Abstract
Background
Loss of muscle protein is a common feature of wasting diseases where currently treatment is limited. This study investigates the potential of epigallocatechin-3-gallate (EGCg), the most abundant catechin in green tea, to reverse the increased protein degradation and rescue the decreased protein synthesis which leads to muscle atrophy.
Methods
Studies were conducted in vitro using murine C2C12 myotubes. Increased protein degradation and reduced rates of protein synthesis were induced by serum starvation and tumour necrosis factor-α (TNF-α).
Results
EGCg effectively attenuated the depression of protein synthesis and increase in protein degradation in murine myotubes at concentrations as low as 10 μM. Serum starvation increased expression of the proteasome 20S and 19S subunits, as well as the proteasome ‘chymotrypsin-like’ enzyme activity, and these were all attenuated down to basal values in the presence of EGCg. Serum starvation did not increase expression of the ubiquitin ligases MuRF1 and MAFbx, but EGCg reduced their expression below basal levels, possibly due to an increased expression of phospho Akt (pAkt) and phospho forkhead box O3a (pFoxO3a). Attenuation of protein degradation by EGCg was increased in the presence of ZnSO4, suggesting an EGCg-Zn2+ complex may be the active species.
Conclusion
The ability of EGCg to attenuate depressed protein synthesis and increase protein degradation in the myotubule model system suggests that it may be effective in preserving skeletal muscle mass in catabolic conditions.
Keywords: Epigallocatechin gallate, Protein degradation, Proteasome, Protein synthesis, Zinc, Tumour necrosis factor-α
Introduction
Muscle atrophy plays an important role in many conditions including cancer cachexia, chronic heart failure, chronic kidney failure, AIDS and the sarcopenia of ageing. Muscle loss results in weakness and inability to carry out normal activities, and if severe can result in death due to respiratory impairment. Attention has been directed towards natural products found in certain foods as treatment of this condition. One such compound is epigallocatechin-3-O-gallate (EGCg), one of the major polyphenols found in green tea, which has potent antioxidative [1], chemopreventive [2] and antitumour activity [3]. EGCg has also been shown to attenuate wasting of skeletal muscle in the Lewis lung carcinoma (LLC) model of cancer cachexia [4], as well as improve muscle function in dystrophic mdx5Cv mice [5], and reduce contractile dysfunction in unloaded skeletal muscle [6].
Muscle atrophy results from an imbalance between the rates of protein synthesis and degradation, usually involving both a depression of protein synthesis and increase in degradation [7]. The predominant pathway leading to myofibrillar protein degradation is the ubiquitin-proteasome pathway [8]. The ability of EGCg to attenuate muscle wasting is probably due to its ability to potently and specifically inhibit proteasome activity [9] through acylation of the N-terminal threonine on the β-5 (‘chymotrypsin-like’) catalytic subunit. There have been no studies on the effect of EGCg on the depression of protein synthesis.
The current study examines the effect of EGCg on protein degradation in myotubes produced by serum starvation, as well as the depression of protein synthesis induced by tumour necrosis factor-α (TNF-α).
Methods
Materials
Foetal calf serum (FCS) and horse serum (HS) were purchased from Invitrogen (Paisley, UK). Dulbecco’s modified Eagle’s medium (DMEM) was from PAA (Somerset, UK). L-[2,6-3H] phenylalanine (sp.act.3.7 TBqmmol−1) was from Perkin Elmer (Cambridge, UK). Mouse monoclonal antibodies to 20S proteasome α-subunits and p42 were from Affiniti Research Products (Exeter, UK), and rabbit polyclonal antibodies to pFoxO3a (S253) and FoxO3a were from Abcam (Cambridge, UK). Tumour necrosis factor (TNF-α) and polyclonal rabbit antibody to mouse β-actin were purchased from Sigma-Aldridge (Dorset, UK). EGCg (Teavigo, >95 % EGCg) was from DSM Nutritional Products Ltd (Heanor, UK). Peroxidase-conjugated sheep antirabbit and antimouse antibodies were purchased from GE Healthcare (Bucks, UK), as were Hybond A nitrocellulose membranes. Enhanced chemiluminescence (ECL) development kits were from Pierce through Thermo Fischer Scientific (Northumberland, UK). Rabbit polyclonal antibodies to pAkt 1/2/3 (S473), mouse monoclonal antibody to Akt 1, rabbit polyclonal antisera to atrogin-1/MAFbx and goat polyclonal antisera to MuRF1 were purchased from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany).
Cell culture
C2C12 myoblasts were passaged in DMEM supplemented with 10 % FCS, 1 % glutamine and 1 % penicillin-streptomycin under an atmosphere of 10 % CO2 in air at 37 °C. When the myoblasts reached about 80 % confluency, they were differentiated into myotubes in DMEM containing 2 % HS, with medium changes every 2 days. Differentiation was complete in 5–7 days, and the myotubes remained viable for a further 4–5 days. For experiments on serum deprivation, serum was removed after complete myotube differentiation. There was no evidence for toxicity of EGCg to myotubes at any of the concentrations employed in this study based on the attachment of the myotubes to the substratum, since even mild toxicity causes major detachment.
Measurement of protein synthesis and degradation in myotubes
This was performed as previously described [7]. Briefly for protein degradation, myotubes were labelled for 24 h with L-[2,6-3H] phenylalanine prior to experimentation, washed extensively in PBS followed by a further incubation for 2 h to allow degradation of short-lived proteins. Myotubes were starved in serum-free DMEM media (±EGCg) for further 24 h while negative control was maintained in 2 % horse serum at all times. Protein degradation was determined over a 24-h period in the presence of 2 mM non-radioactive phenylalanine to prevent reincorporation of radioactivity. The extent of protein degradation was determined over a 24-h period from the radioactivity released into the medium, as a fraction of the total radioactivity (expressed as %) incorporated into the myotubes in control cultures with serum compared with serum-deprived cells. Protein synthesis was determined by the incorporation of L-[2,6-3H] phenylalanine into myotubes over a 4-h period in the presence of TNF-α (±EGCg) as described in the figure legend. Protein synthesis was calculated as the radioactivity incorporated into acid (0.2 M perchloric acid) insoluble material as a percentage [7], compared with control cultures with no serum.
Measurement of proteasome activity
Functional 20S proteasome activity was determined as the “chymotrypsin-like” enzyme activity by the release of 7-amino-4-methylcoumarin (AMC) from the fluorogenic peptide succinyl-LLVY-7-AMC as previously described in detail [10]. Activity was measured in the absence and presence of the specific proteasome inhibitor lactacystin (10 μM) and only lactacystin suppressible activity was considered to be proteasome specific.
Western blotting
Samples of cytosolic protein (10–15 μg) were resolved by 10 % sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) as described [7], at 180 V for approximately ∼ 1 h followed by transference onto 0.45 μm nitrocellulose membranes, which were then blocked with 5 % Marvel in Tris–buffered saline, pH 7.5, at 4 °C overnight. Both primary and secondary antibodies were used at a dilution of 1:1,000. Incubation was either for 1 h at room temperature, or overnight, and development was by ECL. Blots were scanned by densitometer to quantitate differences.
Statistical analysis
Results are expressed as mean values ± s.e.m for at least three replicate experiments. Differences in mean values between groups were determined by one-way ANOVA followed by Tukey-Kramer multiple comparison test and p values <0.05 were considered significant.
Results
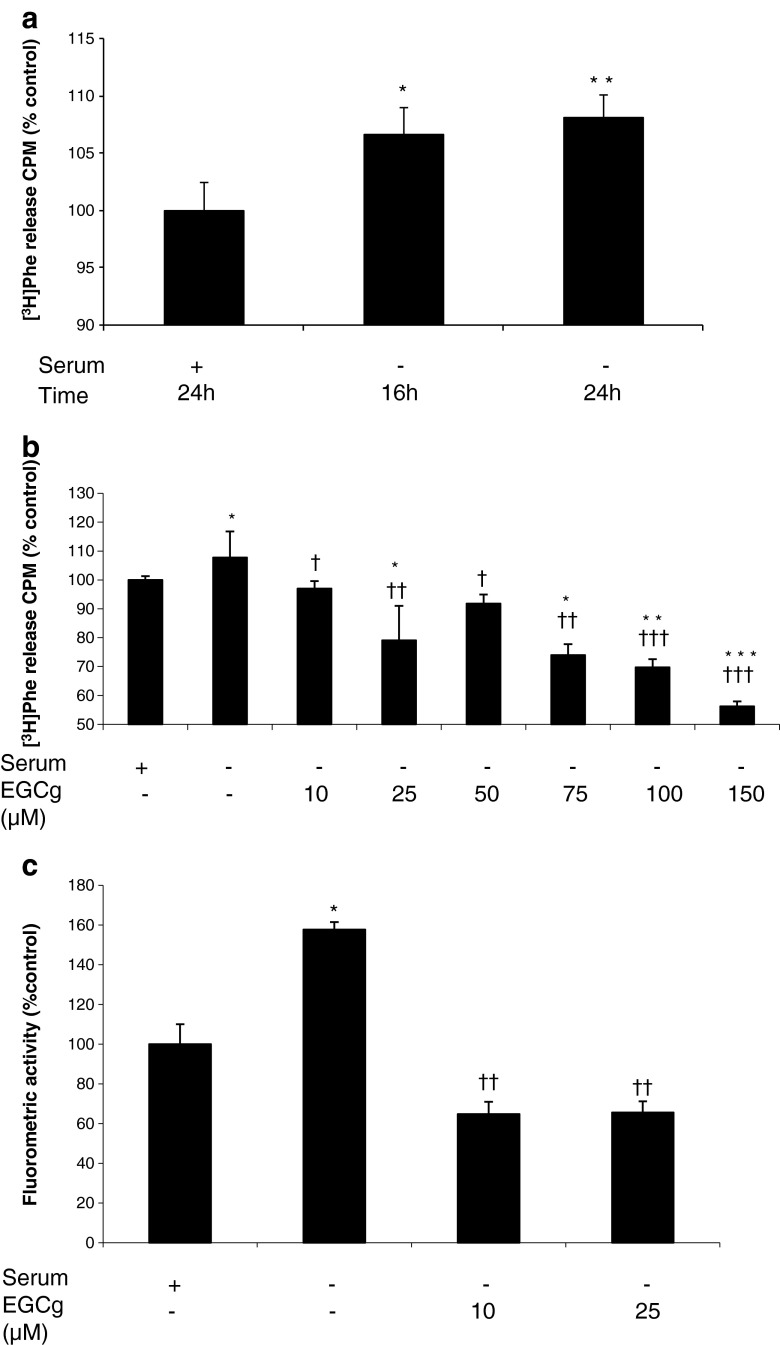
As a model of muscle atrophy in vitro, the C2C12 murine myoblast cell line was induced to differentiate into myotubes and protein degradation was induced by serum starvation. As shown in Fig. 1a, total protein degradation was significantly increased by 16 h serum starvation, and further increased slightly over the following 8 h. All subsequent assays employed 24 h serum starvation to induce protein degradation. The increase in protein degradation achieved by serum starvation was less than that induced by catabolic mediators such as proteolysis-inducing factor (PIF) and angiotensin II (Ang II) [7]. To determine the effect of EGCg on total protein degradation, myotubes were incubated with a wide concentration range (10–150 μM) to determine the most effective concentration that could be used for subsequent studies (Fig. 1b). While low concentrations (10 μM) of EGCg completely attenuated the increased protein degradation induced by serum starvation, higher concentrations further reduced basal levels of protein degradation without having a negative effect on cell viability.
Fig. 1.
a Effect of serum starvation on total protein degradation in C2C12 myotubes after 16 and 24 h, compared with a negative control (NC) incubated with DMEM containing 2 % HS. Control myotubes released 6,563.5 cpm of [3H] Phe over the 24-h period. b Effect of EGCg on total protein degradation in C2C12 myotubes incubated in DMEM without serum for 24 h. c Effect of EGCg on chymotrypsin-like enzyme activity in C2C12 myotubes starved of serum for 24 h. Differences from NC are indicated as * p < 0.05, ** p < 0.01 and *** p < 0.001, while differences from no serum are shown as † p < 0.05, †† p < 0.01 and ††† p < 0.001
The major mechanism for protein degradation in a number of catabolic states is the ubiquitin-proteasome proteolytic pathway [8]. A major proteolytic enzyme in this system is the ‘chymotrypsin-like’ enzyme activity found on the β5 subunit of the proteasome, the activity of which is significantly increased in the absence of serum (Fig. 1c), and attenuated below control values in the presence of EGCg (10 and 25 μM). Serum starvation also increased expression of the proteasome 20S (Fig. 2a) and 19S subunits (Fig. 2b), and also increased expression of the ubiquitin ligases MuRF1 (Fig. 2c) or MAFbx (Fig. 2d). Treatment with EGCg reduced expression of proteasome 20S and 19S subunits down to basal values, while reducing expression of MuRF1 and MAFbx to below the basal levels. Expression of both pAkt (Fig. 2e) and pFoxO3a (Fig. 2f) was increased by EGCg in a dose-related manner, while total Akt and FoxO3a remained unchanged.
Fig. 2.
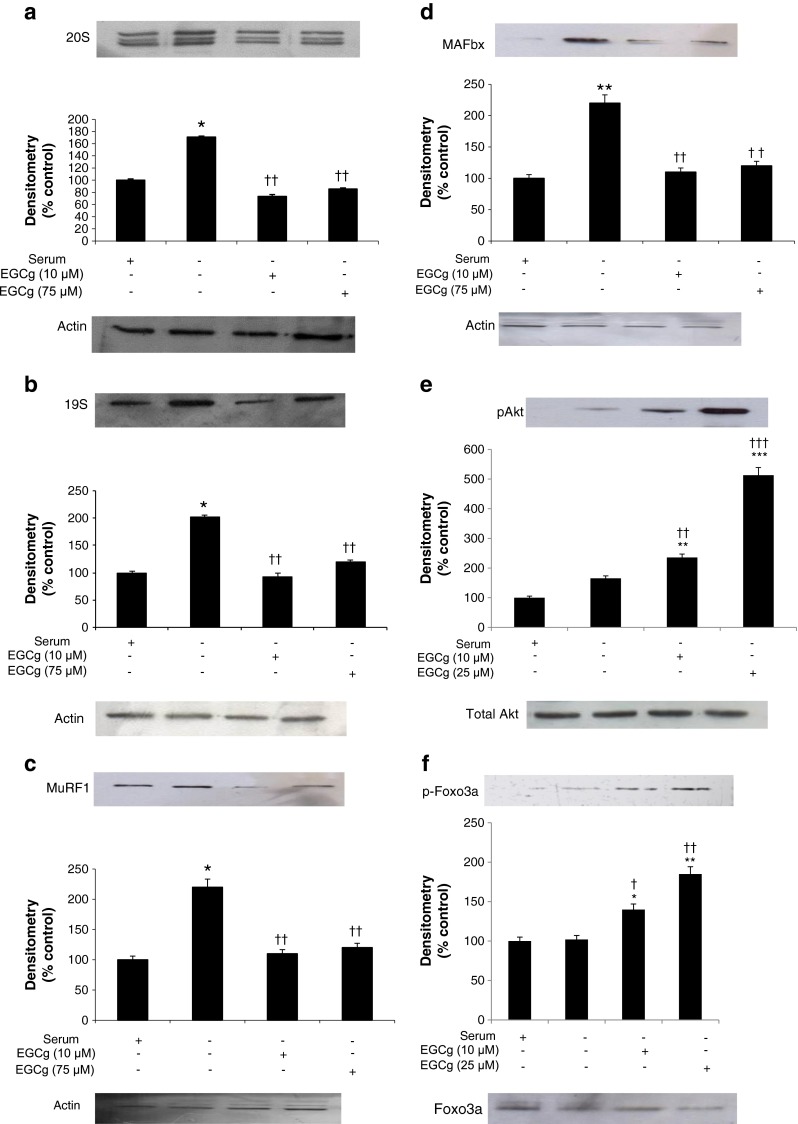
Western blots showing expression of the 20S proteasome (a), 19S proteasome (b) MuRF1 (c), MAFbx (d), with actin as a loading control and pAkt (e) and pFoxo3a (f) with total Akt and FoxO3a as loading controls in C2C12 myotubes after 24 h of serum starvation, with or without EGCg, compared with a negative control (NC) incubated in medium containing serum. The densitometric analysis underneath the blots represents three separate western blots. Differences from NC are shown as * p<0.05 and ** p<0.01 while differences from no serum are shown as † p < 0.05 and †† p < 0.01
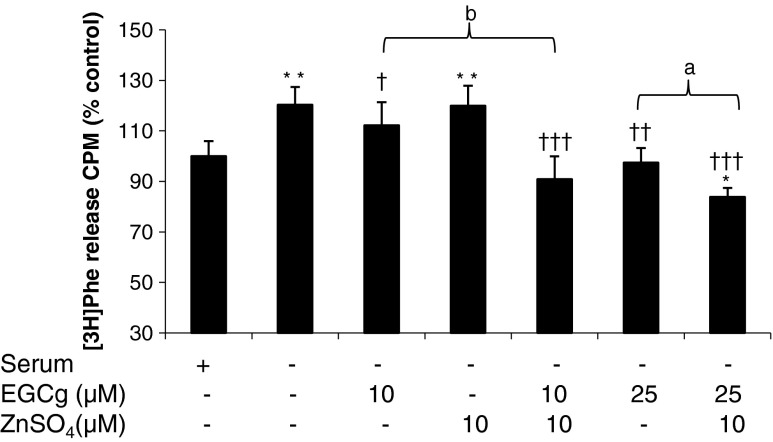
Like other polyphenols, EGCg is a chelating agent, which may remove metal ions such as Zn2+ involved in the increased protein degradation [11]. As shown in Fig. 3, EGCg reduced the increased protein degradation caused by serum deprivation (NS) to values below that seen in the negative control with serum [NC]. In the presence of equimolar concentrations of ZnSO4, protein degradation was further decreased below that of EGCg alone, while Zn2+ alone at this concentration had no effect on protein degradation (Fig. 3). These results suggest that EGCg may act in concert with Zn2+ to reduce protein degradation, possibly through an EGCg-Zn2+ complex.
Fig. 3.
Effect of ZnSO4 on total protein degradation in serum-deprived C2C12 myotubes in the presence of EGCg. Myotubes were incubated in DMEM without serum for 24 h, while the negative control (NC) was in DMEM containing 2 % HS. Differences from NC are shown as * p < 0.05, ** p < 0.01 or *** p < 0.001, while differences from no serum are shown as ††† p < 0.001, and differences between groups as a p<0.05 and b p<0.01
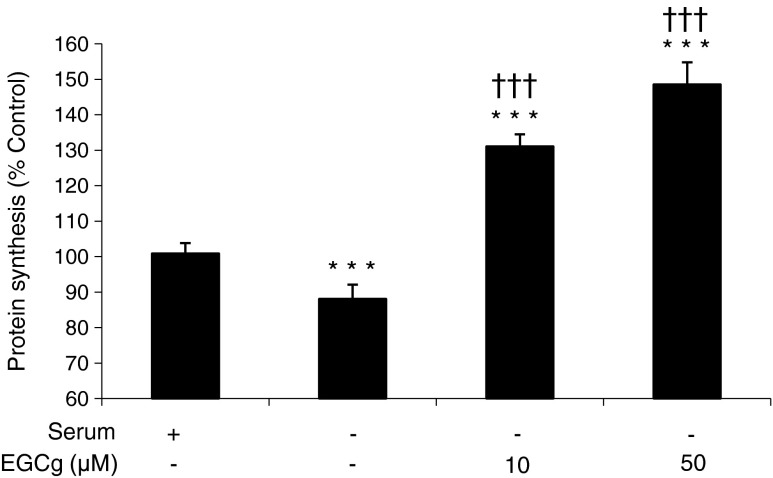
To investigate whether EGCg could reverse the depression of protein synthesis induced by serum deprivation, myotubes were incubated for 4 h in serum-free media in the absence or presence of EGCg (Fig. 4). For both concentrations of EGCg, employed protein synthesis was significantly increased to levels above those in the negative control.
Fig. 4.
Effect of EGCg on protein synthesis in serum-deprived murine myotubes. C2C12 myotubes were incubated in the absence of serum for 4 h, and protein synthesis was measured as described in the methods section. Differences from negative control (NC) are shown as *** p < 0.001, while differences from no serum are shown as ††† p < 0.001
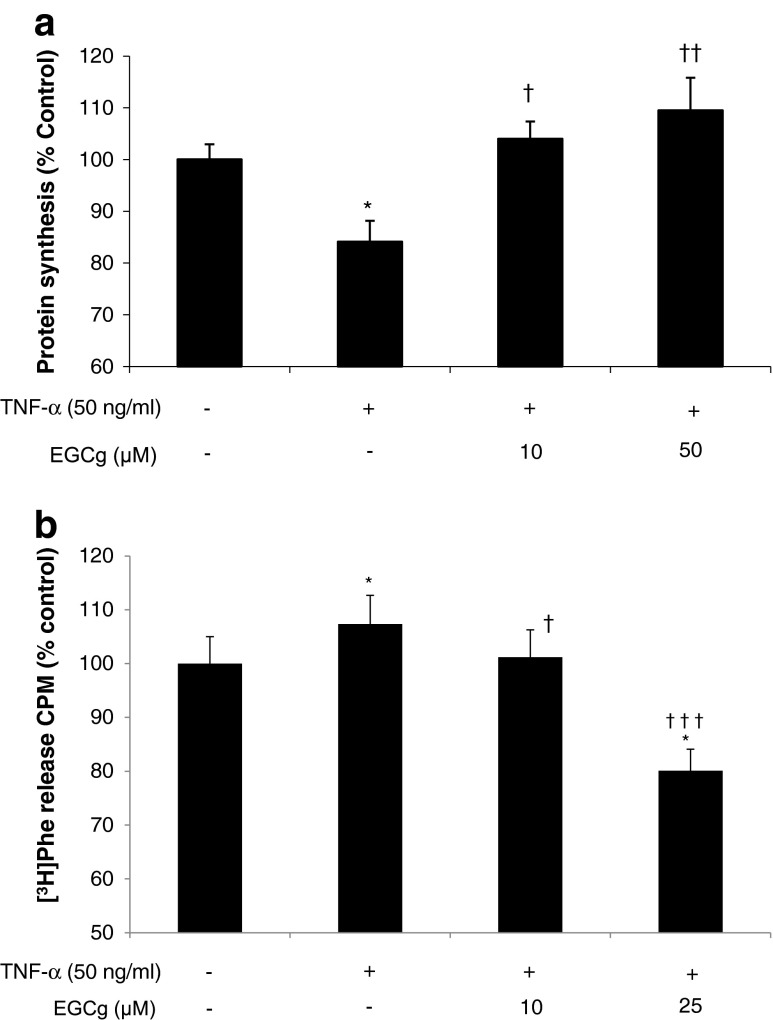
To determine whether EGCg could also overcome the depression of protein synthesis and increase protein degradation caused by inflammatory cytokines, myotubes were exposed to TNF-α (50 ng/ml) for 4 h (Fig. 5a) or 24 h (Fig. 5b) in the absence or presence of EGCg (10 and 50 μM). This concentration of TNF-α was employed because previous studies [12] had indicated that lower concentrations had no significant effect on protein synthesis or degradation in murine myotubes. The results (Fig. 5) show that both concentrations of EGCg attenuated the depression of protein synthesis and increased protein degradation induced by TNF-α, while at 25 μM EGCg reduced protein degradation below basal levels in the negative control (NC).
Fig. 5.
Effect of EGCg on protein synthesis (a) and protein degradation (b) in murine myotubes in the presence of TNF-α. Myotubes were incubated with TNF-α (50 ng/ml) for 2 h in the absence, or presence of EGCg, and protein synthesis was measured over the following 4 h, while protein degradation was measured after 24 h in the presence of TNF-α. Differences from NC are shown as * p < 0.05, while differences from TNFα alone in the presence of EGCg are shown as † p < 0.05, †† p < 0.01 and ††† p < 0.001
Discussion
This study shows that in murine myotubes, EGCg attenuates both the increased protein degradation and depressed protein synthesis induced by serum deprivation and by the inflammatory cytokine TNF-α. The ability of EGCg to counteract the increased protein degradation is linked with its ability to downregulate key components of the ubiquitin-proteasome proteolytic pathway, including the 20S and 19S proteasome subunits, and the ubiquitin ligases (E3) MuRF1 and MAFbx. EGCg has also been shown to significantly reduce expression of MuRF1 and MAFbx when upregulated by 3D clinorotation, but not by dexamethasone [13]. The mechanism by which EGCg downregulates atrogene expression is unclear, but Wang et al. [4] have shown that EGCg decreased expression of NF-κB in muscle of tumour-bearing mice, as well as MuRF1 and MAFbx. Activation of NF-κB has been shown to produce profound muscle wasting through increased expression of proteasome subunits and MuRF1 [14]. At high concentration (100 μM), EGCg has also been shown to stimulate phosphatidyl inositol 3-kinase (PI3K)/Akt, which in turn suppresses forkhead box O (Fox O) activation by phosphorylation, which prevents nuclear accumulation [15]. This study has shown an increase in pAkt and pFoxO in serum starved myotubes in the presence of EGCg, overexpression of FoxO leads to extensive muscle atrophy, and increased expression of MAFbx, but not MuRF1 [16]. The combined effect of inhibiting the activity of NF-κB and FoxO transcriptional activity during cast immobilisation has a combined effect on reducing atrophy gene expression and atrophy [17].
The effect of EGCg on attenuating protein degradation was further enhanced in the presence of ZnSO4. The exact mechanism for this is unclear but there have been previous reports on the synergistic role of EGCg and Zn+2 on inhibiting prostate cancer cell growth [18]. This study postulated an enhanced uptake of EGCg into the cell in the presence of Zn+2. Another study has also demonstrated an enhanced effect of EGCg and Zn on hepatoprotectivity and attributed this to EGCg-Zn complex formation [19].
In addition to attenuating protein degradation induced by serum starvation, EGCg was also effective in attenuating the depression of protein synthesis in myotubes in response to TNF-α and serum starvation. We have shown previously [20] that in this model system, depression of protein synthesis by both lipopolysaccharide (LPS) and TNF-α is due to activation of double-stranded RNA-dependent protein kinase (PKR), with the subsequent phosphorylation of eukaryotic initiation factor 2(eIF2) on the α-subunit, since there is no depression of protein synthesis in myotubes expressing a catalytically inactive PKR variant. Metal ion chelating agents such as D-myo-inositol-1,2,6-triphosphate prevented activation of PKR and the depression of protein synthesis [11]. It is possible that EGCg attenuates the depression of protein synthesis in response to TNF-α via a similar mechanism involving PKR, since EGCg is a strong metal ion chelator.
These results show that EGCg is an effective inhibitor of both the increased protein degradation and depressed protein synthesis in skeletal muscle, and that it may be useful therapeutically in the treatment of conditions of muscle atrophy, such as cancer cachexia, muscle disuse and sarcopenia. This study shows that concentrations of EGCg as low as 10 μM attenuated the depression of protein synthesis and increase in protein degradation in murine myotubes. Average doses of EGCg that have been tested clinically range from 50 to 1,600 mg/day [21]. Maximum absorption occurs in the small intestine, although some can take place through the oral mucosa or the colon. The bioavailability of EGCg is low due to sulphation, glucuronidation and methylation in the liver, with approximately 1 % of the ingested dose appearing in the plasma [22]. Thus at the doses tested in humans, the mean Cmax values ranged from 130 to 3,392 ng/ml [21], giving a maximum plasma concentration of 7.4 μM. However, single oral doses of EGCg up to 1,600 mg/day were safe and well tolerated, suggesting higher doses could be employed and that a plasma concentration of 10 μM is achievable.
Acknowledgments
This work was supported by a grant from Abbott Nutrition, Columbus, OH, USA. Kamran Ali Mirza and Michael J Tisdale declare that they have no conflict of interest. Suzette Pereira and Neile Eden are both employees of Abbott Nutrition, Columbus, OH, USA. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
References
- 1.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, et al. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–44. doi: 10.1016/S1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 3.Tran PL, Kim SA, Choi HS, Yoon JH, Ahn SG. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer. 2010;10:276. doi: 10.1186/1471-2407-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Lai YJ, Chan YL, Li TL, Wu CJ. Epigallocatechin-3-gallate effectively attenuates skeletal muscle atrophy caused by cancer cachexia. Cancer Lett. 2011;305:40–9. doi: 10.1016/j.canlet.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, et al. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–25. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ota N, Soga S, Haramizu S, Yokoi Y, Hase T, Murase T. Tea catechins prevent contractile dysfunction in unloaded murine soleus muscle: a pilot study. Nutrition. 2011;27:955–9. doi: 10.1016/j.nut.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–97. doi: 10.1074/jbc.M610378200. [DOI] [PubMed] [Google Scholar]
- 8.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–37. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 9.Dou QP, Landis-Piwowar KR, Chen D, Huo C, Wan SB, Chan TH. Green tea polyphenols as a natural tumour cell proteasome inhibitor. Inflammopharmacology. 2008;16:208–12. doi: 10.1007/s10787-008-8017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehouse AS, Tisdale MJ. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br J Cancer. 2003;89:1116–22. doi: 10.1038/sj.bjc.6601132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell ST, Siren PM, Siren MJ, Tisdale MJ. Mechanism of attenuation of protein loss in murine C2C12 myotubes by D-myo-inositol 1,2,6-triphosphate. Exp Cell Res. 2010;316:286–95. doi: 10.1016/j.yexcr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-α and angiotensin II by β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1417–26. doi: 10.1152/ajpendo.90567.2008. [DOI] [PubMed] [Google Scholar]
- 13.Hemdan DI, Hirasaka K, Nakao R, Kohno S, Kagawa S, Abe T, et al. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J Med Invest. 2009;56:26–32. doi: 10.2152/jmi.56.26. [DOI] [PubMed] [Google Scholar]
- 14.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Bartholome A, Kampkötter A, Tanner S, Sies H, Klotz LO. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch Biochem Biophys. 2010;501:58–64. doi: 10.1016/j.abb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–23. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 17.Reed SA, Senf SM, Cornwell EW, Kandarian SC, Judge AR. Inhibition of IkappaB kinase alpha (IKKα) or IKKbeta (IKKβ) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem Biophys Res Commun. 2011;18:491–6. doi: 10.1016/j.bbrc.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun SL, He GQ, Yu HN, Yang JG, Borthakur D, Zhang LC, et al. Free Zn2+ enhances inhibitory effects of EGCG on the growth of PC-3 cells. Mol Nutr Food Res. 2008;52:465–71. doi: 10.1002/mnfr.200700172. [DOI] [PubMed] [Google Scholar]
- 19.Kagaya N, Kawase M, Maeda H, Tagawa Y, Nagashima H, Ohmori H, et al. Enhancing effect of zinc on hepatoprotectivity of epigallocatechin gallate in isolated rat hepatocytes. Biol Pharm Bull. 2002;25:1156–60. doi: 10.1248/bpb.25.1156. [DOI] [PubMed] [Google Scholar]
- 20.Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1409–16. doi: 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- 21.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 22.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]