Abstract
Sarcopenia is now defined as a decline in walking speed or grip strength associated with low muscle mass. Sarcopenia leads to loss of mobility and function, falls, and mortality. Sarcopenia is a major cause of frailty, but either condition can occur without the other being present. Sarcopenia is present in about 5 to 10 % of persons over 65 years of age. It has multiple causes including disease, decreased caloric intake, poor blood flow to muscle, mitochondrial dysfunction, a decline in anabolic hormones, and an increase in proinflammatory cytokines. Basic therapy includes resistance exercise and protein and vitamin D supplementation. There is now a simple screening test available for sarcopenia—SARC-F. All persons 60 years and older should be screened for sarcopenia and treated when appropriate.
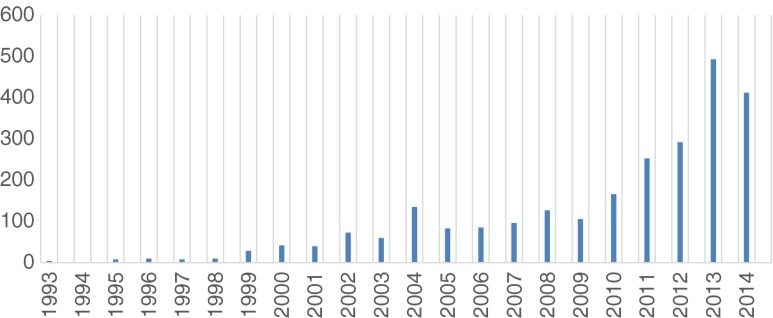
Sarcopenia was originally defined as an excessive loss of muscle mass that is associated with aging [1]. Subsequently, it was recognized that the key element was a loss of muscle strength (dynapenia) rather than a loss of muscle mass [2]. This has led to a change in the definition of sarcopenia to include strength (grip strength) or function (walking speed or distance). Based on this concept, a number of societies around the world have provided revised definitions of sarcopenia (Table 1) [3–8]. These definitions have to some extent de-emphasized the importance of aging, recognizing that sarcopenia has a variety of causes in addition to the physiological effects of aging. Sarcopenia, cachexia, and anorexic disorders (protein-energy malnutrition) represent the major causes of muscle-wasting disorders [9]. The increased interest in sarcopenia is clearly seen by the number of publications published in the last few years compared to previously (Fig. 1). This article represents the third update on sarcopenia published in the journal [10, 11].
Table 1.
Comparison of sarcopenia definitions
| Definition | Function | Muscle mass |
|---|---|---|
| SIG: cachexia-anorexia in chronic wasting disease [3] | Gait speed <0.8 m/s, OR other physical performance test | Low muscle mass (2SD) |
| EWGSOP [4] | Gait speed <0.8 m/s; grip strength 40 kg males, 30 kg females | Low muscle mass (not defined) |
| IWGS Sarcopenia Task Force [5] | Gait speed <1.0 m/s, grip strength | Low appendicular lean mass (<7.23 kg/m2 in men, 5.67 in women) |
| Sarcopenia with limited mobility (SCWD) [6] | 6 min walk <400 m, OR gait speed <1.0 m/s | Low appendicular lean mass/height2 |
| Asian Working Group for Sarcopenia [7] | Gait speed <0.8 m/s; grip strength 26 kg males, 18 kg females | Low appendicular lean mass/height2 |
| Foundation for the National Institutes of Health [8] | Gait speed <0.8 m/s; grip strength 26 kg males, 16 kg females | Appendicular lean mass/BMI |
EWGSOP European Working Group of Sarcopenia in Older Persons, SCWD Sarcopenia, Cachexia and Wasting Disorders, IANA International Association of Nutrition and Aging)
Fig. 1.
Publications on sarcopenia as shown in PubMed (1993 to August 2014)
Prevalence of sarcopenia
Since Baumgartner et al. [12, 13] originally defined sarcopenia as being two standard deviations below the normal appendicular muscle mass divided by height squared, numerous groups have examined the prevalence of low muscle mass. Multiple techniques have been used to measure muscle mass, e.g., dual-energy X-ray absorptiometry (DEXA), computed tomography, MRI, ultrasound, bioelectrical impedance, and anthropometry. On the average, 5–13 % of older persons over 60 years of age have low muscle mass, with the prevalence increasing to as high as 50 % in persons over the age of 80 years [11, 14]. Normal aging is associated with approximately a 1 % loss of muscle from 30 years of age, and this loss tends to accelerate after the age of 70 years [15]. In a recent study, 25 % of patients in a geriatric ward were deemed to be sarcopenic [16]. Coin et al. [17] found that about 20 % of community-dwelling persons in Italy had low muscle mass [18]. In Korea, the prevalence of sarcopenia using the Baumgartner criteria was 0.8 % in women and 1.3 % in men over 60 years of age [19]. In Barcelona, low lean mass was present in 33 % of elderly women and 10 % of males [20]. In Taiwan, low muscle mass was present in 2.5 % of community-dwelling women and 5.4 % of males [21]. Two medical conditions, stroke and hip fracture, rapidly lead to an increase in muscle loss [22, 23]. This appears to be due to disuse and inflammation, and in the case of stroke, denervation. In addition, persons with diabetes mellitus have accelerated muscle loss [24, 25].
Persons with muscle loss in combination with excess fat are considered to have obese sarcopenia [26–28]. These persons are at a much higher risk of functional decline and mortality.
Validation of the new sarcopenic definitions
Using the European Working Group on Sarcopenia in Older People (EWGSOP) definition, 4.6 % of males and 7.9 % of women in Hertfordshire had sarcopenia [29]. In Japan, 21.8 % of men and 22.9 % of women aged 65 to 89 years had sarcopenia [30]. In a population of persons 80 years and older, 12.5 % had sarcopenia by the EWGSOP definition [31]. In nursing home residents, 32.8 % had sarcopenia [32]. Sarcopenia was highly predictive of earlier mortality [33–36]. Sarcopenic individuals had a greater than threefold increase in falls [21, 37]. Sarcopenia has also been shown to prospectively predict mobility and instrumental activities of daily living (IADL) disability [38].
The prevalence of sarcopenia was slightly less common using the International Working Group on Sarcopenia (IWGS) criteria compared to the EWGSOP [39]. The Sarcopenia, Cachexia and Wasting Disorders (SCWD) definition was found to predict ADL and IADL difficulties, frailty, and mortality in a longitudinal study [40].
The Foundation for the National Institutes of Health (FNIH) criteria were based on developing cutoffs using a variety of large epidemiological studies [8]. These criteria are more restrictive with only 1.3 % of men and 2.3 % of women being defined as having sarcopenia. While there was a strong negative percent agreement with the other definition, the positive percent agreements were low ranging from 5 to 32 %. There is a need to compare the FNIH criteria to other definitions to determine which has the best predictive ability.
Can sarcopenia be predicted by a questionnaire without measurements?
Recently, it has been determined that fracture risk can be determined almost as accurately by the FRAX questions as by measuring bone mineral density [41]. This raises the question of whether or not a simple questionnaire can be used to identify persons with sarcopenia. Two such questionnaires have been developed [42, 43]. The SARC-F questionnaire has been validated in two published studies (Table 3) [44, 45]. Woo et al. [44] found that SARC-F had comparable predictive activity to EWGSOP, IWGS, and the Asian Working Group for Sarcopenia. Like the others in this Asian population, it had modest predictive value for 4-year physical limitation. Cao et al. [45] have also provided evidence for validity of the SARC-F.
Table 3.
Biomarkers for sarcopenia
| Biomarker | Aging | Exercise | Comments |
|---|---|---|---|
| Creatine kinase | ↓ | ↑ | |
| Aldolase (A) | ↓ | Correlates with walking speed in old | |
| Coenzyme Q | ↓ | More strongly correlated with FFM | |
| MLC1 | ↓ | ||
| Troponin T | ? | ↑ | |
| Creatine (U) | Correlates with muscle mass | ||
| Myoglobin | ↓ | ↑ | |
| Creatinine (U) (?/cystatin C) | ↓ | Correlates with muscle J-shaped curve with function | |
| N-terminal propeptide III collagen | ↓ | Increased with testosterone | |
| C-terminal agrin fragment | ↓ | Correlates with degeneration of the neuromuscular junction |
Differentiating between frailty and sarcopenia
The physical frailty phenotype as originally defined by Fried et al. [46] has been demonstrated to be highly predictive of poor outcomes [47, 48]. A simple questionnaire—the FRAIL (Table 2)—has been developed and shown to be equally predictive of poor outcomes [49–53]. While sarcopenia is clearly a major component of frailty, they are not identical conditions. Overlap between sarcopenia and frailty ranges from 50 to 70 %.
Table 2.
Comparison of the brief screens for sarcopenia and frailty
| (a) SARC-F | (b) FRAIL | ||
|---|---|---|---|
| Component | Question | Scoring | |
| Strength | How much difficulty do you have in lifting and carrying 10 lb? | None = 0 | Fatigue |
| Some = 1 | Resistance (Can you climb a flight of stairs?) | ||
| A lot or unable = 2 | Aerobic (Can you walk a block?) | ||
| Assistance in walking | How much difficulty do you have walking across a room? | None = 0 | Illness (>5) |
| Some = 1 | Loss of weight (5% in 6 months) (three or more positive answers = frail; one or two positive answers = prefrail) |
||
| A lot, use aids, or unable = 2 | |||
| Rise from a chair | How much difficulty do you have transferring from a chair or bed? | None = 0 | |
| Some = 1 | |||
| A lot or unable without help = 2 | |||
| Climb stairs | How much difficulty do you have climbing a flight of 10 stairs? | None = 0 | |
| Some = 1 | |||
| A lot or unable = 2 | |||
| Falls | How many times have you fallen in the past year? | None = 0 | |
| 1–3 falls = 1 | |||
| ≥4 falls = 2 | |||
Sarcopenia and biomarkers
Diagnostic imaging and functional tests are the basic biomarkers for the diagnosis of sarcopenia [54]. A consensus statement has provided a long list of possible serum biomarkers [55]. These are listed in Table 3. To these needs to be added the measurement of motor unit number index, which can be used to assess the number and the size of the motor units [56]. This is important as loss of motor unit input to muscle is an important cause of sarcopenia in at least half of older persons.
Pathophysiology of sarcopenia
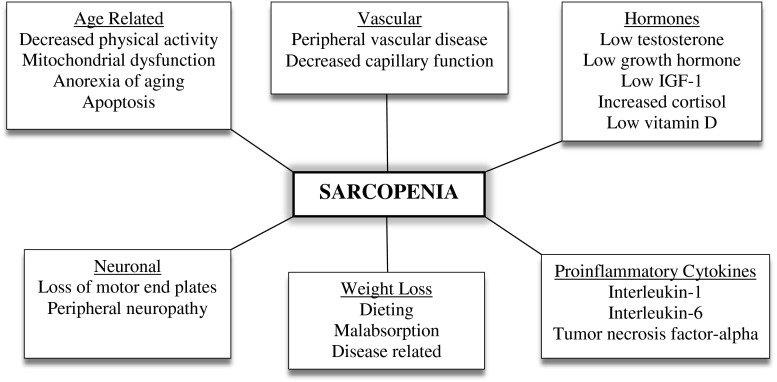
The factors leading to sarcopenia are multifactorial (Fig. 2) [57, 58]. Disuse coupled with aging is the major underlying cause: Poor blood flow to muscle, especially the muscle capillaries due to a decline in nitric oxide production, is another important age-related cause of sarcopenia. Aging is associated with an increase in mitochondrial abnormalities leading to damage to the mitochondrial membrane permeability pore and apoptosis.
Fig. 2.
The causes of sarcopenia
Aging is associated with a physiological anorexia of aging that leads to weight loss [59, 60]. Weight loss results in a 75 % loss of fat and a 25 % loss of muscle and bone. Only a very small amount of muscle is regained when a person gains weight. The increase of fat during weight regain is one of the major causes of sarcopenic obesity.
The age-related loss of motor neuron end plates is a major component of sarcopenia [56]. It leads not only to muscle wasting but also to a decrease in muscle function. Loss of anabolic hormones, such as testosterone, DHEA, growth hormone, and insulin-growth factor 1, occurs with aging. Of these, testosterone has been shown to be the hormone that most closely determines the decline in muscle mass and strength [61]. Testosterone is important not only for protein synthesis but also for the maintenance of satellite cells [62, 63]. Insulin resistance, which occurs with aging and obesity, plays an important role in decreasing available glucose and protein for muscle anabolism [64]. Obesity and disease lead to an increase in proinflammatory cytokines (e.g., interleukin-6, interleukin-1, and tumor necrosis factor alpha). These lead to protein catabolism through the activation of NFkB [65].
Therapeutic approaches
It is now clear that resistance exercise is the primary therapeutic strategy to prevent and reverse sarcopenia [66–70]. The LIFE study has also shown a therapeutic role for aerobic exercise [71]. There is evidence that leucine-enriched essential amino acid supplementation will increase muscle mass and probably function [72–75]. Vitamin D has been shown to enhance muscle function in persons with low muscle function (<50 nmol) [76, 77].
There is a small amount of data suggesting that testosterone will increase muscle mass and strength and may improve function in older frail persons with hypogonadism [78–81]. However, its safety is not clearly established. Selective androgen receptor modulators (SARMs) have shown some promise in increasing muscle mass and stair climb [82]. A number of antibodies that modulate myostatin and the activin II receptor are in clinical trials [83]. Ghrelin agonists, which increase food intake and release growth hormone, are also under evaluation [84].
Conclusion
Sarcopenia represents a major cause of falls and functional deterioration in older persons. Loss of muscle mass is commonly associated with loss of bone, making these individuals at high risk for hip fractures [85]. Persons with muscle loss develop accelerated loss of muscle mass and strength when they develop a variety of diseases such as heart failure, chronic obstructive pulmonary disease, and renal failure [86–89]. Like osteoporosis, sarcopenia is becoming recognized as a definable condition [90]. It is time for physicians to screen for sarcopenia and provide treatment for it—at a minimum resistance exercise and protein and vitamin D supplementation.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle 2010; 1:7–8 (vonHaehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
No outside funding was received for the writing of this article. All authors (JEM, SDA, SH) declare that they have no conflicts of interest involving this work.
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Clark BC, Manini TM. Sarcopenia ≠ dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 3.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker SD, Coats AJ, Morley JE, Rosano G, Bernabei R, von Haehling S, et al. Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle. 2014;5:1–3. [DOI] [PMC free article] [PubMed]
- 10.von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle. 2012;3:213–7. [DOI] [PMC free article] [PubMed]
- 11.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. [DOI] [PMC free article] [PubMed]
- 12.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 14.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29(Suppl 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 15.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc. 2014;15:267–272. doi: 10.1016/j.jamda.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Coin A, Sarti S, Ruggiero E, Giannini S, Pedrazzoni M, Minisola S, et al. Prevalence of sarcopenia based on different diagnostic criteria using DEXA and appendicular skeletal muscle mass reference values in an Italian population aged 20 to 80. J Am Med Dir Assoc. 2013;14:507–512. doi: 10.1016/j.jamda.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Rossi AP, Fantin F, Micciolo R, Bertocchi M, Bertassello P, Zanadrea V, et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc. 2014;15:303.e7–e12. doi: 10.1016/j.jamda.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the fourth Korean national health and nutritional examination surveys. J Gerontol A Biol Sci Med Sci. 2012;67:1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 20.Masanes F, Culla A, Navarro-Gonzalez M, Navarro-Lopez MC, Sacanella E, Torres B, et al. Prevalence of sarcopenia in healthy community-dwelling elderly in an urban area of Barcelona (Spain) J Nutr Health Aging. 2012;16:184–187. doi: 10.1007/s12603-011-0108-3. [DOI] [PubMed] [Google Scholar]
- 21.Wu IC, Lin CC, Hsiung CA, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int. 2014;14(Suppl 1):52–60. doi: 10.1111/ggi.12193. [DOI] [PubMed] [Google Scholar]
- 22.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Villani AM, Miller MD, Cameron ID, Kurrle S, Whitehead C, Crotty M. Development and relative validity of a new field instrument for detection of geriatric cachexia: preliminary analysis in hip fracture patients. J Cachexia Sarcopenia Muscle. 2013;4:209–16. [DOI] [PMC free article] [PubMed]
- 24.Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14:585–592. doi: 10.1016/j.jamda.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):115–121. doi: 10.1111/ggi.12189. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 27.Rolland Y, Lauwers-Cances V, Cristini C, Van Kan Abelland G, Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) study. Am J Clin Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 28.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–7. [DOI] [PubMed]
- 29.Patel HP, Al-Shanti N, Davies LC, Barton SJ, Grounds MD, Tellam RL, et al. Lean mass, muscle strength and gene expression in community dwelling older men: findings from the Hertfordshire sarcopenia study (HSS). Calcif Tissue Int 2014;Jul 24. [DOI] [PubMed]
- 30.Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, et al. Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14:911–915. doi: 10.1016/j.jamda.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Legrand D, Vaes B, Matheï C, Swine C, Degryse JM. The prevalence of sarcopenia in very old individuals according to the European consensus definition: insights from the BELFRAIL study. Age Ageing. 2013;42:727–734. doi: 10.1093/ageing/aft128. [DOI] [PubMed] [Google Scholar]
- 32.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 33.Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652–658. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13:121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Lim S, Choi SH, Kim KM, Yoon JW, Kim KW, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci 2014;April 10. [DOI] [PubMed]
- 36.Vetrano DL, Landi F, Volpato S, Corsonello A, Meloni E, Bernabei R, et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci. 2014;69:1154–1161. doi: 10.1093/gerona/glu034. [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. J Am Med Dir Assoc. 2010;11:391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Da Silva AT, de Oliveira Duarte YA, Ferreira Santos JL, Wong R, Lebrão ML. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for disability in the elderly. J Nutr Health Aging. 2014;18:547–553. doi: 10.1007/s12603-014-0465-9. [DOI] [PubMed] [Google Scholar]
- 39.Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK, ILAS Research Group Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc. 2013;14:528.e1–7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Malmstrom TK, Miller DK, Herning MM, Morley JE. Low appendicular skeletal muscle mass (ASM) with limited mobility and poor health outcomes in middle-aged African Americans. J Cachexia Sarcopenia Muscle. 2013;4:179–86. [DOI] [PMC free article] [PubMed]
- 41.Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX® with and without bone mineral density. Calcif Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 42.Evans CJ, Chiou CF, Fitzgerald FA, Evans WJ, Ferrell BR, Dale W, et al. Development of a new patient-reported outcome measure in sarcopenia. J Am Med Dir Assoc. 2011;12:226–233. doi: 10.1016/j.jamda.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc. 2014;15:630–4. [DOI] [PubMed]
- 45.Cao L, Chen S, Zou C, Ding X, Gao L, Liao Z, et al. A pilot study of the SARC-F scale on screening sarcopenia and physical disability in the Chinese older people. J Nutr Health Aging. 2014;18:277–283. doi: 10.1007/s12603-013-0410-3. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 47.Morley JE. Developing novel therapeutic approaches to frailty. Curr Pharm Des. 2009;15:3384–3395. doi: 10.2174/138161209789105045. [DOI] [PubMed] [Google Scholar]
- 48.Morley JE, von Haehling S, Anker SD, Vellas B. From sarcopenia to frailty: a road less traveled. J Cachexia Sarcopenia Muscle. 2014;5:5–8. [DOI] [PMC free article] [PubMed]
- 49.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:394–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Haehling S, Anker SD, Doehner W, Morley JE, Vellas B. Frailty and heart disease. Int J Cardiol. 2013;168:1745–7. [DOI] [PubMed]
- 51.Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi: 10.1111/j.1532-5415.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 52.Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010;95:3165–3172. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- 53.Ravindrarajah R, Lee DM, Pye SR, Gielen E, Boonen S, Vanderschueren D, et al. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Scharf G, Heineke J. Finding good biomarkers for sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:145–8. [DOI] [PMC free article] [PubMed]
- 55.Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–90. [DOI] [PMC free article] [PubMed]
- 56.Drey M, Krieger B, Sieber CC, Bauer JM, Hettwer S, Bertsch T, et al. Motoneuron loss is associated with sarcopenia. J Am Med Dir Assoc. 2014;15:435–439. doi: 10.1016/j.jamda.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Rolland Y, Czerwinski S, Van Kan Abellan G, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 59.Morley JE. Weight loss in older persons: new therapeutic approaches. Curr Pharm Design. 2007;13:3637–3647. doi: 10.2174/138161207782794149. [DOI] [PubMed] [Google Scholar]
- 60.MacIntosh C, Morley JE, Chapmen IM. The anorexia of aging. Nutrition. 2000;16:983–995. doi: 10.1016/S0899-9007(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 61.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Develop. 1999;107:123–136. doi: 10.1016/S0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009;55:121–130. doi: 10.1016/j.eururo.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 63.Haren MT, Siddiqui AM, Armbrecht HJ, Kevorkian RT, Kim MJ, Haas MJ, et al. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl. 2011;34:55–68. doi: 10.1111/j.1365-2605.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 64.Sinclair A, Morley JE, Rodriguez-Mañas L, Paolisso G, Bayer T, Zeyfang A, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13:497–502. doi: 10.1016/j.jamda.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 65.von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol. 2013;45:2257–2265. doi: 10.1016/j.biocel.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 66.Cadore EL, Ezquierdo M. New strategies for the concurrent strength-, power-, and endurance-training prescription in elderly individuals. J Am Med Dir Assoc. 2013;14:623–624. doi: 10.1016/j.jamda.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Yamada M, Arai H, Sonoda T, Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc. 2012;13:507–511. doi: 10.1016/j.jamda.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc. 2012;13:418–428. doi: 10.1016/j.jamda.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Rolland Y, Onder G, Morley JE, Gillette-Guyonet S, Van Kan Abellan G, Vellas B. Current and future pharmacologic treatment of sarcopenia. Clin Geriatr Med. 2011;27:423–447. doi: 10.1016/j.cger.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2012;13:24–30. doi: 10.1016/j.jamda.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malafarina V, Iriz-Otano F, Iniesta R, Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in older age: a systematic review. J Am Med Dir Assoc. 2013;14:10–17. doi: 10.1016/j.jamda.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;July 17:jc20141742. [DOI] [PubMed]
- 77.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 78.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 79.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–1099. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure: a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 82.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–61. [DOI] [PMC free article] [PubMed]
- 83.Morley JE, von Haehling S, Ankder SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle. 2014;5:83–7. [DOI] [PMC free article] [PubMed]
- 84.Ebner N, Steinbeck L, Doehner W, Anker SD, von Haehling S. Highlights from the 7th Cachexia Conference: muscle wasting pathophysiological detection and novel treatment strategies. J Cachexia Sarcopenia Muscle. 2014;5:27–34. [DOI] [PMC free article] [PubMed]
- 85.Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 2014; April 17. [DOI] [PMC free article] [PubMed]
- 86.Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 87.Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626–634. doi: 10.1007/s12020-012-9836-3. [DOI] [PubMed] [Google Scholar]
- 88.Reid J, Noble HR, Porter S, Shields JS, Maxwell AP. A literature review of end-stage renal disease and cachexia: understanding experience to inform evidence-based healthcare. J Ren Care. 2013;39:47–51. doi: 10.1111/j.1755-6686.2013.00341.x. [DOI] [PubMed] [Google Scholar]
- 89.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 90.Roman D, Mahoney K, Mohamadi A. Sarcopenia: what’s in a name? J Am Med Dir Assoc. 2013;14:80–82. doi: 10.1016/j.jamda.2012.09.021. [DOI] [PubMed] [Google Scholar]