Abstract
Background
Anamorelin HCl (ANAM) is a novel, orally active, ghrelin receptor agonist in clinical development for the treatment of cancer cachexia. We report in vitro and in vivo studies evaluating the preclinical pharmacologic profile of ANAM.
Methods
Fluorescent imaging plate reader and binding assays in HEK293 and baby hamster kidney cells determined the agonist and antagonist activity of ANAM, and its affinity for the ghrelin receptor. Rat pituitary cells were incubated with ANAM to evaluate its effect on growth hormone (GH) release. In vivo, rats were treated with ANAM 3, 10, or 30 mg/kg, or control orally, once daily for 6 days to evaluate the effect on food intake (FI) and body weight (BW), and once to assess GH response. In pigs, single (3.5 mg/kg) or continuous (1 mg/kg/day) ANAM doses were administered to assess GH and insulin-like growth factor (IGF-1) response.
Results
ANAM showed significant agonist and binding activity on the ghrelin receptor, and stimulated GH release in vitro. In rats, ANAM significantly and dose-dependently increased FI and BW at all dose levels compared with control, and significantly increased GH levels at 10 or 30 mg/kg doses. Increases in GH and IGF-1 levels were observed following ANAM administration in pigs.
Conclusion
ANAM is a potent and highly specific ghrelin receptor agonist with significant appetite-enhancing activity, leading to increases in FI and BW, and a stimulatory effect on GH secretion. These results support the continued investigation of ANAM as a potential treatment of cancer anorexia-cachexia syndrome.
Keywords: Anamorelin HCl, Ghrelin receptor agonist, Cancer anorexia-cachexia syndrome, Body weight, Growth hormone
Introduction
Cancer anorexia-cachexia syndrome (CACS) was recently defined by an international expert consensus group as “a multifactorial syndrome characterized by an ongoing loss of skeletal mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment” [1]. CACS has severe consequences, such as reduction of treatment tolerance, reduction of response to therapy, and shortened survival, and can adversely affect a person’s quality of life [1–6]. The pathogenesis of CACS is complex and not completely understood, but altered metabolism, and reduced food intake contribute to loss of muscle mass and decrease in body weight [7–9].
Ghrelin is an octanoylated 28-amino acid peptide gastric hormone with widespread peripheral expression and is the endogenous ligand for the ghrelin receptor (formally known as the growth hormone [GH] secretagogue type 1α receptor) [10]. The effects of ghrelin on signaling nutrient availability to the central nervous system, upregulating food intake, lowering energy expenditure, and stimulating GH release are relevant in the management of diseases such as CACS. In patients with CACS, elevated levels of ghrelin are often observed [11, 12] perhaps as a physiological response to the decrease of body weight and food intake.
The administration of ghrelin in animal studies has resulted in an improvement in food intake, body weight, and lean body mass (LBM) retention [13, 14]. Several clinical trials in human cancer patients have shown that ghrelin was well tolerated and increased food intake [15, 16] and appetite scores [17]. However, due to its short half-life (approximately 30 min) and intravenous administration, the clinical effectiveness of ghrelin remains limited.
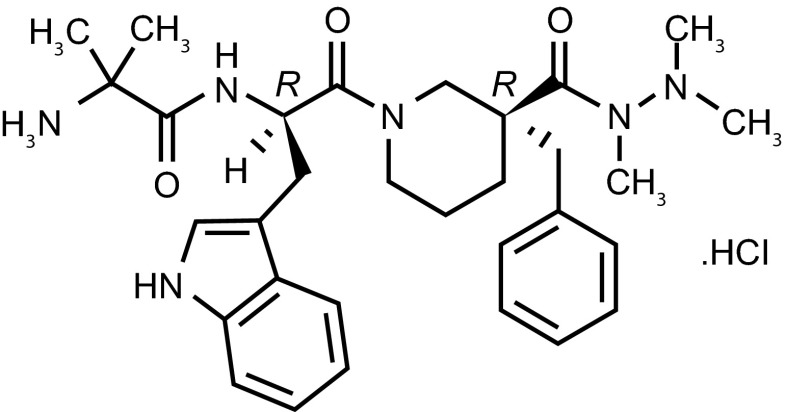
Anamorelin HCl (ONO-7643; ANAM) is a potent and selective novel ghrelin receptor agonist that mimics the N-terminal active core of ghrelin [18] (Fig. 1). ANAM has the advantage of being orally active and having a longer half-life (approximately 7 h) than ghrelin [19, 20]. Similar to ghrelin, ANAM stimulates neuroendocrine responses and can induce rapid positive effects on appetite and metabolism, which can lead to an increase in body weight and LBM [21, 22]. The compound is currently undergoing evaluation as a potential agent for the treatment of CACS in Phase III clinical trials in non-small cell lung cancer (NSCLC) [23].
Fig. 1.
Chemical structure of anamorelin
The aim of the present manuscript is to describe the preclinical profile of ANAM, both in vitro and in vivo, by evaluating its agonist effects on the ghrelin receptor and its activities on food intake, body weight gain, and GH and insulin-like growth factor 1 (IGF-1) secretion.
Materials and methods
Cell lines and reagents
HEK293 and baby hamster kidney (BHK) cell lines were obtained from ATCC. HDB then engineered the cell lines to express human ghrelin receptor, and the cell line was maintained under standard conditions and authenticated with functional calcium influx and immunoprecipitation-1 assays every 2 weeks.
For the in vitro assays, ghrelin (Sigma) and ANAM (Helsinn) were diluted with 100 % dimethyl sulfoxide (DMSO) to make 30 mM stock. On the test day, serial dilutions had been prepared starting from the stock solution, and test solutions were obtained by diluting the stock solutions 1/200 in assay buffer (DMSO final concentration 0.5 %).
For the in vivo rat and pig studies, ANAM (Helsinn) was stored frozen at −20 °C under protection from light until use, when it was dissolved and serially diluted in distilled water to concentrations of 6, 2, and 0.6 mg/mL for administration. These working solutions were stored protected from light at 5 °C and used within 6 days.
Animals
To isolate the rat pituitary cells, Sprague-Dawley male albino rats (250 g ± 25 g) were purchased from Møllegaard, Lille Skensved, Denmark. Rats were housed in group cages at 19–24 °C with a 12-h light cycle.
For the in vivo experiments, 7-week-old male Crl:CD (SD) rats (Charles River Japan, Inc.) were housed individually under controlled temperature (24 ± 2 °C), humidity (55 ± 15 %), outside air ventilation (15 ± 5 cycles/h), and light/dark cycle (12 h). Animals were acclimatized for at least 1 week before use in experiments. Animals were provided solid feed (CRF-1, Oriental Bioservices Co. Ltd.) and tap water ad libitum.
Female slaughter pigs (Danish Landrace) with an initial weight of 40–45 kg at first dosing were acclimatized 1 week before blood sampling (vena jugularis and arteria carotis) and dosing (intragastric) catheters were inserted under general anesthesia (propofol/isoflurane). The animals were then given at least 5 days to recover before the commencement of the study period. Experiments were performed in compliance with the “Guidance for Animal Experiments” established by Research Headquarters, ONO Pharmaceutical Co., Ltd.
In vitro fluorescent imaging plate reader and binding assays
HEK293 cells stably expressing recombinant human ghrelin receptor (HEK293/GRLN) were used in the fluorescent imaging plate reader (FLIPR) assay. One day before the test, cells were seeded at a density of 1.5 × 104/well in a Matrigel® coated 384-well plate with 30 μL of complete Dulbecco’s Modified Eagle’s Medium, and incubated at 37 °C in 5 % CO2 for 22–26 h. On the test day, 4× loading dye was added into each well (10 μL per well for 384-well plates). Assay plates were incubated at 37 °C in the dark for 30 min. The dye content was then removed by centrifugation at 300 rpm for 30 s, and 30 μL Hanks’ Balanced Salt Solution/Hepes with 1 mM probenecid was added with Platemate Matrix (low-speed setting; Thermo). The plate was then placed in FLIPR Tetra (Molecular Device) and 10 μL 4× working concentrations of test compounds were added by FLIPR (agonist mode). Fluorescence signal was detected with FLIPR at room temperature according to standard settings for 10 min before cells were exposed to 10 μL 5× working concentrations of agonists (compound added by FLIPR). The fluorescence signal was detected in the subsequent 3 min (antagonist mode).
BHK cells stably expressing human ghrelin receptor were used for binding assay membrane preparations. Cells were cultured under continuous antibiotics selection. Cells were collected when confluence reached 90 %. Gentle cell dissociate buffer (PBS/EDTA) was used to dissociate cells. Upon dissociation, the cells were homogenized (8,000 rpm for 30 s, three times) in assay buffer. The homogenates were centrifuged at 4,000g at 4 °C for 15 min. The pellets were discarded and the supernatant was centrifuged at 40,000g at 40 °C for 30 min. The pellet was re-suspended and protein concentrations were measured by BCA kit (Thermo).
For the binding assay, the membrane protein was diluted in assay buffer to yield 20 μg/well in 120 μL. The binding assay was set up in a 96-well plate as the following: 120 μL membrane preparations, 15 μL [125I] ghrelin (final concentration 1 nM) and 15 μL compound (10×) diluted in the assay buffer. The reaction mixture was incubated at room temperature for 30 min before terminating by quick filtration onto a glass fibre/B (GF/B) filtration plate presoaked in 0.3 % polyethylenimine (PEI) using cell harvester (PerkinElmer). The filter was washed three times and dried at 37 °C overnight. The radioactivity bound to filter membrane was measured with MicroBeta® TriLux (Perkin Elmer).
For the competition assay, ANAM concentrations (1 pM–10 μM) were added to the membranes together with 35S-MK-677. Nonspecific binding was determined by adding 10 μM nonlabeled MK-677. The mixture was incubated at 30 °C for 60 min, followed by application of the samples to GF/B filters (Whatman), which had been pretreated with 0.5 % PEI for 60 min. The filters were subsequently washed in 0.9 % NaCl and counted using an OptiPhase counter (Wallac).
Rat pituitary cell isolation and GH assay
To isolate the rat pituitary cells, the pituitaries were dissected from Sprague-Dawley male albino rats. The neurointermediate lobes were removed and the remaining tissue was immediately placed in ice-cold isolation buffer. The tissue was cut into small pieces and transferred to isolation buffer supplemented with trypsin and DNase and incubated at 70 rpm for 35 min at 37 °C. The tissue was then aspirated into single cells and the cells were filtered through a nylon filter (160 μm) to remove undigested tissue. The cell suspension was washed with isolation buffer supplemented with trypsin inhibitor and then re-suspended in culture medium. The cells were seeded 4 × 104 cells/well and cultured for 3 days at 37 °C. Following the culture period, the cells were washed with stimulation buffer and pre-incubated for 1 h. The buffer was then exchanged and the cells were incubated for 15 min with concentrations of ANAM ranging from 0.01 nM to 10 μM. The medium was then decanted and analyzed for GH content in a recombinant (r)GH ELISA test system (Novo Nordisk).
Assessment of food intake and body weight
Four groups of seven rats were selected from a pool of 40 rats according to body weight, food intake, and general condition during a 6-day period before grouping, and received ANAM at 3, 10, or 30 mg/kg, or vehicle control. In order to keep groups homogeneous, individual animals were first arranged in order of food intake over the previous 3-day period and assigned to groups using a stratified selection method. A random number was generated for each group and the four groups were established in ascending order of random number. Body weight and leftover feed for the calculation of food intake were measured on Day −6, Day −3, and Day 0. Leftover feed was calculated as the total weight of the feed and feed container. ANAM and vehicle control were administered orally once daily at a dose of 5 mL/kg for 6 days starting from Day 1 using a flexible tube for rats (RZ-2; CLEA Japan, Inc.) attached to a syringe. The dosing volume was calculated for each animal based on body weight on the day of dosing. Body weights and leftover feed for the calculation of food intake were measured daily until Day 7. Food intake on each day from Day 2 to Day 7 was calculated as the difference between the amount of leftover feed on the previous day and that on the day of dosing. Food intake on Day 1 was calculated by dividing by 4 the difference in leftover feed on Day −3 and Day 1 to obtain the food intake at baseline. Cumulative change in food intake, weight gain, and time course of food intake and body weight were evaluated. The cumulative change in food intake was calculated as the sum of the difference in food intake after dosing compared to baseline from Day 2 to Day 7. The difference in body weight between Day 7 and Day 1 was calculated as the weight gain in grams.
Assessment of GH and IGF-1 in vivo
For the assessment of food intake and body weight, rats were divided into four groups: ANAM 3 mg/kg (n = 7), 10 mg/kg (n = 7), or 30 mg/kg (n = 7), or vehicle control (n = 8), and 100 μL blood samples were collected before and 0.25, 0.5, 1, 2, 3, 4, 5, and 6 h after single dosing. Rats were anesthetized with sodium pentobarbital 64.8 mg/kg. A catheter filled with heparinized saline solution was inserted in the left femoral artery for blood collection and fitted with an extension tube, 1-mL sampling syringe, and a three-way cock to allow excess blood to return. Plasma levels of GH were measured immunochemically using a Rat Growth Hormone EIA kit (A05104, SPI-bio) and microplate reader (SpectraMax™ 250, Molecular Devices). Measurements were performed in duplicate. Area under the GH concentration curve from 0 to 6 h (AUC0–6h) postdose and the time course of GH plasma concentrations were evaluated.
In pigs (n = 6 per group), anamorelin was dosed directly into the gastric lumen via the dosing catheter. Blood samples were collected for the stimulation profile of GH at 30 and 15 min before, and 0, 5, 15, 30, 45, 60, and 120 min following dosing. Animals received either a single dose (3.5 mg/kg), or once-daily administration (1 mg/kg) for 7 days and stimulation profiles were taken after the first and seventh dose of ANAM. To assess IGF-1 levels, pigs received either placebo or ANAM for 7 days (1 mg/kg/day), and the following 7 days the two treatments were crossed over. A single blood sample was taken once a day immediately before dosing.
Statistical analysis
Data analyses were performed using SAS software, version 9.1.3 Service Pack 4 (SAS Institute, Japan), EXSAS, version 7.5.2 (Arm Systex, Co., Ltd) and Graphpad®. Data are expressed as mean ± standard error (SE). The half maximal effective concentration (EC50) and binding affinity (Ki) measures for ghrelin and ANAM were calculated by sigmoidal regression analysis. The cumulative changes in food intake and weight gain in ANAM-treated groups were compared to those in the control group by the Williams test. GH AUC0–6h was calculated using the trapezoidal method, and the Williams test was used to compare differences between ANAM-treated and control groups. All tests were one-sided and differences were considered significant for P < 0.05.
Results
In vitro studies
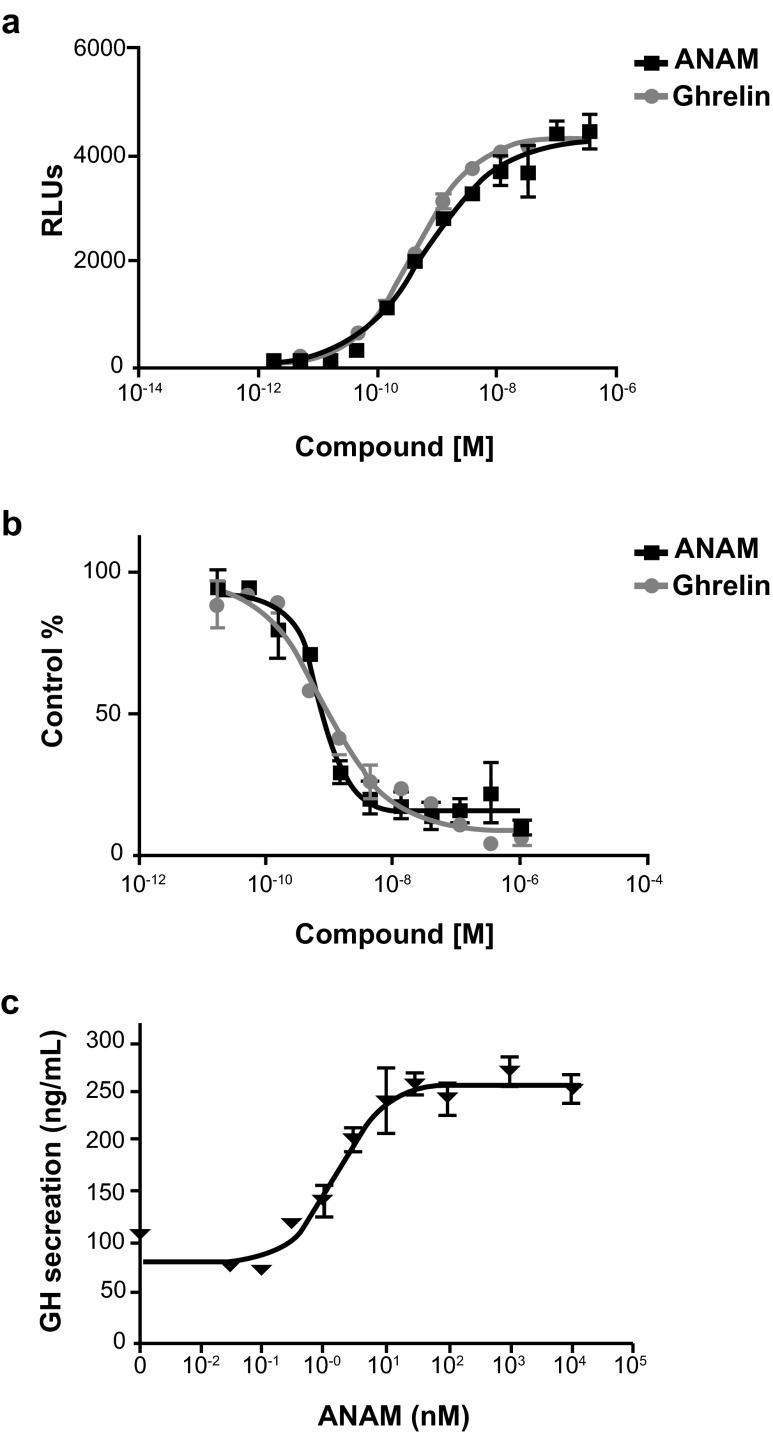
In the FLIPR assay, ghrelin and ANAM showed significant agonist activity on the ghrelin receptor (Fig. 2a), with EC50 values of 0.67 nM (95 % confidence interval [CI] 0.60–0.76) and 0.74 nM (95 % CI 0.50–1.12), respectively. No significant antagonist activity was observed with ANAM at concentrations of up to 1,000 nM. In the binding experiments, ghrelin and ANAM bound to the ghrelin receptor (Fig. 2b) with a binding affinity constant (Ki) of 0.58 nM (95 % CI 0.51–0.66) and 0.70 nM (95 % CI 0.55–0.96), respectively.
Fig. 2.
a Concentration-response curve of ghrelin and ANAM in the HEK293/GRLN FLIPR assay. Data are shown as the mean ± SE (n = 3). b Concentration-response curve of ghrelin and ANAM in the binding assay using HEK293/GRLN membrane preparations and [125I] ghrelin. Data are shown as the mean ± SE (n = 3). c The effect of ANAM in rat pituitary cell assay in doses ranging from 0.01 nM to 10 μM (n = 4). ANAM anamorelin; FLIPR fluorescent imaging plate reader; GH growth hormone; RLUs relative light units; SE standard error
In the competition assay with radiolabeled ibutamoren (35S-MK-677; another ghrelin receptor agonist) ANAM was also found to bind with high affinity to the ghrelin receptor (IC50 = 0.69 nM). In rat pituitary cells incubated with ANAM, there was a dose-dependent stimulatory effect on GH release (Fig. 2c) and the potency (EC50) was 1.5 nM.
ANAM was screened for activity against a set of over 100 receptors, ion channels, transporters, and enzymes. ANAM demonstrated binding to the tachykinin neurokinin 2 (NK2) site (IC50 = 0.021 μM); however, a subsequent NK2 functional assay demonstrated no functional activity. At the screening concentration of 10 μM, ANAM demonstrated weak binding to the calcium channel L-type receptors (benzothiazepine and phenylalkylamine), the serotonin transporter, and the sodium channel (data on file).
In vivo effects on food intake and body weight
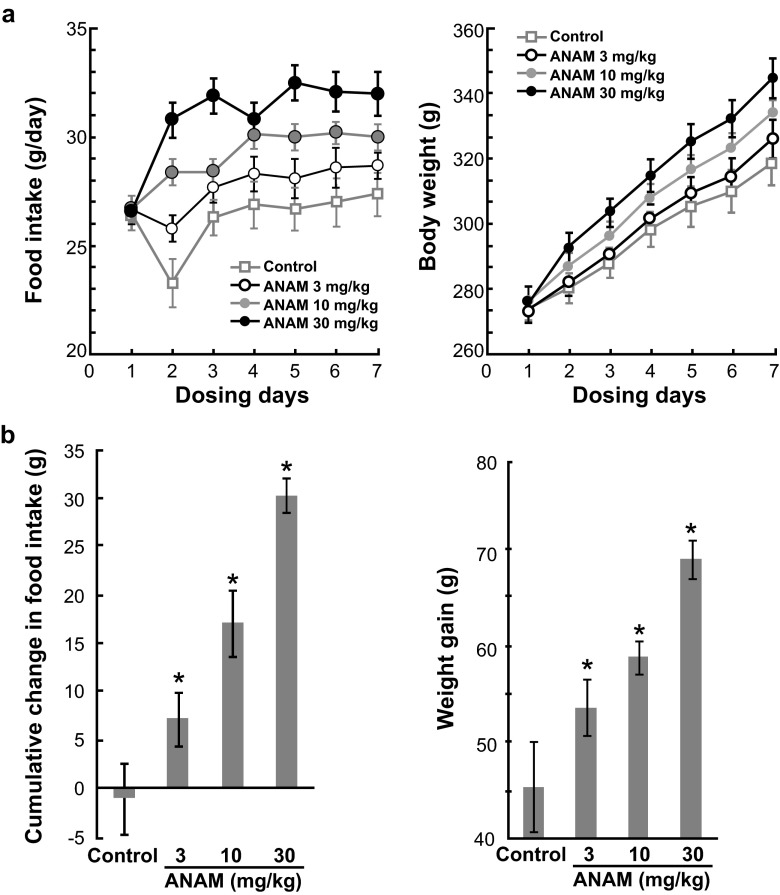
In rats, ANAM at an oral dose of 3, 10, or 30 mg/kg once daily significantly increased both food intake and body weight from Day 2 to Day 7 of treatment compared with the vehicle control (Fig. 3a). The cumulative change in food intake and weight gain increased dose-dependently, and these changes were significant at all dose levels (P < 0.05) compared to the control (Fig. 3b).
Fig. 3.
a Increasing effect of ANAM on food intake and body weight following repeated oral administration in rats. Data are shown as the mean ± SE. b Cumulative effect of ANAM on food intake and body weight following 7 days of repeated oral administration in rats. Data are shown as the mean ± SE; *P < 0.05 compared to control group. ANAM anamorelin; SE standard error
In vivo effects on GH and IGF-1 secretion
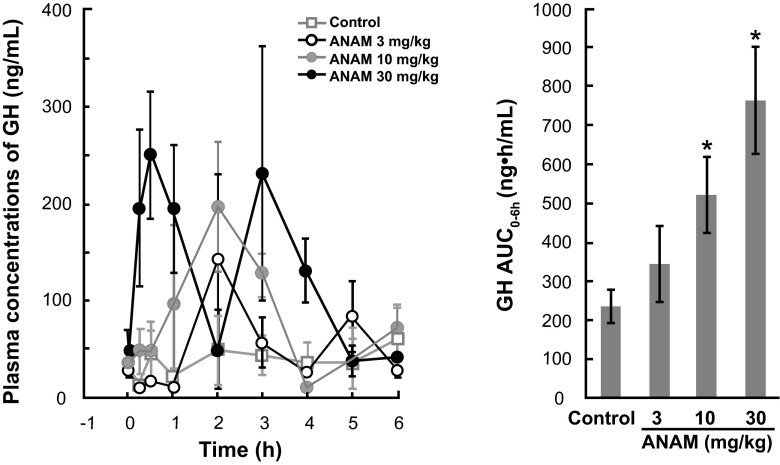
Administration of ANAM at a single oral dose of 3, 10, or 30 mg/kg induced a dose-dependent increase in plasma GH levels and GH AUC0-6h in rats (Fig. 4). The mean plasma GH concentration ranged from 10.0 to 143.0 ng/mL in the 3 mg/kg dose group, 11.3–197.6 ng/mL in the 10 mg/kg group, and 38.7–251.3 ng/mL in the 30 mg/kg group. The maximum plasma GH concentration for the ANAM groups was reached at 0.5–2 h postdose. In contrast, the mean plasma concentration of GH in the control group remained more constant and ranged from 13.5–61.0 ng/mL. Thus, ANAM stimulated a maximum increase in GH concentration ranging from 2.3-fold for the 3 mg/kg dose to 4.1-fold for the 30 mg/kg dose. At the doses of 10 and 30 mg/kg, GH AUC0–6h was significantly (P < 0.05) higher than the control vehicle-treated animals (Fig. 4).
Fig. 4.
Stimulatory effect of ANAM on growth hormone secretion following a single oral administration in rats. Data are shown as the mean ± SE; *P < 0.05 compared to control group. ANAM anamorelin; AUC 0–6h area under the GH concentration curve from 0 to 6 h; GH growth hormone; SE standard error
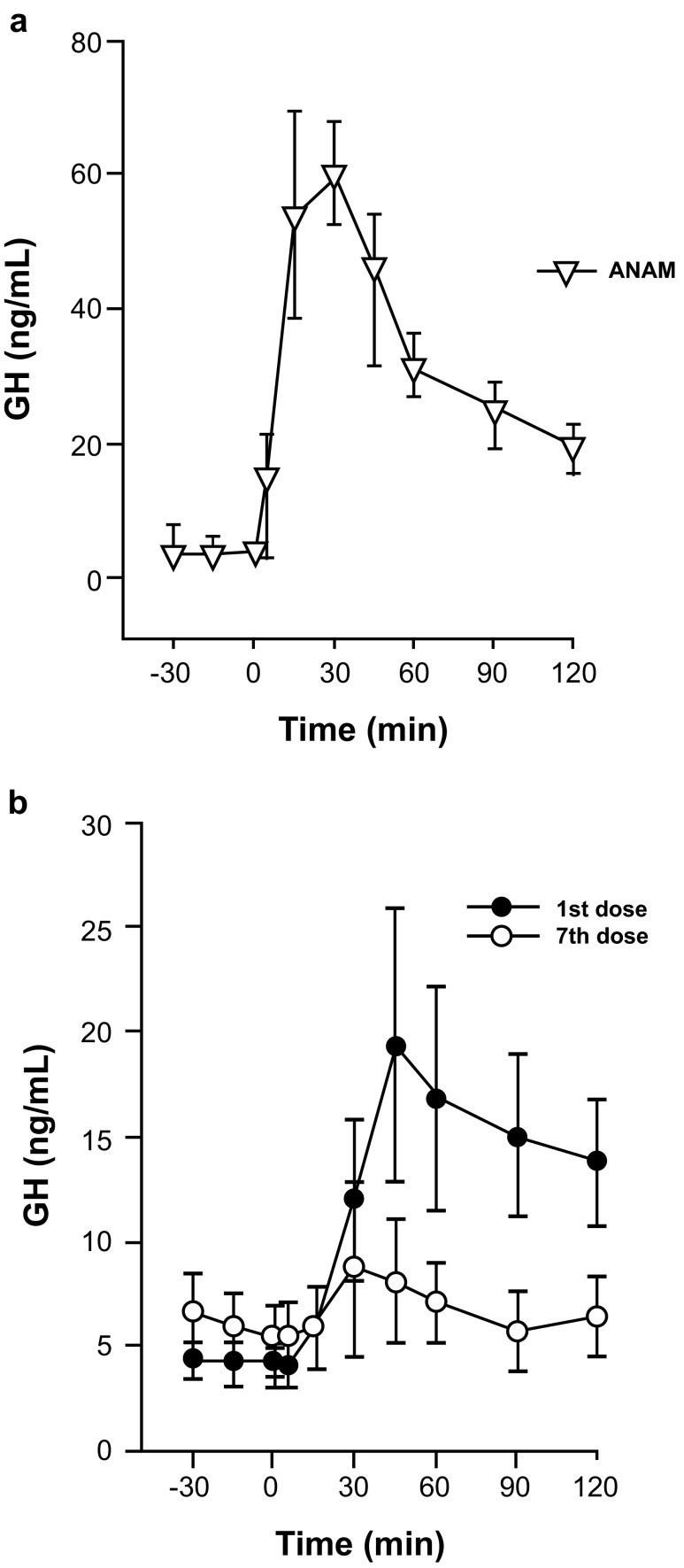
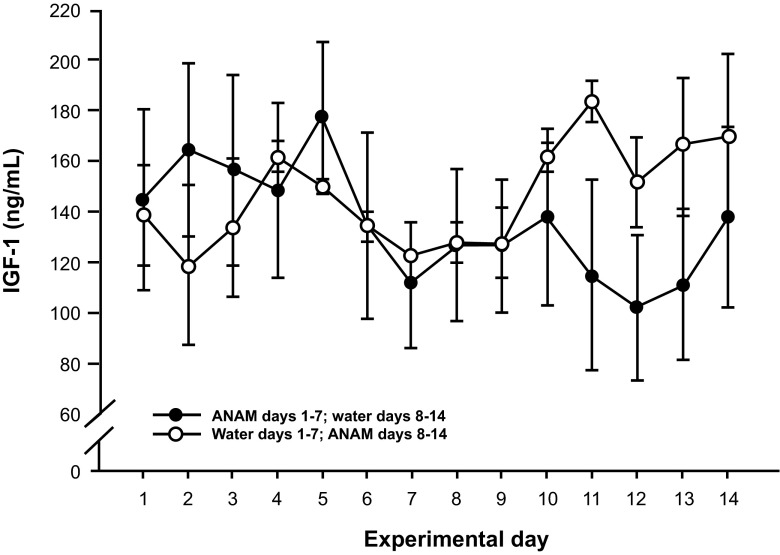
In pigs, following the administration of a single oral dose of ANAM 3.5 mg/kg there was a significant rise in GH levels (CmaxP < 0.0001) (Fig. 5a). Similar GH stimulatory activity was also observed in dogs [data on file]. Repeated administration of ANAM at 1 mg/kg/day for 7 days in pigs showed an acute GH release on both the first and seventh dose; however, the response was considerably reduced by the seventh dose (Fig. 5b). Furthermore, treatment with either control or ANAM (1 mg/kg/day) for 7 days, after which treatments were crossed over and continued for another 7 days, showed that mean IGF-1 concentrations were significantly elevated by 21 % (P < 0.001) with ANAM treatment compared with the control (Fig. 6).
Fig. 5.
a Acute GH release following a single dose of ANAM (3.5 mg/kg) in pigs. b GH response on Day 1 and on Day 7 following repeated daily ANAM dosing (1.5 mg/kg/day) in pigs. ANAM anamorelin; GH growth hormone
Fig. 6.
Mean IGF-1 plasma concentrations in pigs during repeated dosing with ANAM (1 mg/kg/day) or control for 7 days. Pigs were either first treated with ANAM from Days 1–7 and then crossed over to control from Days 8–14 (solid symbols), or vice versa (open symbols). The change in mean IGF-1 levels in the individual animals is shown and the mean ± SE in the ANAM or control periods is represented by the gray diamonds. ANAM anamorelin; IGF-1 insulin-like growth factor 1; SE standard error
Discussion
The in vitro data, utilizing various assays, consistently showed that ANAM is a potent synthetic ghrelin agonist with an affinity at the nanomolar level for the ghrelin receptor. Furthermore, these findings demonstrate that the agonist effects of ANAM are not associated with antagonism. In the screening for ANAM activity, 10 μM ANAM showed weak binding to the calcium channel L-type receptors, the serotonin transporter, and the sodium channel. Therefore, the compound exhibited a high selectivity versus ghrelin receptors, and given the high concentrations (nM) of ANAM required to elicit binding, none of these effects are likely to be of any clinical significance except in a massive overdose setting.
In vivo results showed that ANAM had significant appetite-enhancing activity, with multiple-dose administration in rats resulting in an increase in food intake and body weight after a short 6-day treatment. The appetite-enhancing effects were apparent as early as on Day 2 of treatment in the 10 and 30 mg/kg dose groups, suggesting that effects on appetite were elicited after the initial dose. The cumulative change in food intake and weight gain occurred in a dose-dependent manner. Similar findings were previously reported in another preclinical study that investigated the effects of ghrelin and ANAM in a lung cancer mouse xenograft model [24]. Although daily food consumption remained unchanged in this model, the investigators reported a significant increase in mean body weight in animals administered with ANAM at a dose of 10 or 30 mg/kg, compared to control. Significant increases in body weight relative to placebo have also been reported in healthy volunteers [21, 25] and patients with CACS [22, 26] after a short course of ANAM treatment. Additionally, in patients with CACS, food intake was increased compared to placebo after a 3-day course of ANAM, although this increase was not statistically significant [22].
The appetite-enhancing effects of ANAM are consistent with data from other ghrelin receptor agonists in development, including capromorelin and MK-677. In a Phase II exploratory study investigating the effects of capromorelin in older (≥65 years) subjects with mild functional limitation, capromorelin was found to increase body weight, LBM, tandem walk speed, and stair climbing power, although the study was terminated early according to predetermined treatment effect criteria [27]. MK-677 was also found to increase body weight in healthy subjects aged ≥60 years in a Phase I/II trial [28].
In the study exposing rat pituitary cells to ANAM, GH release was induced. In vivo, single-dose administration of ANAM produced a dose-dependent increase in GH AUC0–6h in rats, indicating that at the same doses as its appetite-enhancing effects, ANAM has a stimulatory effect on GH secretion. Increases in plasma GH concentrations from 2.3- to 4.1-fold higher than control were observed following ANAM treatment, reflecting the findings of an earlier preclinical study [24]. Furthermore, ANAM administered at a single dose was found to induce GH release in pigs. However, repeated administration of ANAM in pigs led to a reduced GH response and an increase in IGF-1 levels. This effect may be explained by the negative feedback exerted by IGF-1 on GH secretion. IGF-1 can influence GH-releasing hormone (GHRH) and somatostatin production in the hypothalamus, and also influence GHRH action in the pituitary, while GH can inhibit its own secretion in the hypothalamus [29, 30].
Several clinical trials have found an increase in GH secretion following ANAM treatment in humans [22, 25]. One of these studies also demonstrated that repeated administration of ANAM led to a decrease in GH response and increase in IGF-1 levels when the Day 1 and corresponding Day 5/6 parameters were compared [25]. These findings support the presence of somatotrophic control of GH levels in both animals and humans, and show that the GH axis cannot be hyperstimulated by ANAM.
As with ANAM, treatments with other ghrelin receptor agonists have also led to increases in GH/IGF-1 secretion. In the Phase II study of capromorelin in elderly patients, capromorelin prompted a rise in peak nocturnal GH levels and a sustained, dose-related rise in IGF-1 concentrations [27]. MK-677 has also been shown to significantly increase GH and IGF-1 levels in healthy subjects [28]. Neither capromorelin nor MK-677 has been studied in the context of CACS. Macimorelin is another ghrelin receptor agonist that is currently in development for the treatment of CACS. In a Phase I clinical study in healthy male volunteers, macimorelin induced a potent release of GH with concentrations peaking about 1 h following administration [31]. A randomized pilot clinical trial is in progress to study the efficacy and safety of macimorelin in patients with CACS [32].
Both GH and IGF-1 treatments have been found to increase LBM [33, 34], but increasing GH levels in the context of ANAM treatment in cancer patients raises concerns over the potential for stimulating tumor growth. In the Northrup et al. study, however, the significant increase in GH produced in response to ANAM (~2.5-fold increase) and even greater rise in GH levels produced in response to ghrelin (~50-fold increase) following repeated daily dosing for 28 days were not associated with promotion of tumor growth in a murine model [24]. Notably, the A549 NSCLC adenocarcinoma tumor cell line used in that study has been shown to possess a high degree of expression of the IGF-1 receptor [35]. Other studies evaluating GH-based therapies in tumor-bearing animal models [14, 36–39] and in formal carcinogenicity studies in tumor-free rats and mice [40] support the findings that ghrelin and ANAM do not stimulate tumor growth. Furthermore, in a Phase II clinical study in NSCLC patients, no statistically significant effect on long-term overall survival was noted after 12-weeks of treatment with 50 mg or 100 mg anamorelin compared with placebo [26].
ANAM is currently being evaluated in two Phase III clinical trials in patients with advanced NSCLC and CACS (NCT01387269, NCT01387282), and a 12-week double-blind safety extension study (NCT01395914). Efficacy and safety findings from these studies are expected to become available shortly.
In conclusion, the results presented suggest that the potent affinity of ANAM for the ghrelin receptor is associated with dose-dependent stimulatory effects on appetite with consequent weight gain in rats. In addition, ANAM has an accompanying stimulatory effect on GH and IGF-1. These findings validate the outcomes observed in clinical trials to date and support the continued investigation of ANAM as a potential treatment of CACS.
Acknowledgments
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Editorial support and medical writing assistance was provided by Eva Polk, PhD (TRM Oncology, The Hague, The Netherlands), funded by Helsinn Healthcare SA, Lugano, Switzerland. The authors are fully responsible for all content in the manuscript.
Conflicts of interest
Claudio Pietra is an employee of Helsinn Healthcare, and Robert Northrup and Elizabeth Manning Duus are employees of Helsinn Therapeutics (US), Inc. Yasuhiro Takeda, Naoko Tazawa-Ogata, and Masashi Minami are employees of ONO Pharmaceutical Co., Ltd., and Xia Yuanfeng is an employee of HD Biosciences.
References
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–7. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Kumar NB, Kazi A, Smith T, Crocker T, Yu D, Reich RR, et al. Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Curr Treat Options Oncol. 2010;11:107–17. doi: 10.1007/s11864-010-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9. doi: 10.1016/S0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 6.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–11. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki H, Asakawa A, Amitani H, Fujitsuka N, Nakamura N, Inui A. Cancer cachexia pathophysiology and translational aspect of herbal medicine. Jpn J Clin Oncol. 2013;43:695–705. doi: 10.1093/jjco/hyt075. [DOI] [PubMed] [Google Scholar]
- 8.Dodson S, Baracos VE, Jatoi A, Evans JW, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–79. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- 9.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 11.Argilés JM, Stemmler B. The potential of ghrelin in the treatment of cancer cachexia. Expert Opin Biol Ther. 2013;13:67–76. doi: 10.1517/14712598.2013.727390. [DOI] [PubMed] [Google Scholar]
- 12.Guillory B, Splenser A, Garcia J. The role of ghrelin in anorexia-cachexia syndromes. Vitam Horm. 2013;92:61–106. doi: 10.1016/B978-0-12-410473-0.00003-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Andersson M, Iresjö BM, Lönnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28:1393–400. [PubMed] [Google Scholar]
- 14.DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–12. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- 15.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–6. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 16.Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer. 2012;118:4785–94. doi: 10.1002/cncr.27430. [DOI] [PubMed] [Google Scholar]
- 17.Lundholm K, Gunnebo L, Körner U, Iresjö BM, Engström C, Hyltander A, et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer. 2010;116:2044–52. doi: 10.1002/cncr.24917. [DOI] [PubMed] [Google Scholar]
- 18.Morozumi N, Hanada T, Habara H, Yamaki A, Furuya M, Nakatsuka T, et al. The role of C-terminal part of ghrelin in pharmacokinetic profile and biological activity in rats. Peptides. 2011;32:1001–7. doi: 10.1016/j.peptides.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Kumor K, Polvino W. Biologic activity of RC-1291, a novel oral ghrelin mimetic for cancer anorexia/cachexia: results from a Phase I randomized, double-blind, placebo-controlled trial in healthy volunteers. Support Care Cancer. 2006;14:593. Abstract 03–009.
- 20.Blum R, Polvino W. Pharmacokinetic (PK) profile of RC-1291, a novel oral ghrelin mimetic for the treatment of cancer anorexia/cachexia. Support Care Cancer. 2006;14:593. Abstract 03–010.
- 21.Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007;12:594–600. doi: 10.1634/theoncologist.12-5-594. [DOI] [PubMed] [Google Scholar]
- 22.Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21:129–37. doi: 10.1007/s00520-012-1500-1. [DOI] [PubMed] [Google Scholar]
- 23.Abernethy AP, Temel JS, Currow D, Gleich LL, Friend J. Phase III clinical trials with anamorelin HCl, a novel oral treatment for NSCLC cachexia. J Clin Oncol. 2013;31(suppl): Abstract TPS9649.
- 24.Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, et al. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer. 2013;21:2409–15. doi: 10.1007/s00520-013-1800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009;19:267–73. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Temel J, Bondarde S, Jain M, Allen S, Mann W. Efficacy and safety of anamorelin HCl in NSCLC patients: results from a randomized, double-blind, placebo-controlled, multicentre phase II study. Eur J Cancer. 2013;49 (Suppl 2): Abstract 1308.
- 27.White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94:1198–206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 28.Nass R, Pezzoli SS, Oliveri MC, Patrie JC, Harrell FE, Jr, Clasey JL, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–11. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato Y, Murakami Y, Sohmiya M, Nishiki M. Regulation of human growth hormone secretion and its disorders. Intern Med. 2002;41:7–13. doi: 10.2169/internalmedicine.41.7. [DOI] [PubMed] [Google Scholar]
- 30.Reichlin S. Neuroendocrinology. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. William's textbook of endocrinology. Philadelphia: Saunders; 1998. pp. 165–248. [Google Scholar]
- 31.Piccoli F, Degen L, MacLean C, Peter S, Baselgia L, Larsen F, et al. Pharmacokinetics and pharmacodynamic effects of an oral ghrelin agonist in healthy subjects. J Clin Endocrinol Metab. 2007;92:1814–20. doi: 10.1210/jc.2006-2160. [DOI] [PubMed] [Google Scholar]
- 32.Ali SA, Garcia JM. Randomized clinical trial of the novel oral ghrelin mimetic macimorelin in the treatment of cancer cachexia: study design and preliminary results. Endocr Rev. 2013;34: Abstract MON-327.
- 33.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–77. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 34.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154:557–68. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507) PLoS One. 2009;4:e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo GC, Huang C, Camacho R, Trainor C, Blake JT, Sirotina-Meisher A, et al. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166:4195–201. doi: 10.4049/jimmunol.166.6.4195. [DOI] [PubMed] [Google Scholar]
- 37.Khan AS, Anscombe IW, Cummings KK, Pope MA, Smith LC, Draghia-Akli R. Effects of plasmid-mediated growth hormone-releasing hormone supplementation on LL-2 adenocarcinoma in mice. Mol Ther. 2003;8:459–66. doi: 10.1016/S1525-0016(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 38.Khan AS, Smith LC, Anscombe IW, Cummings KK, Pope MA, Draghia-Akli R. Growth hormone releasing hormone plasmid supplementation, a potential treatment for cancer cachexia, does not increase tumor growth in nude mice. Cancer Gene Ther. 2005;12:54–60. doi: 10.1038/sj.cgt.7700767. [DOI] [PubMed] [Google Scholar]
- 39.Perboni S, Bowers C, Kojima S, Asakawa A, Inui A. Growth hormone releasing peptide 2 reverses anorexia associated with chemotherapy with 5-fluorouracil in colon cancer cell-bearing mice. World J Gastroenterol. 2008;14:6303–5. doi: 10.3748/wjg.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farris GM, Miller GK, Wollenberg GK, Molon-Noblot S, Chan C, Prahalada S. Recombinant rat and mouse growth hormones: risk assessment of carcinogenic potential in 2-year bioassays in rats and mice. Toxicol Sci. 2007;97:548–61. doi: 10.1093/toxsci/kfm059. [DOI] [PubMed] [Google Scholar]