Abstract
In heart failure, impairment of cardiac muscle function leads to numerous neurohormonal and metabolic disorders, including an imbalance between anabolic and catabolic processes, in favour of the latter. These disorders cause loss of muscle mass with structural and functional changes within the skeletal muscles, known as skeletal myopathy. This phenomenon constitutes an important mechanism that participates in the pathogenesis of chronic heart failure. both its clinical symptoms and the progression of the disease. Attempts to reverse the above-mentioned pathologic processes by exploiting the anabolic action of androgenic hormones could provide a potentially attractive treatment option. The current concepts of anabolic androgen deficiency and resultant skeletal myopathy in patients with heart failure are reviewed, and the potential role of anabolic-androgenic hormones as an emerging therapeutic option for targeting heart failure is discussed.
Keywords: Skeletal muscles, Myopathy, Anabolic androgens, Testosterone, Heart failure
Introduction
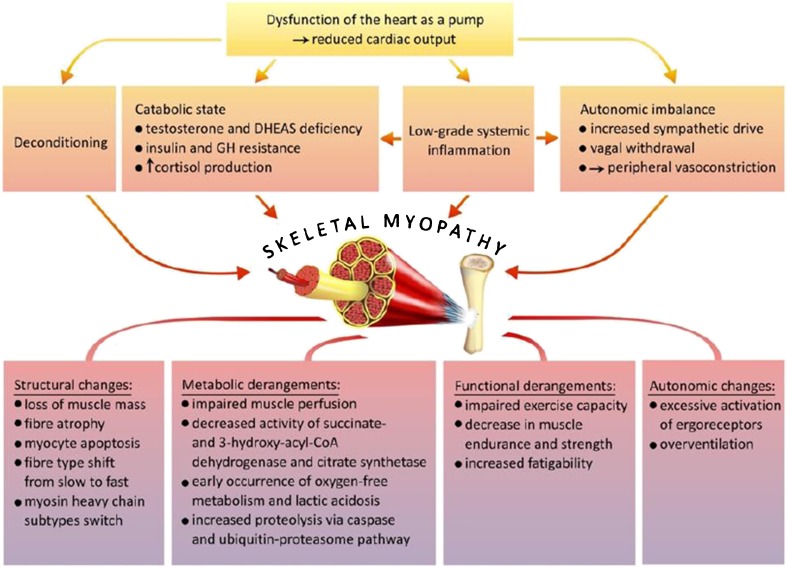
The basic clinical symptom of heart failure lies in an intolerance towards physical effort related with increased fatigability and the occurrence of dyspnoea, while lowered peak oxygen consumption, which is its objective measure, correlates with low quality of life and bad prognosis [1–4]. However, these symptoms do not result directly from the impairment of heart function as a pump or from haemodynamic disorders related with the above. In fact, there is a poor correlation between haemodynamic parameters and clinical symptoms, as well as the degree of functional limitation in patients with chronic heart failure [5]. In recent years, the involvement of the so-called peripheral mechanisms (namely disorders within organs and systems outside the circulatory system) in the pathogenesis of heart failure symptoms has been emphasised [1, 6–8]. As far as this aspect is concerned, the so-called muscle hypothesis is a particularly important concept, since it recognises structural and functional changes within skeletal muscles (i.e. skeletal myopathy) as the main reason for impaired exercise capacity in patients with chronic heart failure [1, 2, 9, 10] (Fig. 1).
Fig. 1.
The muscle hypothesis in heart failure: pathogenesis of skeletal myopathy—modified from [10]
Skeletal myopathy in patients with chronic heart failure
The muscle hypothesis assumes that development of skeletal myopathy constitutes the second crucial mechanism, after neurohormonal activation, that participates in the pathogenesis of chronic heart failure and that it is responsible for the occurrence of the symptoms and progression of the disease. Impairment of cardiac muscle function leads to numerous neurohormonal and metabolic adjustments and compensatory mechanisms, including sympathetic activation, vagal withdrawal, and peripheral vasoconstriction, with the teleological effect of maintaining effective perfusion of vital organs (heart, kidney, brain). These changes are beneficial and life saving in the short term; however, in the long term, they result in deleterious consequences, including an imbalance between anabolic and catabolic processes, in favour of the latter, causing a loss of muscle mass with subsequent development of macroscopic and microscopic disorders within the structure, metabolism and functioning of skeletal muscles [8, 11]. Moreover, a decrease in muscular blood flow has been observed in patients with heart failure, which is related not only with decreased cardiac output, but also with disorders of vascular endothelium functions, decreased volume and capillarisation of skeletal muscles and the maintenance of vasoconstriction caused by increased activity of the sympathetic nervous system [8, 12, 13].
Muscle mass
Structural changes, which can be observed on the macroscopic level, are associated with a loss of muscular mass, especially within lower and upper limbs, and this is particularly visible in patients with cardiac cachexia syndrome [14, 15]. These changes lead to functional disorders, reflected mainly in a decrease in muscle endurance and strength. In patients with chronic heart failure, both the mass of skeletal muscles, as well as muscle strength, constitute significant determinants of physical fitness and capacity, evaluated as peak oxygen consumption, and these relationships are independent of age, New York Heart Association (NYHA) functional class or the level of neurohormonal activation [3, 15, 16]. A decrease in muscle mass is related to a general decrease in the cross section area and is also a consequence of a loss of muscular fibres.
Apoptosis
The degree of skeletal muscle fibre atrophy correlates with the number of cell nuclei displaying features of apoptosis, which suggests that this process might play an important role in muscle mass loss [17]. The phenomenon of apoptosis is almost absent in skeletal muscles in healthy persons, while in experimental models and humans with heart failure, both the activation of intracellular pathways promoting myocyte programmed death (a decrease in the activity of the Bcl-2 antiapoptotic system, an increase in caspase-3 activity and ubiquitin, increased expression of induced NO synthase), as well as an increase in the number of cellular nuclei with apoptotic features, have been observed [17–20]. Apoptosis in skeletal muscles in patients with heart failure is probably triggered by proinflammatory cytokines, in particular, the TNF alpha and its second messenger—sphingosine [21, 22], and its intensity displays a negative correlation with exercise capacity, evaluated by peak oxygen consumption [17, 19]. However, the role of apoptosis in the development of muscle fibre atrophy in heart failure still arouses controversy. The DNA in situ end-labelling technique (terminal deoxynucleotidyl transferase end labelling (TUNEL)) frequently used to detect DNA fragmentation, which is a hallmark of apoptosis, may lack specificity, i.e. false positive TUNEL reactions may be observed in living muscle cells during active gene transcription [23]. Conraads et al. analysed skeletal muscle biopsies of 16 patients with mild-to-moderate chronic heart failure, but they searched for apoptotic myocyte nuclei in a more rigorous way than the authors of previous studies, enhancing the classical TUNEL technique with confirmatory tests. Notably, they did not confirm the presence of apoptosis [23]. These results might, thus, question the role of apoptosis in the pathogenesis of skeletal myopathy, particularly in the early stages of heart failure.
Fibre composition
Structural abnormality characteristics of skeletal myopathy related to heart failure also include qualitative changes within the content of muscular fibres secondary to the changed expression of specific subtypes of heavy myosin chains. These lesions seem to be independent of skeletal muscle atrophy [18]. In addition, they result in a decrease in the number of slow muscle fibres—type I based on oxygen metabolism, with a simultaneous growth in the number of type IIa fast fibres based on oxygen metabolism and type IIb based on oxygen-free metabolism, which is more susceptible to fatigue and more rapidly achieves the anaerobic threshold [17, 20–22, 24]. It is also worth mentioning that structural changes in muscle tissue occurring in the course of chronic heart failure are not limited solely to muscles in the limbs, but they also cover respiratory muscles, which may additionally induce dyspnoea [25].
Metabolic capacity
The above-mentioned structural changes are also associated with biochemical and metabolic disorders expressed in the decreased activity of mitochondrial enzymes participating in the final stages of oxidative phosphorylation, namely succinate dehydrogenase and citrate synthetase, as well as 3-hydroxyacyl-CoA dehydrogenase which plays an important role in the B-oxidation process of fatty acids [26]. These changes are responsible for the early occurrence of an oxygen-free metabolism and the accelerated development of lactic acidosis during muscle activity in patients with heart failure, which leads to a decrease in tolerance of physical effort and contributes to the occurrence of dyspnoea [27].
Muscle reflex (ergoreflex)
Excessive activation of the muscle ergoreceptor is a significant consequence of structural and functional disorders within skeletal muscle levels in patients with heart failure, and this plays a crucial role in the pathogenesis of impaired physical tolerance [28]. These receptors include both a mechanoreceptor (sensitive to passive movements), as well as metaboreceptors stimulated by metabolic products of skeletal muscle work (such as prostaglandin, lactate bradykinin, cations) and through afferent fibres inform the brain stem on the level of muscular activity. Within the reflexive pathway, they increase the activity of the sympathetic nervous system and provoke physiological, ventilatory and haemodynamic adjustments according to the skeletal muscle needs in oxidised blood [29–31]. However, metabolic disorders in active skeletal muscles observed in patients with chronic heart failure evoke extensive activation of the reflex from ergoreceptors, which on the one hand, overstimulate the ventilation response to physical effort, thus contributing to the experience of dyspnoea. On the other hand, this leads to an increase in the activity of the sympathetic system with subsequent generalised vasoconstriction responsible for an increased vasoconstrictive drive in the inactive muscles, the growth of peripheral resistance and a subsequent increase in the afterload of the left ventricle. Moreover, further deterioration of the heart function, as well as a subsequent loss of muscle mass resulting from the increase in catabolic processes, is observed [2, 8]. Activation of muscle ergoreceptors is particularly intensified in patients suffering from cardiac cachexia and generates the greatest loss of muscle mass in the course of heart failure. This suggests that complex disorders within the level of skeletal muscles are responsible for the creation of a vicious circle and reflect the intensification of the disease; and they inevitably lead to the progression of the disease [32].
Therapeutic option
Attempts to reverse the above-mentioned adverse changes within the skeletal muscles in patients with chronic heart failure could form potentially attractive therapeutic alternatives. Currently, the only therapeutic approach with sufficient clinical evidence for counteracting skeletal myopathy in heart failure is exercise training [33]. In clinical and experimental studies, this has been shown to inhibit the pathomechanisms and pathways involved in muscle wasting, such as the overactivation of the ubiquitin-proteasome system, increased oxidative stress, and the overexpression of myostatin and proinflammatory cytokines, leading to an improvement in muscle strength and peak oxygen consumption [33]. Another approach could include involvement of the anabolic activity of androgenic hormones aiming to reconstruct the proper skeletal muscle structure and function. It is well known, mainly due to experiences related with the use of anabolic steroids for doping in sports, that these hormones play a significant role in the quantitative and qualitative regulation of muscle fibre content, leading to increases in muscle mass and strength, as well as improvement in physical capacity [34–36]. It has also been shown that anabolic-androgenic steroid concentrations are strongly correlated with muscle strength and mass, both in the case of healthy persons, as well as in people suffering from chronic diseases [37, 38].
Significance of anabolic-androgenic hormones in patients with chronic heart failure
Three main anabolic hormonal axes, two of which—the adrenal and gonadal axes—are related with the production of anabolic androgens: the adrenal (dehydroepiandrosterone sulphate (DHEAS)) and gonadal androgen (testosterone), respectively, in men. These hormones constitute trophic factors essential for the proper development of mammals during the process of ontogenesis [39]; however, their secretion decreases with age, favouring the development of disadvantageous lesions in ageing men, known as testosterone deficiency syndrome (TDS). This syndrome, apart from a decrease in total testosterone concentration below 3.5 ng/l, is characterised by a drop in muscle mass and strength, decreased bone tissue density, abdominal obesity, as well as a worsened quality of life [40–42]. Although testosterone and dehydroepiandrosterone (DHEA) levels decrease with age, this phenomenon is, however, much faster in men with heart failure than in healthy ones, and this is more evident in younger men (less than 45 years old) [43]. This phenomenon is independent of aetiology (observed both in ischaemic and non-ischaemic heart failure) [43]. In our study, which included 208 men with chronic heart failure due to significant left ventricle systolic dysfunctional deficiency of the anabolic hormones, including adrenal and gonadal androgens, the phenomenon significantly affected the clinical course and prognosis [43]. Testosterone and DHEAS deficiencies correlated with the severity of heart failure symptoms (NYHA functional class), independently of left ventricular ejection fraction (LVEF) and N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) serum concentration. Nevertheless, the observed correlation, although statistically significant, was relatively weak, indicating that androgen deficiency level is one element of a complex hormonal and metabolic syndrome, covering, among others, an imbalance between anabolic and catabolic processes, which develops at an earlier stage and contributes to its further development [43]. Low concentrations of testosterone and DHEAS were also related with increased risk of death, independently of LVEF, NT-proBNP serum concentration, NYHA functional class or glomerular filtration rate. During a 3-year follow-up, the magnitude of the anabolic imbalance, expressed by the number of anabolic axes involved (i.e. somatotropic: growth hormone/insulin-like growth factor-1, adrenal: DHEAS and gonadal: testosterone), was related to the mortality due to cardiac and vascular causes [43].
Anabolic hormone deficiency in patients with CHF is also related with the loss of bone mass, a feature of the cardiac cachexia syndrome, and this favours the occurrence of anaemia and constitutes an independent factor determining the incidence of depression syndromes [44–46]. Low testosterone concentrations in men with CHF are also related with low peak oxygen consumption, independently of age, disease severity and lower limb muscle mass. This relationship seems to be a result of the effect of this hormone on the circulatory system and on peripheral tissues, including the structure and function of skeletal muscles [47].
Effect of androgens on skeletal muscles
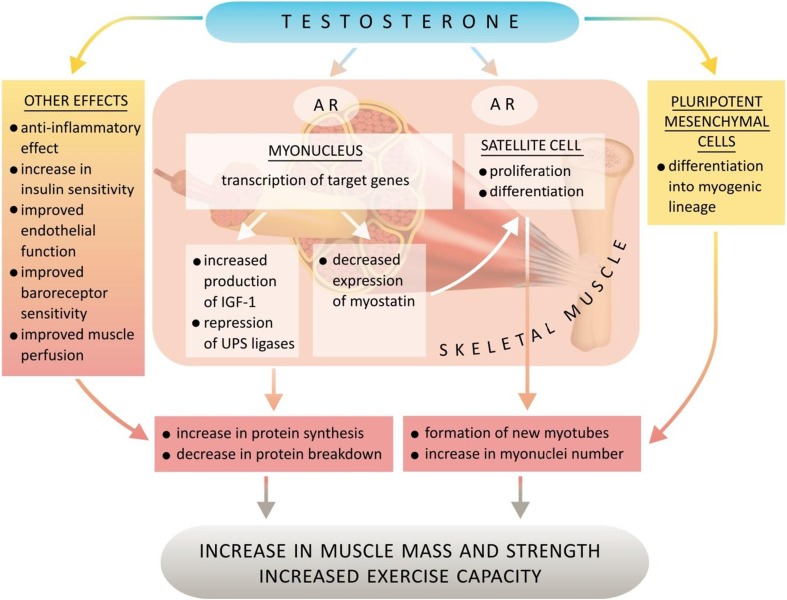
Skeletal muscles contain steroid hormones (testosterone, DHEA/DHEAS, estradiol) and specific androgen and oestrogen receptors, as well as enzymes participating in mutual transformations of these hormones [48–52]. The mechanisms of androgenic hormones action on skeletal muscles are summarised in Fig. 2.
Fig. 2.
Mechanisms of action of anabolic hormones on skeletal muscles. AR androgen receptor, UPS ubiquitin-proteasome system)
Muscle structure
The results of several meta-analyses concerning randomised placebo-controlled trials performed in middle-aged and senile men suffering from hypogonadism or testosterone concentrations close to the lower standard border indicate that testosterone therapy increases muscle mass and improves the muscle strength of the upper and lower limbs and the trunk [37, 53, 54]. Similar results have been observed when synthetic testosterone derivatives, which are characterised by stronger anabolic activity, are used in men with cachexia in the course of severe chronic diseases, such as AIDS and COPD [55, 56]. Testosterone replacement therapy has also been proved to increase muscle mass in women with androgen deficiency due to hypopituitarism [57]. Testosterone influences androgen receptors and induces the hypertrophy of type I and II muscular fibres; it also increases the number of active satellite cells in skeletal muscles, as well as promotes the differentiation of pluripotent mesenchymal cells towards muscle cellular lines, simultaneously hampering adipogenesis [52, 58, 59]. The positive effect of testosterone on satellite cells which play an important role in muscle growth and repair processes may also be related to the negative regulation of myostatin expression. In an animal experimental model, testosterone also decreased the messenger (m)RNA for myostatin—a protein from the transforming growth factor beta superfamily inhibiting satellite cell activation and, thus, being an autocrine, strong inhibitor of muscle fibre growth and development [60]. Trophic effects of testosterone on skeletal muscles also include a negative influence on ubiquitin-proteasome system (UPS) activity, a system responsible for most proteolysis in cells. Testosterone largely represses expression of atrogin-1 and Murf-1, which are ubiquitin ligases involved in the regulation of the UPS activity and specifically upregulated in muscle atrophy, thus inhibiting muscle proteolysis [61].
Muscle metabolism
Detailed mechanisms of the above-mentioned positive testosterone influence on the structure and function of skeletal muscles are not fully known; however, it is highly likely that the major part of this activity results from stimulation of the skeletal muscle local insulin-like growth factor (IGF) system [50, 51]. Testosterone increases the level of insulin-like growth factor-1 (IGF-1), stimulating its production by hepatocytes, as well as locally within skeletal muscles by muscle and satellite cells [62]. In young healthy men, pharmacological suppression of gonad activity simultaneously leads to a decrease in mRNA expression for IGF-1 in skeletal muscles, together with a significant reduction of peripheral blood testosterone concentrations and an associated muscle mass reduction [63]. IGF-1, as it is a pluripotent trophic factor, stimulates proliferation, differentiation processes and protein synthesis within skeletal muscles. IGF-1 probably also mediates testosterone action towards UPS activity. It activates a phosphatidyloinositol-3 kinase (PI3K)/Akt pathway leading to phosphorylation of a FOXO3a transcription factor and a subsequent decrease in atrogin-1 expression [61]. Due to the above, activation of the local system of this factor in skeletal muscles leads to a decrease in proteolysis and an increase in protein synthesis and counters apoptosis and muscle atrophy [50, 51, 64, 65]. It is worth mentioning that IGF-1 participates not only in the regeneration of existing skeletal muscles, but also in the recruitment, activation, proliferation and final differentiation of progenitor cells [64, 65].
Anti-inflammatory effect
Excessive activation of inflammatory processes during the course of heart failure has a hampering influence on the IGF system in skeletal muscles [66, 67]. Testosterone has anti-inflammatory properties, expressed by the reduction of proinflammatory cytokines, including interleukin-1β, interleukin-6 and TNF-α, by circulating monocytes and by simultaneous stimulation of anti-inflammatory interleukin-10 secretion from CD4+ lymphocytes [68, 69]. Hence, anti-inflammatory testosterone activity leads to an inhibition of the inflammatory-related catabolic effect, with simultaneous improvement in muscle sensitivity to the anabolic activity of the IGF system.
Insulin sensitivity
Testosterone and DHEA improve the insulin sensitivity of peripheral cells, among others by increasing translocation of type 1 and 4 glucose transporters into cell membranes, which may be partially responsible for the enhanced physical capability of men with proper levels of these hormones [70–72]. Insulin resistance, leading to impairment of glucose transportation into muscle cells and as a result causing impairment of the effectiveness of myocyte contraction energetic and increased muscle tiredness, which means a deterioration of tolerance to physical effort, is quite a significant element of metabolic disorders observed in patients with CHF [73]. Decreased insulin sensitivity is also related with a proteolysis increase within caspase and ubiquitin-proteasome pathway activating mechanisms, which as a consequence leads to muscle protein degradation, and then to muscle atrophy and weakening [74]. Results of studies performed on animal models and also those performed in men with hypogonadism indicate the existence of a relationship between androgen deficiency and insulin resistance in men [72, 73, 75].
Other effects
The beneficial influence of testosterone on the functioning of skeletal muscles and physical capability in men suffering from CHF may also partially result from its other qualities. These may include an improvement of vascular endothelium function and the biomechanics of respiratory muscles. In addition, it may be possible to observe an increase in heart rate variability and an improvement of baroreceptor sensitivity, together with a degree of influence on the intracellular calcium metabolism, as well as vasodilatation activity, generating an improvement in perfusion within the pulmonary vascular bed and in systemic circulation within the scope of skeletal muscles [76–80].
Determinants of androgen activity
When analysing the effect of anabolic androgens on skeletal muscles, it is essential to take into consideration the fact that the observed results relating to the activity of these hormones on target tissues do not necessarily have to directly depend on their peripheral blood concentration. The assumption that the circulatory pool of a given hormone is proportional to its concentrations obtained in target organs seems to be an excessive simplification. Transfer of the hormone from the circulating pool into the peripheral tissues depends not only on the gradient of concentrations between these compartments, but also on carrier proteins. Additionally, peripheral tissues, including skeletal muscles, contain local hormonal systems, where steroid hormones are metabolised and synthesised from their precursors. Both 3- and 17β-hydroxysteroid dehydrogenase transforming DHEA collected from circulating blood into testosterone and aromatase converting testosterone into oestrogens have been observed in skeletal muscles [48]. These issues require further research. Peripheral tissue response to hormone activity also depends on the sensitivity of specific receptors, which is genetically conditioned. CAG or GGN nucleotide polymorphisms in the androgen receptor gene may affect the influence of testosterone on skeletal muscles [81, 82]. For example, in the case of CAG polymorphism, the smaller the number of CAG sequence repetitions, the greater the ability of androgen receptor transactivation [83]. Further research on steroid hormone metabolism in peripheral tissues and skeletal muscles are also required, as it cannot be excluded that certain beneficial testosterone or DHEAS effects result from their aromatisation to oestrogens and the true activity by interaction with oestrogen receptors [84]. Androgen aromatisation occurring within skeletal muscles may constitute a significant source of the amount of oestrogens circulating in men [85].
Testosterone—an emerging alternative for skeletal myopathy treatment in patients with CHF?
Therapy with testosterone or its analogues has previously been used in severe ailments associated with cachexia, such as AIDS, chronic obstructive pulmonary disease or severe burns; in the case of which, the anabolic activity of these hormones was reflected in an increase in muscle mass and strength [38, 86, 87]. There are few reports concerning the use of testosterone in men suffering from chronic heart failure, and to date there are no data on DHEA activity. The first study performed by Pugh et al. comprised 20 men with symptomatic heart failure (HF) and demonstrated that treatment with testosterone for 3 months improved walking distance, but there were no effects on muscle strength or body weight [88]. Malkin et al. evaluated the influence of a 12-month-long testosterone therapy on the form of the transdermal system and observed a prolongation of marching distance, an increase in muscle strength and an improvement of NYHA functional class compared to controls. Apart from burdensome skin reactions, no significant adverse events were observed [89]. In another study, the same authors evaluated the influence of a 4-week treatment with a combination of testosterone esters on insulin resistance, and they reported its improvement, as well as an increase in overall body mass and a reduction of fatty tissue mass in a group treated with testosterone in comparison to the group treated with a placebo [90]. In the largest research project which has so far been performed, Caminiti et al. evaluated the results of a 12-week-long treatment with testosterone of prolonged activity, administered intramuscularly in a group of 70 older men (35 in the group of active treatment and 35 in placebo group) with systolic HF. The project demonstrated considerable improvements in peak oxygen consumption and quadriceps muscle strength, insulin sensitivity and baroreceptor sensitivity within the group treated with testosterone. No effect on the left ventricle ejection fraction was observed, which would suggest that a positive influence of testosterone on physical capability depended on the influence of peripheral mechanisms [91]. The same research team performed the first and so far only randomised placebo-controlled study assessing the effects of a 6-month transdermal testosterone supplementation on functional capacity and insulin resistance in women with chronic HF [92]. The trial, comprising 36 elderly women, demonstrated a significant increase in 6-min walking distance (similar to that observed in the above-mentioned study of men), peak oxygen consumption and muscle strength, as well as a decline in insulin resistance in the active treatment arm [92].
Results of the above-mentioned studies, although inevitably promising, are still perceived only as “hypothesis-generating” studies, due to the small study populations treated with testosterone. Only large prospective randomised controlled clinical trials will be able to determine the prognostic benefits, as well the influence on skeletal muscles and physical capability, resulting from testosterone therapy in patients suffering from CHF. Further studies addressing this issue are particularly needed in female patients, because, although androgens also decline with age, their pathophysiological effects on cardiovascular systems in women remain controversial. It is also worth mentioning that in the above-mentioned studies, testosterone was administered in subjects with HF, regardless of whether or not there was an initial deficiency of this hormone. Nonetheless, testosterone in men and in women with an initial deficiency of this hormone is much more beneficial. In the study by Caminiti et al., a greater improvement of peak oxygen consumption and muscle strength was observed in patients with low initial testosterone concentrations [91]. Similarly, a more marked increase in 6-min walking distance was found by Iellamo et al. in women with lower baseline testosterone levels than in those with normal levels at the initiation of testosterone therapy [92]. In the case of hormones, there usually exists a clear U-shaped dependence between the peripheral blood concentration of a given hormone and clinical results of its activity, which means that there is a range of optimal values concerning the peripheral blood concentration of this hormone and exceeding this range, both towards surplus or deficiency, would be related with a deterioration of clinical parameters. In relation to the above, the correction of testosterone deficiency should most probably be characterised by an increased safety profile in comparison to administering this hormone to patients with its proper initial level. Experiences related with the use of anabolic-androgen steroids in sport doping indicate the harmfulness of supraphysiological doses and concentrations of these hormones. Long-term usage of this type of pharmacological support may lead, inter alia, to mood changes, development of proatherogenic lipid profiles, increases in thrombotic and inflammatory processes and impairment of vascular endothelium functions. It may also increase the risk of sudden cardiac death and myocardial infarction [93–95]. In the studies performed so far on patients with heart failure, testosterone administration at replacement levels, i.e. much smaller doses, has been shown to be well tolerated with no significant adverse effects [87, 88, 90, 91]. Notably, such a therapy seems also to be safe in elderly women [92].
Conclusion
Anabolic deficiency in patients with chronic heart failure results in skeletal myopathy which constitutes an important mechanism in heart failure pathogenesis and favours progression of the disease. Therefore, treatment with testosterone aimed at counteracting anabolic imbalance and reconstructing the proper skeletal muscle structure and function could provide a potentially attractive option for patients with heart failure.
Several aspects, including safety, modality of administration and dosages, should be addressed by further investigations before these novel therapeutic options will be able to enter daily clinical practice.
Acknowledgments
All the authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Conflict of interest
Krystian Josiak, Ewa Anita Jankowska, Massimo F. Piepoli, Waldemar Banasiak and Piotr Ponikowski declare that they have no conflict of interest.
References
- 1.Al C, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Ponikowski P, Clark AL, et al. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–1056. doi: 10.1016/S0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 3.Cicoira M, Davos CH, Francis DP, et al. Prediction of mortality in chronic heart failure from peak oxygen consumption adjusted for either body weight or lean tissue. J Card Fail. 2004;10:421–462. doi: 10.1016/j.cardfail.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 4.De Groote P, Dagorn J, Soudan B, et al. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43:1584–1589. doi: 10.1016/j.jacc.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Lipkin DP, Canepa-Anson R, Stephens MR, Poole-Wilson PA. Factors determining symptoms in heart failure: comparison of fast and slow exercise test. Br Heart J. 1986;55:439–445. doi: 10.1136/hrt.55.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker SD, Coots AJ. Metabolic, functional, and hemodynamic staging for CHF? Lancet. 1996;348:1530–1531. doi: 10.1016/S0140-6736(05)66163-6. [DOI] [PubMed] [Google Scholar]
- 7.Coats AJS. What causes the symptoms of heart failure? Heart. 2001;86:574–578. doi: 10.1136/heart.86.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coats AJS, Clark AL, Piepoli M, et al. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72(Suppl):536–539. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coats AJS. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol. 1996;28:2255–2262. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- 10.Piepoli MF, Coats AJS. The ‘skeletal muscle hypothesis in heart failure’ revised. Eur Heart J. 2013;34:486–488. doi: 10.1093/eurheartj/ehs463. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.CIR.96.2.526. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JR, Wiener DH, Fink LI, Ferraro N. Vasodilatatory behavior of skeletal muscle arteriole in patients with nonedematous chronic heart failure. Circulation. 1986;74:775–779. doi: 10.1161/01.CIR.74.4.775. [DOI] [PubMed] [Google Scholar]
- 13.Zelis R, Nellis S, Longhust J, et al. Abnormalities in the regional circulations accompanying congestive heart failure. Prog Cardiovasc Dis. 1975;18:181–199. doi: 10.1016/0033-0620(75)90010-9. [DOI] [PubMed] [Google Scholar]
- 14.Anker SD, Swan JW, Volterrani M, et al. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18:259–269. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- 15.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/S0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Omiya K, Yamada S, et al. Relations between strength and endurance of leg skeletal muscle and cardiopulmonary exercise testing parameters in patients with chronic heart failure. J Cardiol. 2004;43:59–68. [PubMed] [Google Scholar]
- 17.Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84:431–437. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vescovo G, Zennaro R, Sandri M, et al. Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. J Moll Cell Cardiol. 1998;30:2449–2459. doi: 10.1006/jmcc.1998.0807. [DOI] [PubMed] [Google Scholar]
- 19.Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959–965. doi: 10.1016/S0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 20.Vescovo G, Ambrosio GB, Dalla Libera L. Apoptosis and changes in contractile protein pattern in the skeletal muscle in heart failure. Acta Physiol Scand. 2001;171:305–310. doi: 10.1046/j.1365-201x.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Libera LD, Vescovo G. Muscle wastage in chronic heart failure, between apoptosis, catabolism and altered anabolism: a chimaeric view of inflammation? Curr Opin Clin Nutr Metab Care. 2004;7:435–441. doi: 10.1097/01.mco.0000134374.24181.5b. [DOI] [PubMed] [Google Scholar]
- 22.Vescovo G, Dalla Libera L. Skeletal muscle apoptosis in experimental heart failure: the only link between inflammation and skeletal muscle wastage? Curr Opin Clin Nutr Metab Care. 2006;9:416–422. doi: 10.1097/01.mco.0000232902.97286.35. [DOI] [PubMed] [Google Scholar]
- 23.Conraads VM, Hoymans VY, Vermeulen T, et al. Exercise capacity in chronic heart failure patients is related to active gene transcription in skeletal muscles and not apoptosis. Eur J Cardiovasc Prev Rehabil. 2009;16:325–332. doi: 10.1097/HJR.0b013e3283244436. [DOI] [PubMed] [Google Scholar]
- 24.Vescovo G, Serafini L, Facchin L, et al. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsay DC, Lovegrove CA, Dunn M, et al. Histological abnormalities of diaphragmatic muscle may contribute to dyspnea in heart failure. Circulation. 1992;86(Suppl.I):1–514. [Google Scholar]
- 26.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.CIR.81.2.518. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT, Janicki JS. Lactate production during maximal and submaximal exercise in patients with chronic heart failure. J Am Coll Cardiol. 1985;6:717–724. doi: 10.1016/S0735-1097(85)80472-1. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Chua TP, Francis D, et al. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 29.Scott AC, Wensel R, Davos CH, et al. Skeletal muscle reflex in heart failure patients: role of hydrogen. Circulation. 2003;107:300–306. doi: 10.1161/01.CIR.0000042704.37387.29. [DOI] [PubMed] [Google Scholar]
- 30.Scott AC, Wensel R, Davos CH, et al. Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on ACE-inhibitor therapy for chronic heart failure. Eur Heart J. 2004;25:1806–1813. doi: 10.1016/j.ehj.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Mark AL, Victor RG, Nerhed C, Walkin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 32.Piepoli MF, Kaczmarek A, Darrel P, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114:126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]
- 33.von Haehling S, Steinbeck L, Doehner W, et al. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol. 2013;45:2257–2265. doi: 10.1016/j.biocel.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 35.Bahrke MS, Yesalis CE. Abuse of anabolic androgenic steroids and related substances in sport and exercise. Curr Opin Pharmacol. 2004;4:614–620. doi: 10.1016/j.coph.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Kadi F. Cellular and molecular mechanism responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154:522–528. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, et al. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54:1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casaburi R, Bhasin S, Cosntino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 39.de Delenmarre-van de Waal HA, van Coeverden SC, Rotteveel J. Hormonal determinants of pubertal growth. J Pediatr Endocrinol Metab. 2001;14(suppl 6):1521–1526. [PubMed] [Google Scholar]
- 40.Nieschlag E, Swerdloff R, Behere HM, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM and EAU recommendations. Int J Androl. 2005;28:125–127. doi: 10.1111/j.1365-2605.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 41.Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 42.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 43.Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact n survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 44.Anker SD, Sharma R. The syndrome of cardia cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/S0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 45.Jankowska EA, Jakubaszko J, Cwynar A, et al. Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail. 2009;11:28–38. doi: 10.1093/eurjhf/hfn004. [DOI] [PubMed] [Google Scholar]
- 46.Jankowska EA. Znaczenie kliniczne i prognostyczne niedoboru hormonów anabolicznych u mężczyzn z niewydolnością serca [dissertation]. Akademia Medyczna we Wrocławiu 2008
- 47.Jankowska EA, Filippatos G, Ponikowska B, et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Card Fail. 2009;15:442–450. doi: 10.1016/j.cardfail.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Aizawa K, Iemitsu M, Maeda S, et al. Expression of steroidogenic enzymes and synthesis of sex steroid hormones from DHEA in skeletal muscle of rats. Am J Physiol Endorinol Metab. 2007;292:E577–E584. doi: 10.1152/ajpendo.00367.2006. [DOI] [PubMed] [Google Scholar]
- 49.Labrie F, Belanger A, Luu-The V, et al. DHEA ad the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 50.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 51.Urban RJ, Bodenberg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 52.Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- 54.Isidori AM, Giannetta E, Greco E, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira IM, Verreschi IT, Nery LE. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest. 1998;114:19–28. doi: 10.1378/chest.114.1.19. [DOI] [PubMed] [Google Scholar]
- 56.Strawford A, Barbieri T, Neesa R, et al. Effects of nandrolone decanoate therapy in borderline hypogonadal men with HIV-associated weight loss. J Acquir Immune Defic Syndr Hum Retroviral. 1999;20:137–146. doi: 10.1097/00042560-199902010-00005. [DOI] [PubMed] [Google Scholar]
- 57.Miller K, Biller B, Beauregard C, et al. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2006;91:1683–1690. doi: 10.1210/jc.2005-2596. [DOI] [PubMed] [Google Scholar]
- 58.Bhasin S, Calof O, Strorer T, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhasin S, Taylo WE, Singh R, et al. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A: Biol Med Sci. 2003;58:M1103–M1110. doi: 10.1093/gerona/58.12.M1103. [DOI] [PubMed] [Google Scholar]
- 60.Mendler L, Baka Z, Kovacs-Simon A, Dux L. Androgens negatively regulate myostatin expression in an androgen-dependent skeletal muscle. Biochem Biophys Res Commun. 2007;361:237–242. doi: 10.1016/j.bbrc.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Pires-Oliveira M, Margano AL, Perreiros-e-Silva LT, et al. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen sensitive rat skeletal muscle in vivo. J Appl Physiol. 2010;108:266–273. doi: 10.1152/japplphysiol.00490.2009. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y, Zhao W, Zhao J, et al. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology. 2007;148:2984–2993. doi: 10.1210/en.2006-1653. [DOI] [PubMed] [Google Scholar]
- 63.Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 64.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytkine Growth Factor Rev. 2006;17:305–323. doi: 10.1016/j.cytogfr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Celemin L, Pasko N, Blomant V, Thissen JP. Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2002;283:E1279–E1290. doi: 10.1152/ajpendo.00054.2002. [DOI] [PubMed] [Google Scholar]
- 67.Schulze PC, Gielen S, Adams V, et al. Muscular levels of proinflammatory cytokines correlate with a reduced expression f insulin-like growth factor-I in chronic heart failure. Basic Res Cardiol. 2003;98:267–274. doi: 10.1007/s00395-003-0411-1. [DOI] [PubMed] [Google Scholar]
- 68.Corrales JJ, Almeida M, Burgo R, et al. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-resenting cells i aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189:595–604. doi: 10.1677/joe.1.06779. [DOI] [PubMed] [Google Scholar]
- 69.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 70.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 71.Perrini S, Natalicchio A, Laviola L, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- 72.Simon P, Charles MA, Lahlou N, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-mont randomized placebo-controlled trial. Diabetes Care. 2001;24:2149–2151. doi: 10.2337/diacare.24.12.2149. [DOI] [PubMed] [Google Scholar]
- 73.Doehner W, Rauchhaus M, Ponikowski P, et al. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Hu Z, Hu J, et al. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 75.Muthusamy T, Dhevika S, Murugeson P, Balasubramanian K. Testosterone deficiency impairs glucose oxidation through defective insulin and receptor gene expression in target tissues of adult male rats. Life Sci. 2007;81:534–542. doi: 10.1016/j.lfs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci. 2007;28:263–270. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Svartberg J, Schirmer H, Medbo A, et al. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromso study. Eur J Epidemiol. 2007;22:107–112. doi: 10.1007/s10654-006-9095-9. [DOI] [PubMed] [Google Scholar]
- 78.El-Mas MM, Aify EA, Mohy El-Din MM, et al. Testosterone facilitates the baroreceptor control o reflex bradycardia: role of cardiac sympathetic and parasympathetic compartments. J Cardiovasc Pharmacol. 2001;38:754–763. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 79.Jaimovich E, Espinosa A. Possible link of different slow calcium signals generated by membrane potential and hormones to differential gene expression in cultured muscle cells. Biol Res. 2004;37:625–633. doi: 10.4067/S0716-97602004000400018. [DOI] [PubMed] [Google Scholar]
- 80.Jones RD, Jones TH, Channer KS. The influence of testosterone upon vascular reactivity. Eur J Endocrinol. 2004;151:29–37. doi: 10.1530/eje.0.1510029. [DOI] [PubMed] [Google Scholar]
- 81.Lapauw B, Goemaere S, Crabbe P, et al. Is the effect of testosterone on body composition modulated by the androgen receptor gene CAG repeat polymorphism in elderly men? Eur J Endocrinol. 2007;156:395–401. doi: 10.1530/EJE-06-0607. [DOI] [PubMed] [Google Scholar]
- 82.Lundin KB, Giwercman A, Dizeyi N, Giwercman YL. Functional in vitro characterization of the androgen receptor GGN polymorphism. Mol Cell Endocrinol. 2007;264:184–187. doi: 10.1016/j.mce.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Zitzmann M, Nieschlag E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl. 2003;26:76–83. doi: 10.1046/j.1365-2605.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- 84.Lombardi G, Zarilli S, Colao A, et al. Estrogens and health in males. Mol Cel Endocrinol. 2001;178:51–55. doi: 10.1016/S0303-7207(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 85.Larinov AA, Vasyliev DA, Mason J, et al. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol. 2003;84:485–492. doi: 10.1016/S0960-0760(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 86.Coodley GO, Coodley MK. A trial of testosterone therapy for HIV-associated weight loss. AIDS. 1997;11:1347–1352. doi: 10.1097/00002030-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–810. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pugh PJ, Jones RD, West JN, et al. Testosterone treatment for men with chronic heart failure. Heart. 2004;90:446–447. doi: 10.1136/hrt.2003.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 90.Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur JHeart Fail. 2007;9:44–50. doi: 10.1016/j.ejheart.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional capacity, skeletal muscle performance, and baroreflex sensitivity in elderly patients with chronic heart failure. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 92.Iellamo F, Volterrani M, Calminti G, et al. Testosterone therapy in women with chronic heart failure. A pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol. 2010;56:1310–1316. doi: 10.1016/j.jacc.2010.03.090. [DOI] [PubMed] [Google Scholar]
- 93.Bronson FH, Matherre CM. Exposure to anabolic-androgenic steroids shortens life span of ale mice. Med Sci Sports Exerc. 1997;29:615–619. doi: 10.1097/00005768-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 95.Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41:1–15. doi: 10.1016/S0033-0620(98)80019-4. [DOI] [PubMed] [Google Scholar]