAST, (G) adult GAST, (H) young QUAD, and (I) adult QUAD muscles from tumor-bearing mice and age-matched controls. Each of the control distributions was determined to be significantly different from the C-26 distribution by Chi squared analysis; n = 4–7 per group. Asterisks denote p <0.05. (PDF 829 kb)
C-26 tumors alter gene expression. Gene expression data from Fig. 4a-d is represented in (A-D), with the young control average set equal to 1. (E) mRNA expression of collagen1a1 (Col1a1) and collagen3a1 (Col3a1) were decreased by C-26 tumors, with interaction effects present in addition to main effects for both tumor status and age. Additionally, fibronectin1 (FN1) demonstrated main effects for both tumor status and age; n = 6–11 per group. Asterisks (*) denote a main effect for tumor status, pound (#) denotes a main effect for age, and plus (+) denotes an interaction effect of age and tumor status, p <0.05. (PDF 2104 kb)
(PDF 53 kb)