Abstract
Malnutrition and sarcopenia often occur in rehabilitation settings. The prevalence of malnutrition and sarcopenia in older patients undergoing rehabilitation is 49–67 % and 40–46.5 %, respectively. Malnutrition and sarcopenia are associated with poorer rehabilitation outcome and physical function. Therefore, a combination of both rehabilitation and nutrition care management may improve outcome in disabled elderly with malnutrition and sarcopenia. The concept of rehabilitation nutrition as a combination of both rehabilitation and nutrition care management and the International Classification of Functioning, Disability and Health guidelines are used to evaluate nutrition status and to maximize functionality in the elderly and other people with disability. Assessment of the multifactorial causes of primary and secondary sarcopenia is important because rehabilitation nutrition for sarcopenia differs depending on its etiology. Treatment of age-related sarcopenia should include resistance training and dietary supplements of amino acids. Therapy for activity-related sarcopenia includes reduced bed rest time and early mobilization and physical activity. Treatment for disease-related sarcopenia requires therapies for advanced organ failure, inflammatory disease, malignancy, or endocrine disease, while therapy for nutrition-related sarcopenia involves appropriate nutrition management to increase muscle mass. Because primary and secondary sarcopenia often coexist in people with disability, the concept of rehabilitation nutrition is useful for their treatment. Stroke, hip fracture, and hospital-associated deconditioning are major causes of disability, and inpatients of rehabilitation facilities often have malnutrition and sarcopenia. We review the concept of rehabilitation nutrition, the rehabilitation nutrition options for stroke, hip fracture, hospital-associated deconditioning, sarcopenic dysphagia, and then evaluate the amount of research interest in rehabilitation nutrition.
Keywords: Rehabilitation nutrition, Stroke, Hip fracture, Hospital-associated deconditioning, Sarcopenic dysphagia
Introduction
Rehabilitation nutrition is a combination of both rehabilitation and nutrition care management, and this concept is used with International Classification of Functioning, Disability and Health guidelines to evaluate nutrition status and to maximize functionality in the elderly and other people with disability. Rehabilitation nutrition may further improve physical and mental function, activities of daily living, and quality of life. The term “rehabilitation nutrition” is quite different from that of “nutritional rehabilitation.” Nutritional rehabilitation usually refers to nutritional improvement of malnourished children in developing countries. In contrast, rehabilitation nutrition not only refers to nutritional improvement but also to rehabilitation in people with disability [1, 2]. Rehabilitation nutrition is similar to sports nutrition. The key aims of rehabilitation nutrition assessment [2] are to assess the following: (1) the presence and cause of malnutrition; (2) the presence and cause of sarcopenia; (3) the presence and cause of dysphagia; (4) the adequacy of nutrition care management with prediction of future nutritional status; and (5) whether rehabilitation for functional improvement, such as resistance training and endurance training, can be conducted.
The prevalence of malnutrition in rehabilitation settings is high. In elderly patients hospitalized for rehabilitation, the prevalence of compromised nutrition status was estimated to be 49–67 % [3]. In Australia, 33 and 51.5 % of patients admitted to rehabilitation hospitals were classified as malnourished and at nutritional risk using the Mini Nutritional Assessment (MNA) and the MNA short-form (MNA-SF) [4]. One study using pooled MNA data found that the prevalence of malnutrition in elderly people was highest in rehabilitation settings (rehabilitation, 50.5 %; hospital, 38.7 %) [5]. Another study using the MNA-SF revealed a 40.8 % prevalence of malnutrition in rehabilitation settings [6]. A systematic review found that malnutrition in older adults admitted for rehabilitation has a negative effect on functional recovery and quality of life following discharge to the community [7]. Furthermore, rehabilitation outcome has been shown to be poor in malnourished patients with stroke [8], hip fracture [9], hospital-associated deconditioning [10, 11], and a variety of other diseases.
The prevalence of sarcopenia in rehabilitation settings is also high: 10–30 % in community-dwelling elderly [12] and 40 % in ambulatory rehabilitation facility-dwelling elderly 60 years and older [13]. Another study revealed that 46.5 % patients admitted to a subacute geriatric care unit who underwent a rehabilitation intervention met the diagnostic criteria for sarcopenia [14].
The European Working Group on Sarcopenia in Older People categorized sarcopenia into primary sarcopenia (age-related sarcopenia) and secondary sarcopenia (i.e., activity-, disease-, or nutrition-related sarcopenia) [15]. Assessment of the multifactorial causes of primary and secondary sarcopenia is indispensable because rehabilitation nutrition for sarcopenia differs depending on its etiology. Treatment of age-related sarcopenia includes resistance training, protein and amino acid supplementation, smoking cessation, and pharmaceutical therapies [16, 17]. Pharmaceutical therapy of sarcopenia is likely to advance in the near future because our understanding of the role of regulators in sarcopenia has increased [18, 19]. Early ambulation, exercise, and avoiding bed rest are important for preventing and treating activity-related sarcopenia. Treatment of disease-related sarcopenia includes therapies for advanced organ failure, inflammatory disease, malignancy, and endocrine disease, while treatment of nutrition-related sarcopenia includes appropriate nutrition management to increase muscle mass [16, 17]. In cases of age-, activity-, disease-, and nutrition-related sarcopenia, rehabilitation nutrition can be used to maximize functionality.
Stroke, hip fracture, and hospital-associated deconditioning are major causes of disability in inpatient rehabilitation facilities. In the USA, the six largest diagnostic impairment categories receiving inpatient rehabilitation include stroke, lower extremity fracture, lower extremity joint replacement, debility, neurologic disorders, and brain dysfunction [20]. Hip fracture is a leading cause of disability in lower extremity fracture patients, and debility is synonymous with hospital-associated deconditioning. In Japan, common causes of inpatient rehabilitation in convalescent rehabilitation wards are stroke (47.9 %); orthopedic diseases, including hip fracture (35.2 %); disuse syndrome (10.5 %); and traumatic brain and spinal cord injury (5.4 %) [21]. Disuse syndrome is synonymous with hospital-associated deconditioning. These data indicate that management of patients with stroke, hip fracture, and hospital-associated deconditioning is an important part of inpatient rehabilitation. The term “sarcopenic dysphagia” refers to difficulty swallowing due to sarcopenia of generalized skeletal muscles and swallowing muscles [22, 23]. Age-related loss of the tongue and geniohyoid muscle mass has been studied in the elderly [24, 25]. Sarcopenic dysphagia is an important current and future public health issue, because it is common in the elderly and can lead to aspiration pneumonia, the prevalence of which is increasing with the aging of society [23]. Therefore, we review rehabilitation nutrition for stroke, hip fracture, hospital-associated deconditioning, and sarcopenic dysphagia, and then assess the level of research interest in rehabilitation nutrition.
Stroke
Stroke is the leading cause of disability in Western and East Asian countries. More than 60 % of patients remain disabled, 50 % of patients suffer from hemiparesis, and 30 % remain unable to walk without assistance [26]. As the benefits of rehabilitation are beyond doubt, rehabilitation strategies play center stage in optimizing functional recovery after stroke [27, 28].
Both malnutrition and obesity are nutritional problems in stroke. According to a recent systematic review, malnutrition and dysphagia respectively occur in 8.2–49.0 % and 24.3–52.6 % of subjects following stroke [29]. In subgroup analysis, the odds of malnutrition were significantly increased during the rehabilitation stage (odds ratio (OR), 2.445; 95 % confidence interval (CI), 1.009–5.925) [29]. Tissue wasting, sarcopenia, and cachexia may impair and delay poststroke rehabilitation and worsen the prognosis, and increasing evidence suggests that patients who are overweight and mildly obese may actually have a better outcome [30]. Analysis of data from the China National Stroke Registry on patients grouped according to their body mass index (BMI) into underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23–27.4 kg/m2), obese (27.5–32.4 kg/m2), or severely obese (≥32.5 kg/m2) [31] found that overweight was independently associated with favorable 3-month functional recovery (OR, 1.24; 95 % CI, 1.12–1.38), but severe obesity was independently associated with higher 3-month mortality (OR, 2.01; 95 % CI, 1.10–3.69) [31]. In stroke patients admitted to a rehabilitation hospital, the underweight group had the lowest functional independence measure (FIM) efficiency, followed by the obese and normal-weight subgroups [32]. The overweight group had the highest FIM efficiency (p = 0.05) when compared with the obese subgroup [32]. These results indicate that outcome is better in overweight stroke patients than in underweight stroke patients. However, the obesity paradox seems not to be applicable to poststroke rehabilitation.
Skeletal muscles are the main effector organs impacted by disability in stroke, but little attention is paid to structural, metabolic, and functional alterations of muscle tissue after stroke [27, 28]. Stroke-induced sarcopenia is difficult to differentiate from hemiparesis in terms of evaluating muscle strength and physical performance. Therefore, diagnosis of stroke-induced sarcopenia is a challenging task. In a systematic review of loss of skeletal muscle mass after stroke [33], lean tissue mass was significantly less in the paretic than the nonparetic lower limb (median, 342.3 g; 95 % CI, 247.0–437.6 g) and upper limb (median, 239.9 g; 95 % CI, 181.7–298.2 g), and midthigh muscle cross-sectional area (median, 15.4 cm2; 95 % CI, 13.8–16.9 cm2) was significantly less in individuals at least 6 months poststroke. Mechanisms of muscle wasting in stroke-related sarcopenia include disuse atrophy, spasticity, inflammation, denervation, reinnervation, impaired feeding, and intestinal absorption [28]. Further research will be required to diagnose and treat stroke-induced sarcopenia.
Nutritional supplements can improve outcomes in poststroke rehabilitation [34]. A randomized study comparing intensive nutritional supplementation to routine nutritional supplementation was performed in 116 undernourished stroke inpatients [34]. Compared with those on standard nutritional supplements, patients receiving intensive nutritional supplementation improved more on measures of motor function (total FIM, FIM motor subscore, 2 and 6-min timed walk tests, p < 0.002) [34]. In a randomized, controlled trial comparing routine care with individualized, nutritional care aiming to prevent weight loss in acute stroke patients at nutritional risk [35], 20.7 % of the intervention group lost ≥5 % weight compared with 36.4 % of the control group (p = 0.055) at follow-up. The intervention group had a significantly higher increase in QoL score (p = 0.009) and in handgrip strength (p = 0.002) [35]. In a Cochrane Database of Systematic Review [36], nutritional supplementation in acute and subacute stroke was associated with reduced frequency of pressure sores (OR: 0.56; 95 % CI: 0.32–0.96), and increased energy intake (mean differences (MD), 430.18 kcal/day; 95 % CI, 141.61–718.75) and protein intake (MD, 17.28 g/day; 95 % CI, 1.99–32.56). These results indicate that nutrition support for stroke rehabilitation patients at malnutrition or nutritional risk seems to improve nutrition intake and rehabilitation outcome.
Hip fracture
Hip fractures are associated with more disability, health care costs, and mortality than all other osteoporotic fractures combined [37]. In 2005, hip fractures in the USA were estimated to account for 14 % of total fractures but 72 % of total fracture-related health care costs [37]. Compared with its pre-fracture level, post-fracture function is deteriorated in 60 % of patients with hip fracture [38]. The demographic trend worldwide is that more and more people are suffering from hip fracture. The number of hip fractures is expected to rise from 1.6 million in 2000 up to 6.3 million in 2050 [37]. Hip fracture is the most common condition requiring geriatric musculoskeletal rehabilitation.
The prevalence of malnutrition in hip fracture depends on the method of nutrition assessment. Malnutrition prevalence was lowest when assessed by BMI (13 %), followed by MNA-SF (27 %), International Classification of Disease, 10th Revision, Australian Modification (ICD10-AM) (48 %), albumin (53 %), and geriatrician individualized assessment (55 %) [39]. Malnutrition prevalence in hip fracture was 37.5 % using ICD10-AM criteria in another study [40]. Nutrition status assessed by MNA in one hip fracture study revealed that 8.8 % of elderly patients were undernourished, 43.7 % at risk of malnutrition, and 47.5 % well-nourished [41]. Nutrition status in another hip fracture study revealed that 11.6 % were malnourished, 44.2 % at risk of malnutrition, and 44.2 % were well-nourished [42]. MNA predicted gait status and mortality 6 months after hip fracture [43]. Serum albumin level (p = 0.0004; OR, 5.8541) and BMI (p = 0.0192; OR, 1.1693) significantly influenced mortality after hip fracture [44]. Malnutrition and being at risk for malnutrition are common in patients with hip fracture and seem to affect rehabilitation outcome.
The prevalence of sarcopenia in patients with hip fracture is high. In the Sarcopenia and Hip Fracture study [45], 71 % of participants were sarcopenic. Another study in women with hip fracture revealed that 58 % were sarcopenic [46]. Using normative data from the New Mexico Elder Health Study [47], 64.0 % of female hip fracture inpatients and 95.0 % of male hip fracture inpatients admitted to rehabilitation wards had sarcopenia. Analysis of other data revealed that 21.8 % of female hip fracture patients and 86.7 % of male hip fracture patients had sarcopenia [47]. In 357 Japanese patients immediately after hip fracture, 44.7 % of women and 81.1 % of men had sarcopenia, and the presence of sarcopenia was independently associated with the occurrence of hip fracture [48]. On the other hand, only 4 of the 71 hip fracture patients (5.6 %) were identified as cachectic [49]. Sarcopenia not cachexia seems to be common in elderly patients with hip fracture.
A Cochrane Database Systematic Review of nutritional supplementation in elderly patients with hip fracture found weak evidence for the effectiveness of protein and energy supplements [50]. One trial of multinutrient intravenous feeding followed by oral supplements found a reduction in the number of participants with complications (RR, 0.21; 95 % CI, 0.10–0.46), but not in mortality rate (RR, 0.11; 95 % CI, 0.01–2.00) [50]. A controlled prospective cohort study in patients with hip fracture found a significant association of multidisciplinary postoperative nutritional care with a decline in the number of malnourished patients and a decline in the EuroQol (p = 0.004) after 3 months of the intervention [51]. In a randomized, controlled study [52], nutritional support actively supervised by a dietician and guided by repeated measurements of resting energy requirements was achievable and improved outcomes in geriatric patients following surgery for hip fractures. Multidisciplinary nutritional care reduced nutritional deterioration during admission (5.4 vs. 20.5 %; p = 0.049), and increased the rate of discharge directly back to the community (48.0 vs. 17.6 %; p = 0.012) in a pragmatic intervention study [53]. A high-protein nutritional intervention-based study on β-hydroxy-β-methylbutyrate, vitamin D3, and calcium in obese and lean aged patients with hip fractures and sarcopenia will be implemented [54]. These results indicate that nutrition support for hip fracture patients may improve nutrition status and rehabilitation outcome.
Hospital-associated deconditioning
Hospital-associated deconditioning is characterized by the functional decline that occurs during acute hospitalization due to illness or injury, or both, and is unrelated to a specific neurological or orthopedic insult, or both [55]. Several concepts have been proposed to explain the consequences of inactivity and disuse in the hospital, and include debility [20], disuse syndrome [10, 21], hospital-associated deconditioning [11, 55], hospitalization-associated disability [56], and post-hospital syndrome [57]. During hospitalization, patients are commonly deprived of sleep, experience disruption of normal circadian rhythms, are nourished poorly, have pain and other discomfort, confront a baffling array of mentally challenging situations, receive medications that can alter cognition and physical function, and become deconditioned by bed rest or inactivity [57]. Hospitalization-associated disability occurs in approximately one-third of patients older than 70 years of age and may be triggered even when the illness that necessitated the hospitalization is successfully treated [56]. Therefore, hospital-associated deconditioning represents an important condition in geriatric rehabilitation medicine [11].
Malnutrition is associated with poor rehabilitation outcome in hospital-associated deconditioning. In an acute rehabilitation setting, obese patients with deconditioning show greater improvement in FIM scores, compared with patients whose BMI is in the normal range or lower (BMI ˂ 18.5) [58]. This lower BMI group shows the smallest increase in FIM motor scores with rehabilitation [58]. In elderly patients with deconditioning, admission Norton scale scores were correlated with discharge walking FIM scores (r = 0.32; p = 0.003), discharge transfer FIM scores (r = 0.30; p = 0.005), and length of rehabilitation (r = −0.37; p < 0.0001) [59]. In our previous prospective cohort study [11], 87.6 % of patients were malnourished, 12.4 % were at risk for malnutrition, and there were none with normal nutritional status. In multiple regression analysis, the MNA-SF score, albumin level, and cachexia status were significantly associated with the Barthel Index score at discharge [11]. These results indicated that patients with hospital-associated deconditioning may experience not only activity-related sarcopenia but also nutrition-related and disease-related sarcopenia [11]. Nutrition management and sarcopenia treatment in patients with hospital-associated deconditioning may lead to improvement of disability, although further studies are required.
Sarcopenic dysphagia
Sarcopenic dysphagia is characterized by the loss of swallowing muscle mass and function associated with generalized loss of skeletal muscle mass and function. The prevalence of dysphagia has been reported to be 11.4–38 % in community-dwelling elderly individuals [60–64] and 40–68 % in nursing home residents [65–67]. Dysphagia management is important because dysphagia is common in the elderly and increases the risk of related complications such as aspiration pneumonia, choking, dehydration, malnutrition, and a lower quality of life following the loss of the joy of eating. Furthermore, sarcopenic dysphagia is not only the result of aspiration pneumonia, but also an important cause of recurrent aspiration pneumonia [23]. Sarcopenic dysphagia may be common in elderly subjects with sarcopenia and dysphagia [23].
Age-related loss of swallowing muscles has been studied [24, 25]. Swallowing muscles include the intrinsic muscle of the tongue and the mimic, masticatory, suprahyoid, infrahyoid, palatal, pharyngeal, and esophageal muscles. Tamura et al. [24] evaluated thickness of the central part of the tongue in the elderly using ultrasonography and showed mid-arm muscle area and age were associated independently with tongue thickness. These results indicate that tongue muscle mass is associated with generalized skeletal muscle mass and aging. Feng et al. [25] assessed the geniohyoid muscle in healthy older adults using computed tomography. A decrease in the cross-sectional area of the geniohyoid muscle has been shown to occur with increasing age, with this area being significantly smaller in aspirators compared with non-aspirators, but only in older men [25]. These findings suggest that geniohyoid muscle atrophy may be a component of decreased swallowing safety and aspiration in older adults with presbyphagia or frailty of swallowing.
Mid-upper arm circumference and calf circumference were correlated with dysphagia [22, 68]. The circumference of the mid-upper arm in older Japanese adults with suspected swallowing disorders was correlated significantly with swallowing function [22]. This finding suggested that swallowing impairment was related to thinness. It is likely that the general reduction in lean body mass, including the swallowing muscle mass, is responsible for the association between mid-upper arm circumference and swallowing function, and indicates the presence of sarcopenic dysphagia [22]. Another study revealed that swallowing measures had significant correlations with the functional and nutritional measures including serum albumin levels, mid-upper arm circumference, and calf circumference but not with age [68]. Given that sarcopenia is exacerbated by disease, inactivity, and malnutrition, sarcopenia involving the swallowing muscle mass and its function may account for this result [68].
Malnutrition can cause dysphagia [69, 70]. Malnutrition results in both increased adductor pollicis muscle fatigability and an altered pattern of muscle contraction and relaxation which are reversible by nutritional supplementation [71]. No experimental evidence shows that malnutrition would affect the loss of swallowing muscle fibers. However, deglutition muscles that have a moderate to high percentage of type II fibers may be among the first to atrophy at malnutrition because malnutrition affects type II muscle fibers to a much greater extent than it does type I fibers [69, 70]. Furthermore, malnutrition was associated with dysphagia and head lifting strength which reflects the strength of the suprahyoid muscles in frail older adults [72].
Therapy for sarcopenic dysphagia includes dysphagia rehabilitation, treatment of sarcopenia, and nutrition improvement. The core components of dysphagia rehabilitation are oral health care, rehabilitative techniques, and food modification. Malnutrition contributes to the etiology of secondary sarcopenia and sarcopenic dysphagia. Therefore, nutrition management to increase muscle mass is indispensable for sarcopenic dysphagia rehabilitation, and the concept of rehabilitation nutrition is useful. Further research on sarcopenic dysphagia is required, although consensus diagnostic criteria for sarcopenic dysphagia have been proposed [23].
Research interest in rehabilitation nutrition
The rehabilitation medicine literature lacks research focused on nutrition and sarcopenia. We searched seven major rehabilitation journals cited in the article “Publishing in physical and rehabilitation medicine” [73] and indexed by PubMed. These rehabilitation journals were the Archives of Physical Medicine and Rehabilitation, Clinical Rehabilitation, Journal of Rehabilitation Medicine, the European Journal of Physical and Rehabilitation Medicine, the American Journal of Physical Medicine and Rehabilitation, Disability and Rehabilitation, and International Journal of Rehabilitation Research. Of 24,214 PubMed entries for these seven journals, 185 (0.8 %) and 8 (0.03 %), respectively, contained the words “nutrition” and “sarcopenia” on 25 April 2014 (Table 1). Four articles (one editorial and three reviews) published in the European Journal of Physical and Rehabilitation Medicine contained the word “sarcopenia” and were about sarcopenia and muscular modifications in disabling pathologies [74–77]. Though the importance of nutrition in rehabilitation was already recognized in the 1940s [78], interest in nutrition and sarcopenia in rehabilitation medicine has remained very low.
Table 1.
Number of PubMed entries retrieved in a search of seven rehabilitation journals for the terms “nutrition” and “sarcopenia.” Accessed on 25 April 2014 from www.pubmed.gov
| Journal name | Total no. of entries | Nutrition | Sarcopenia |
|---|---|---|---|
| Archives of Physical Medicine and Rehabilitation | 11,856 | 96 | 2 |
| Clinical Rehabilitation | 1,768 | 10 | 1 |
| Journal of Rehabilitation Medicine | 1,499 | 6 | 0 |
| European Journal of Physical and Rehabilitation Medicine | 523 | 5 | 5 |
| American Journal of Physical Medicine and Rehabilitation | 3,123 | 30 | 0 |
| Disability and Rehabilitation | 3,638 | 27 | 0 |
| International Journal of Rehabilitation Research | 1,807 | 11 | 0 |
| Total | 24,214 | 185 (0.8 %) | 8 (0.03 %) |
In Japan, interest in rehabilitation nutrition has increased in recent years. Using the Japan Medical Abstracts Society Database, we searched for articles in four major Japanese rehabilitation journals including the Japanese Journal of Rehabilitation Medicine, Sogo Rihabiriteshon, Journal of Clinical Rehabilitation, and Medical Rehabilitation. Of the 38,898 entries of these four journals, 1092 (2.8 %) and 55 (0.1 %), respectively, contained the words “nutrition” and “sarcopenia” on 25 April 2014 (Table 2). When the search was limited to entries after 2010, 4.4 and 0.7 %, respectively, contained the words “nutrition” and “sarcopenia”.
Table 2.
Number of Japan Medical Abstracts Society Database entries retrieved in a search of four Japanese rehabilitation journals for the words “nutrition” and “sarcopenia.” Accessed on 25 April 2014 from http://www.jamas.or.jp/about/english.html
| Entire period | From 2010 | |||||
|---|---|---|---|---|---|---|
| Journal name | Total | Nutrition | Sarcopenia | Total | Nutrition | Sarcopenia |
| The Japanese Journal of Rehabilitation Medicine | 24,457 | 545 | 17 | 4,419 | 136 | 15 |
| Sogo Rihabiriteshon | 7,759 | 136 | 8 | 1,100 | 31 | 5 |
| Journal of Clinical Rehabilitation | 4,602 | 180 | 9 | 839 | 53 | 8 |
| Medical Rehabilitation | 2,080 | 231 | 21 | 778 | 97 | 20 |
| Total | 38,898 | 1,092 (2.8 %) | 55 (0.1 %) | 7,136 | 317 (4.4 %) | 48 (0.7 %) |
We established the Japanese Association of Rehabilitation Nutrition in 2011; its membership in April 2014 had increased to more than 3300 people and included physical therapists, registered dietitians, speech-language-hearing therapists, etc. Moreover, 629 people attended the 3rd Congress of the Japanese Association of Rehabilitation Nutrition held in 2013.
Interest in rehabilitation nutrition is increasing in Japan because of the emergence of a rapidly aging society, high number of convalescent rehabilitation beds, and high number of nutrition support teams in hospitals. The aging rate in Japan is the highest in the world (i.e., 25.1 % in October 2013). The number of convalescent rehabilitation beds available under the Japanese Medical Insurance System has increased since 2000 to 68,316 in March 2014. The mean age of the patients in convalescent rehabilitation wards is 73.0 years [21]. These data suggest that the number of disabled elderly with malnutrition and sarcopenia is increasing at an accelerated pace. The number of hospitals that have nutrition support teams certified by the Japan Council for Nutritional Therapy was 1001 in 2013. Many physical therapists, occupational therapists, and speech-language-hearing therapists are actively involved in nutrition support teams and interested in nutrition care management. Collaborative studies of rehabilitation nutrition have been undertaken by the Japanese Association of Rehabilitation Nutrition [72]. Furthermore, the Japanese Society for Sarcopenia, Cachexia and Wasting Disorders was established in 2014. Further, more focused, research on rehabilitation nutrition will be needed because the number of elderly with disability is expected to increase in developed countries as the population ages [79, 80].
Conclusion
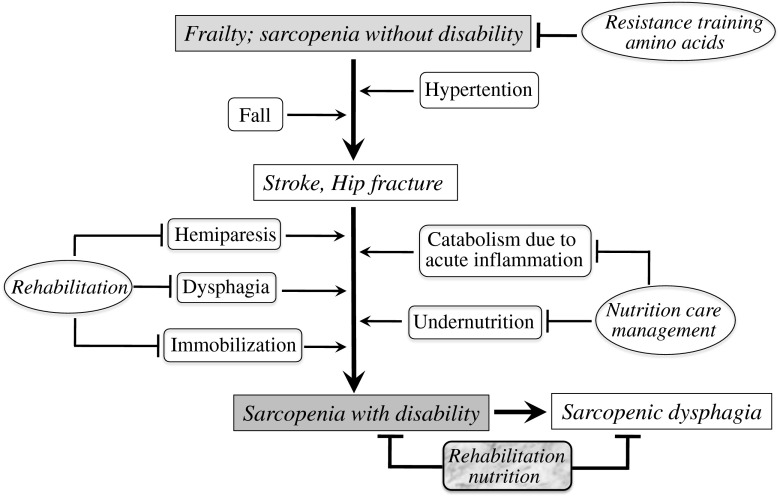
The prevalence of malnutrition and sarcopenia in physically disabled elderly patients who undergo rehabilitation is high. In contrast, the amount of research focused on nutrition and sarcopenia in rehabilitation medicine is very low. The major causes of disability in inpatients of rehabilitation facilities, including stroke, hip fracture, and hospital-associated deconditioning, are often complicated by malnutrition and sarcopenia. Sarcopenic dysphagia is common in the elderly population and is not only the result of aspiration pneumonia, but also an important cause of recurrent aspiration pneumonia. Because primary and secondary sarcopenia often coexist in people with disability, rehabilitation nutrition can be used to improve their functionality (Fig. 1). Further studies on rehabilitation nutrition are important in a rapidly aging society, where the number of elderly with disability is expected to increase.
Fig. 1.
Mechanism of sarcopenia with disability in frail elderly with stroke and hip fracture. Frail elderly with stroke or hip fracture becomes sarcopenia with disability because of hemiparesis, dysphagia, immobilization, catabolism due to acute inflammation, and undernutrition. Rehabilitation for hemiparesis, dysphagia, immobilization, and nutrition care management for catabolism due to acute inflammation and undernutrition are usually provided separately. Sarcopenia with disability induces sarcopenic dysphagia which is characterized by the loss of swallowing muscle mass and function associated with generalized loss of skeletal muscle mass and function. Rehabilitation nutrition can be used to improve functionality in people with sarcopenic dysphagia and sarcopenia with disability
Acknowledgments
All authors of this manuscript have complied with the guidelines of ethical authorship and publishing as stated in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD). This work was supported by a research Grant-in-Aid for Scientific Research C (no. 25350611) from the Ministry of Education, Science, Culture, Sports, Science, and Technology of Japan.
Conflict of interest
Hidetaka Wakabayashi and Kunihiro Sakuma declare that they have no conflict of interest.
References
- 1.Wakabayashi H. Seamless community coordination of rehabilitation nutrition care management in patients with dysphagia. Gan To Kagaku Ryoho. 2010;37:198–200. [PubMed] [Google Scholar]
- 2.Wakabayashi H. Rehabilitation and clinical nutrition. Jpn J Rehabil Med. 2011;48:270–81. doi: 10.2490/jjrmc.48.270. [DOI] [Google Scholar]
- 3.Strakowski MM, Strakowski JA, Mitchell MC. Malnutrition in rehabilitation. Am J Phys Med Rehabil. 2002;81:77–8. doi: 10.1097/00002060-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Charlton KE, Nichols C, Bowden S, Lambert K, Barone L, Mason M, et al. Older rehabilitation patients are at high risk of malnutrition: evidence from a large Australian database. J Nutr Health Aging. 2010;14:622–8. doi: 10.1007/s12603-010-0307-3. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser MJ, Bauer JM, Rämsch C, Uter W, Guigoz Y, Cederholm T, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–8. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser MJ, Bauer JM, Uter W, Donini LM, Stange I, Volkert D, et al. Prospective validation of the modified mini nutritional assessment short-forms in the community, nursing home, and rehabilitation setting. J Am Geriatr Soc. 2011;59:2124–8. doi: 10.1111/j.1532-5415.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 7.Marshall S, Bauer J, Isenring E. The consequences of malnutrition following discharge from rehabilitation to the community: a systematic review of current evidence in older adults. J Hum Nutr Diet. 2014;27:133–41. doi: 10.1111/jhn.12167. [DOI] [PubMed] [Google Scholar]
- 8.Davis JP, Wong AA, Schluter PJ, Henderson RD, O’Sullivan JD, Read SJ. Impact of premorbid undernutrition on outcome in stroke patients. Stroke. 2004;35:1930–4. doi: 10.1161/01.STR.0000135227.10451.c9. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, John M, Pedersen PU, Raguso C, Cicoira M, Dardai E, et al. ESPEN guidelines on enteral nutrition: cardiology and pulmonology. Clin Nutr. 2006;20:311–8. doi: 10.1016/j.clnu.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi H, Sashika H. Association of nutrition status and rehabilitation outcome in the disuse syndrome: a retrospective cohort study. Gen Med. 2011;12:69–74. doi: 10.14442/general.12.69. [DOI] [Google Scholar]
- 11.Wakabayashi H, Sashika H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital-associated deconditioning a prospective cohort study. J Rehabil Med. 2014;46:277–82. doi: 10.2340/16501977-1258. [DOI] [PubMed] [Google Scholar]
- 12.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaxley A, Miller MD, Fraser RJ, Cobiac L, Crotty M. The complexity of treating wasting in ambulatory rehabilitation: is it starvation, sarcopenia, cachexia or a combination of these conditions? Asia Pac J Clin Nutr. 2012;21:386–93. [PubMed] [Google Scholar]
- 14.Sánchez-Rodríguez D, Marco E, Miralles R, Fayos M, Mojal S, Alvarado M, et al. Sarcopenia, physical rehabilitation and functional outcomes of patients in a subacute geriatric care unit. Arch Gerontol Geriatr. 2014 doi: 10.1016/j.archger.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi H, Sakuma K. Comprehensive approach to sarcopenia treatment. Curr Clin Pharmacol. 2014;9:171–80. doi: 10.2174/1574884708666131111192845. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi H, Sakuma K. Nutrition, exercise, and pharmaceutical therapies for sarcopenic obesity. J Nutr Ther. 2013;2:100–11. [Google Scholar]
- 18.Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012;3:77–94. [DOI] [PMC free article] [PubMed]
- 19.Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch. 2014. doi:10.1007/s00424-014-1527-x [DOI] [PubMed]
- 20.Ottenbacher KJ, Karmarkar A, Graham JE, Kuo YF, Deutsch A, Reistetter TA, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA. 2014;311:604–14. doi: 10.1001/jama.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyai I, Sonoda S, Nagai S, Takayama Y, Inoue Y, Kakehi A, et al. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil Neural Repair. 2011;25:540–7. doi: 10.1177/1545968311402696. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda Y, Kuroda R. Relationship between thinness and swallowing function in Japanese older adults: implications for sarcopenic dysphagia. J Am Geriatr Soc. 2012;60:1785–6. doi: 10.1111/j.1532-5415.2012.04123.x. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi H. Presbyphagia and sarcopenic dysphagia: association between aging, sarcopenia, and deglutition disorders. J Frailty Aging 2014;3:97–103. [DOI] [PubMed]
- 24.Tamura F, Kikutani T, Tohara T, Yoshida M, Yaegaki K. Tongue thickness relates to nutritional status in the elderly. Dysphagia. 2012;27:556–61. doi: 10.1007/s00455-012-9407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, Todd T, Lintzenich CR, Ding J, Carr JJ, Ge Y, et al. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68:853–60. doi: 10.1093/gerona/gls225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–26. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 27.Scherbakov N, Doehner W. Sarcopenia in stroke-facts and numbers on muscle loss accounting for disability after stroke. J Cachexia Sarcopenia Muscle. 2011;2:5–8. [DOI] [PMC free article] [PubMed]
- 28.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. 2009;41:707–13. doi: 10.2340/16501977-0415. [DOI] [PubMed] [Google Scholar]
- 30.Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42:3646–50. doi: 10.1161/STROKEAHA.111.619163. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Du W, Zhao X, Liu L, Wang C, Wang Y, et al. Favorable functional recovery in overweight ischemic stroke survivors: findings from the China National Stroke Registry. J Stroke Cerebrovasc Dis. 2014;23:e201–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Burke DT, Al-Adawi S, Bell RB, Easley K, Chen S, Burke DP. Effect of body mass index on stroke rehabilitation. Arch Phys Med Rehabil. 2014. doi:10.1016/j.apmr.2014.01.019. [DOI] [PubMed]
- 33.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke. 2010;5:395–402. doi: 10.1111/j.1747-4949.2010.00467.x. [DOI] [PubMed] [Google Scholar]
- 34.Rabadi MH, Coar PL, Lukin M, Lesser M, Blass JP. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology. 2008;71:1856–61. doi: 10.1212/01.wnl.0000327092.39422.3c. [DOI] [PubMed] [Google Scholar]
- 35.Ha L, Hauge T, Spenning AB, Iversen PO. Individual, nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized, controlled trial. Clin Nutr. 2010;29:567–73. doi: 10.1016/j.clnu.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD000323.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–42. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 38.Iki M. Epidemiology of osteoporosis in Japan. Clin Calcium. 2012;22:797–803. [PubMed] [Google Scholar]
- 39.Bell JJ, Bauer JD, Capra S, Pulle RC. Concurrent and predictive evaluation of malnutrition diagnostic measures in hip fracture inpatients: a diagnostic accuracy study. Eur J Clin Nutr. 2014;68:358–62. doi: 10.1038/ejcn.2013.276. [DOI] [PubMed] [Google Scholar]
- 40.Bell JJ, Bauer JD, Capra S. The malnutrition screening tool versus objective measures to detect malnutrition in hip fracture. J Hum Nutr Diet. 2013;26:519–26. doi: 10.1111/jhn.12040. [DOI] [PubMed] [Google Scholar]
- 41.Pérez Durillo FT, Ruiz López MD, Bouzas PR, Martín-Lagos A. Nutritional status in elderly patients with a hip fracture. Nutr Hosp. 2010;25:676–81. [PubMed] [Google Scholar]
- 42.Koren-Hakim T, Weiss A, Hershkovitz A, Otzrateni I, Grosman B, Frishman S, et al. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin Nutr. 2012;31:917–21. doi: 10.1016/j.clnu.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Gumieiro DN, Rafacho BP, Gonçalves AF, Tanni SE, Azevedo PS, Sakane DT, et al. Mini nutritional assessment predicts gait status and mortality 6 months after hip fracture. Br J Nutr. 2013;109:1657–61. doi: 10.1017/S0007114512003686. [DOI] [PubMed] [Google Scholar]
- 44.Miyanishi K, Jingushi S, Torisu T. Mortality after hip fracture in Japan: the role of nutritional status. J Orthop Surg (Hong Kong) 2010;18:265–70. doi: 10.1177/230949901001800301. [DOI] [PubMed] [Google Scholar]
- 45.Fiatarone Singh MA, Singh NA, Hansen RD, Finnegan TP, Allen BJ, Diamond TH, et al. Methodology and baseline characteristics for the sarcopenia and Hip fracture study: a 5-year prospective study. J Gerontol A Biol Sci Med Sci. 2009;64:568–74. doi: 10.1093/gerona/glp002. [DOI] [PubMed] [Google Scholar]
- 46.Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 2011;52:71–4. doi: 10.1016/j.archger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr. 2012;55:e48–52. doi: 10.1016/j.archger.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013;13:413–20. doi: 10.1111/j.1447-0594.2012.00918.x. [DOI] [PubMed] [Google Scholar]
- 49.Villani AM, Miller MD, Cameron ID, Kurrle S, Whitehead C, Crotty M. Development and relative validity of a new field instrument for detection of geriatric cachexia: preliminary analysis in hip fracture patients. J Cachexia Sarcopenia Muscle. 2013;4:209–16. [DOI] [PMC free article] [PubMed]
- 50.Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD001880.pub5. [DOI] [PubMed] [Google Scholar]
- 51.Hoekstra JC, Goosen JH, de Wolf GS, Verheyen CC. Effectiveness of multidisciplinary nutritional care on nutritional intake, nutritional status and quality of life in patients with hip fractures: a controlled prospective cohort study. Clin Nutr. 2011;30:455–61. doi: 10.1016/j.clnu.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Anbar R, Beloosesky Y, Cohen J, Madar Z, Weiss A, Theilla M, et al. Tight calorie control in geriatric patients following hip fracture decreases complications: a randomized, controlled study. Clin Nutr. 2014;33:23–8. doi: 10.1016/j.clnu.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Bell JJ, Bauer JD, Capra S, Pulle RC. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients—results of a pragmatic intervention. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Malafarina V, Uriz-Otano F, Gil-Guerrero L, Iniesta R, Zulet MA, Martinez JA. Study protocol: high-protein nutritional intervention based on β-hydroxy-β-methylbutirate, vitamin D3 and calcium on obese and lean aged patients with hip fractures and sarcopenia. The HIPERPROT-GER study. Maturitas. 2013;76:123–8. doi: 10.1016/j.maturitas.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil. 2009;88:66–77. doi: 10.1097/PHM.0b013e3181838f70. [DOI] [PubMed] [Google Scholar]
- 56.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–93. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 57.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–2. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain NB, Al-Adawi S, Dorvlo AS, Burke DT. Association between body mass index and functional independence measure in patients with deconditioning. Am J Phys Med Rehabil. 2008;87:21–5. doi: 10.1097/PHM.0b013e31815e61af. [DOI] [PubMed] [Google Scholar]
- 59.Guy N, Lerman Y, Justo D. Admission Norton scale scores (ANSS) correlate with rehabilitation outcome and length in elderly patients with deconditioning. Arch Gerontol Geriatr. 2012;54:381–4. doi: 10.1016/j.archger.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Bloem BR, Lagaay AM, van Beek W, Haan J, Roos RA, Wintzen AR. Prevalence of subjective dysphagia in community residents aged over 87. BMJ. 1990;300:721–2. doi: 10.1136/bmj.300.6726.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawashima K, Motohashi Y, Fujishima I. Prevalence of dysphagia among community-dwelling elderly individuals as estimated using a questionnaire for dysphagia screening. Dysphagia. 2004;19:266–71. doi: 10.1007/s00455-004-0013-6. [DOI] [PubMed] [Google Scholar]
- 62.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116:858–65. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 63.Serra-Prat M, Hinojosa G, López D, Juan M, Fabré E, Voss DS, et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J Am Geriatr Soc. 2011;59:186–7. doi: 10.1111/j.1532-5415.2010.03227.x. [DOI] [PubMed] [Google Scholar]
- 64.Holland G, Jayasekeran V, Pendleton N, Horan M, Jones M, Hamdy S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: a self-reporting questionnaire survey. Dis Esophagus. 2011;24:476–80. doi: 10.1111/j.1442-2050.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 65.Steele CM, Greenwood C, Ens I, Robertson C, Seidman-Carlson R. Mealtime difficulties in a home for the aged: not just dysphagia. Dysphagia. 1997;12:43–50. doi: 10.1007/PL00009517. [DOI] [PubMed] [Google Scholar]
- 66.Park YH, Han HR, Oh BM, Lee J, Park JA, Yu SJ, et al. Prevalence and associated factors of dysphagia in nursing home residents. Geriatr Nurs. 2013;34:212–7. doi: 10.1016/j.gerinurse.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Nogueira D, Reis E. Swallowing disorders in nursing home residents: how can the problem be explained? Clin Interv Aging. 2013;8:221–7. doi: 10.2147/CIA.S39452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroda Y. Relationship between swallowing function, and functional and nutritional status in hospitalized elderly individuals. Int J Speech Lang Pathol Audiol. 2014;2:20–6. doi: 10.12970/2311-1917.2014.02.01.3. [DOI] [Google Scholar]
- 69.Veldee MS, Peth LD. Can protein-calorie malnutrition cause dysphagia? Dysphagia. 1992;7:86–101. doi: 10.1007/BF02493439. [DOI] [PubMed] [Google Scholar]
- 70.Hudson HM, Daubert CR, Mills RH. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia. 2000;15:31–8. doi: 10.1007/s004559910007. [DOI] [PubMed] [Google Scholar]
- 71.Lopes J, Russell DM, Whitwell J, Jeejeebhoy KN. Skeletal muscle function in malnutrition. Am J Clin Nutr. 1982;36:602–10. doi: 10.1093/ajcn/36.4.602. [DOI] [PubMed] [Google Scholar]
- 72.Wakabayashi H, Sashika H, Matsushima M. Head lifting strength is associated with dysphagia and malnutrition in frail older adults. Geriatr Gerontol Int. 2014 doi: 10.1111/ggi.12283. [DOI] [PubMed] [Google Scholar]
- 73.Franchignoni F, Ozçakar L, Michail X, Vanderstraeten G, Christodoulou N, Frischknecht R. Publishing in physical and rehabilitation medicine. An update on the European point of view. Eur J Phys Rehabil Med. 2013;49:711–4. [PubMed] [Google Scholar]
- 74.Invernizzi M, Cisari C. Sarcopenia and muscular modifications in disabling pathologies of the elderly from the physical and rehabilitation medicine: point of view. Eur J Phys Rehabil Med. 2013;49:107–9. [PubMed] [Google Scholar]
- 75.Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013;49:111–7. [PubMed] [Google Scholar]
- 76.Carda S, Cisari C, Invernizzi M. Sarcopenia or muscle modifications in neurologic diseases: a lexical or patophysiological difference? Eur J Phys Rehabil Med. 2013;49:119–30. [PubMed] [Google Scholar]
- 77.Montero-Fernández N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49:131–43. [PubMed] [Google Scholar]
- 78.Nutrition in convalescence and rehabilitation. Nutr Rev. 1945;3:59–61.
- 79.Palacios-Ceña D, Jiménez-García R, Hernández-Barrera V, Alonso-Blanco C, Carrasco-Garrido P, Fernández-de-Las-Peñas C. Has the prevalence of disability increased over the past decade (2000–2007) in elderly people? A Spanish population-based survey. J Am Med Dir Assoc. 2012;13:136–42. doi: 10.1016/j.jamda.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Annual report on government measures for persons with disabilities (Summary) 2012. In: cabinet office. 2012. http://www8.cao.go.jp/shougai/english/annualreport/2012/pdf/s6.pdf Accessed 25 Apr 2014.