Abstract
Objective:
The aim of the study was to determine sociodemographic, biological epilepsy-specific, and adherence predictors of long-term pediatric seizure outcomes.
Methods:
This study is a prospective, longitudinal, observational study of antiepileptic drug (AED) adherence and seizure outcomes in children with newly diagnosed epilepsy. Patients were recruited from April 2006 to March 2009 and followed for 2 years. Objective, electronic monitors were used to assess AED adherence. Medical chart reviews assessed medical variables and seizure outcomes.
Results:
Participants (n = 109) were 7.3 ± 2.9 years of age, and 62% male. Four adherence trajectory groups were identified: severe early nonadherence (n = 10), variable nonadherence (n = 16), moderate nonadherence (n = 40), and high adherence (n = 43). Two seizure probability trajectory groups were identified: high seizure (n = 28) and low seizure probability (n = 81). Participants with recognizable syndromes were less likely to be a member of the high seizure probability group (b = −2.372; odds ratio [OR] = 0.093; 95% confidence interval [CI]OR = 0.015, 0.595); those with the presence of epileptiform discharges on EEG were more likely to be in the high seizure probability group (b = 1.649; OR = 5.203; 95% CIOR = 1.422, 19.037). Adherence trajectory group status was a significant predictor of seizure trajectory group status (partial max-rescaled R2 = 0.13).
Conclusions:
Adherence trajectories and 2 biological epilepsy-specific variables explain a similar proportion of the variability in longitudinal seizure outcomes. The relationship between AED nonadherence and seizure outcomes is not linear. Early adherence interventions could change the course of seizure outcomes, particularly if variability in adherence was minimized postdiagnosis.
Despite the introduction of multiple new efficacious antiepileptic drugs (AEDs), 20% to 35% of children with newly diagnosed epilepsy continue to have seizures.1–3 Several biologically based factors have been examined to understand this disease course, including underlying brain disorders and structural abnormalities, seizure type/etiology, and genetics.4,5 However, these biological factors, which are nonmodifiable, do not fully explain the variability in seizure outcomes. In contrast, behavioral factors, such as AED nonadherence, are modifiable and may affect long-term seizure outcome. While discrete trajectories of AED nonadherence in children with newly diagnosed epilepsy have been identified,6 similar trajectories of long-term seizure outcomes have not been examined. Our prior work also indicates that children who are nonadherent to their AEDs within the first 6 months of therapy are 3.24 times more likely to have seizures 4 years after diagnosis.7 In adults, AED nonadherence increases short-term risk of seizures.8,9 However, what remains unknown is the relationship between long-term nonadherence and seizure outcome in both adults and children. We conducted a prospective, longitudinal, observational study to determine sociodemographic, epilepsy-specific, and adherence predictors of long-term pediatric seizure outcomes.
METHODS
Participants and procedures.
A consecutive cohort of children (ages 2–12 years inclusive) with newly diagnosed untreated epilepsy were enrolled from April 2006 to March 2009 at Cincinnati Children's Hospital Medical Center. This study was observational in nature. Full details of the cohort have been described6 and included the following exclusion criterion: no concurrent developmental disorder and no comorbid medical illness requiring daily medication. Treating clinicians used a standardized treatment approach to initial AED monotherapy (exclusively involving valproic acid and carbamazepine) that predated the study's onset.
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval was obtained for the prospective, longitudinal adherence study. Written consent was obtained from all parents and child participants (if applicable).
A prospective, consecutive cohort of eligible participants were approached for enrollment at the time of initial diagnosis before AED initiation (i.e., baseline visit). Subsequent study visits coincided with clinic visits and occurred 1 month after diagnosis and every 3 months thereafter until 25 months after diagnosis.
Measures.
At study entry, participant demographics (age, sex, race, and ethnicity), family history of epilepsy, caregiver marital status, and family socioeconomic status were collected. Socioeconomic status was determined using a revised Duncan score based on parental occupation.10
At the initiation of drug therapy, patients and their caregivers were provided with a Medication Event Monitoring System TrackCap (Aardex, Sion, Switzerland), an electronic monitor of medication adherence, to use for their AED. Participants were asked to place their AED in the bottle and use the TrackCap for the duration of the study. The TrackCap records the date and time of each bottle opening. TrackCap data were downloaded at each study visit and this adherence information was not shared with families or health care providers.
At diagnosis, each participant's epilepsy biological variables, including seizure/epilepsy type, epilepsy syndrome and etiology (using the 1989 International Classification of Epilepsy and Epilepsy Syndromes),11 absence or presence of epileptiform discharges on EEG, and clinically significant MRI abnormalities were determined. Caregivers completed the Pediatric Epilepsy Side Effects Questionnaire, a 19-item measure of typical AED side effects.12 Parent report of the presence or absence of seizures since the last visit was obtained as part of the study and confirmed by clinician notes in the medical record.
Statistical analyses.
Summaries of medication adherence (i.e., the number of AED doses taken divided by the number of prescribed AED doses over a specified time multiplied by 100%) and seizure activity across participants within the 3-month period between consecutive clinic visits were used for all analyses. Participants with complete adherence data, defined as 90% or more during the 3 months between clinic visits, were included in data analyses. Mean medication adherence during the 3-month intervals was the unit of analysis. Seizure data were based on the presence (1) or absence (0) of any seizure activity in the same 3-month intervals.
Latent class growth modeling (LCGM; PROC TRAJ) analyses13 were conducted in SAS (version 9.3; SAS Institute, Cary, NC). LCGM is similar to longitudinal analysis models that test for differences between groups, with one key difference. In standard longitudinal analyses that test for group differences, an observed variable in the dataset shows all participants' group membership. LCGM proceeds from the suspicion that there are unobserved subgroups in the dataset, each subgroup having a different longitudinal response variable trajectory. These unobserved groups are extracted based on response variable patterns in the data, and participants are assigned to one and only one of the subgroups using probabilistic estimation techniques (i.e., posterior probabilities). Our goal was to use trajectory subgroups to identify and characterize differential patterns of individual change over time in the population for the 2 outcomes of interest: (1) mean adherence percentage, and (2) binary seizure indicator data. In the LCGM analyses, we examined the possibility of 1 to 7 subgroups for each outcome. The Bayesian information criterion statistic, model estimation convergence, and subgroups containing <5% of the population were all factors in determining the best fitting model for each outcome. Additional diagnostic checks13 were also performed on the LCGMs to examine how well the subgroup solution fit the data and how definitively participants were assigned to the subgroups.
Two additional statistical analyses were performed using the mean adherence and seizure probability subgroups. An omnibus χ2 analysis tested the null hypothesis of independence between adherence and seizure probability subgroups. This prespecified analysis was followed by a logistic regression that identified the following variables as salient predictors of patients being in the high (vs the low) seizure probability subgroup: family socioeconomic status, child age, child sex, child race, and ethnicity, seizure/epilepsy type, seizure etiology, presence of recognizable epilepsy syndrome, family history of epilepsy, presence of epileptiform discharges on EEG, presence of clinically significant MRI abnormalities; total side effects at the 1-month postdiagnosis clinic visit, and adherence trajectory group status. AED was also considered as a predictor. High collinearity between AED and seizure/epilepsy type dictated that only one be used in the analyses. Statistical significance was defined as p < 0.05.
RESULTS
Participants.
A total of 130 eligible participants (children and their primary caregiver) were approached for study participation. Five subjects declined participation because of time constraints (96% recruitment rate). One participant was found to be ineligible after study consent was obtained (because of simultaneous diagnosis of pervasive development disorder). Ten participants were excluded because of lack of follow-up data after their initial visit (n = 4) or 1-month assessment (n = 6). Among the remaining 114, 5 were excluded because of <90% complete data for all visits. Thus, there were 109 participants in the study cohort (84% of those initially eligible).
The cohort's demographics were 7.3 ± 2.9 years of age, 62% male, and 97% non-Hispanic. The majority (76%) were white, 17% were black, and 7% biracial. At diagnosis, the majority of epilepsies were classified as localization-related (59.5% overall; 47% idiopathic, 7% cryptogenic, 5.5% symptomatic) with the remainder split between generalized (24.5% overall; 18% idiopathic, 5.5% cryptogenic, 1% symptomatic) and unclassified (overall 16%; all idiopathic). Twenty-seven percent of children in the cohort had clinically significant MRI abnormalities and 59% had epileptiform discharges on their EEG. Syndromes were present in 19% of children (13% childhood/juvenile absence epilepsy, 6% benign rolandic epilepsy). Initial AED therapy was carbamazepine for 60% and valproic acid for 40% of participants. Over the course of the study, 69% of participants were prescribed only one AED, 20% were on sequential monotherapy of 2 AEDs, 9% were on sequential monotherapy of 3 AEDs, and 2% were on sequential monotherapy of 4 AEDs. By the end of the study, 38% were prescribed carbamazepine, 28% valproic acid, 12% levetiracetam, 8% topiramate, 6% oxcarbazepine, 2% ethosuximide, 1% gabapentin, and 1% other. Caregivers were predominantly mothers (83%) who were married (64%). The mean Duncan score was 53.5 ± 20.5, a score associated with occupations such as property managers, physician's assistants, mail carriers, sheriffs/law enforcement, and fire prevention occupations. This cohort is demographically representative of the US childhood epilepsy population (e.g., higher male and Caucasian prevalence and 17% of families <100% below the federal poverty line).14
Determining adherence behavior and seizure activity trajectories.
LCGM analyses (table 1) resulted in final growth model trajectories for adherence (k = 4, figure 1) and seizure probability (k = 2, figure 2). Based on additional diagnostic criteria13 (table e-1 on the Neurology® Web site at Neurology.org), the trajectory solutions found for adherence and seizure probability provided acceptable fits to the sample data.
Table 1.
Final group-based trajectory models
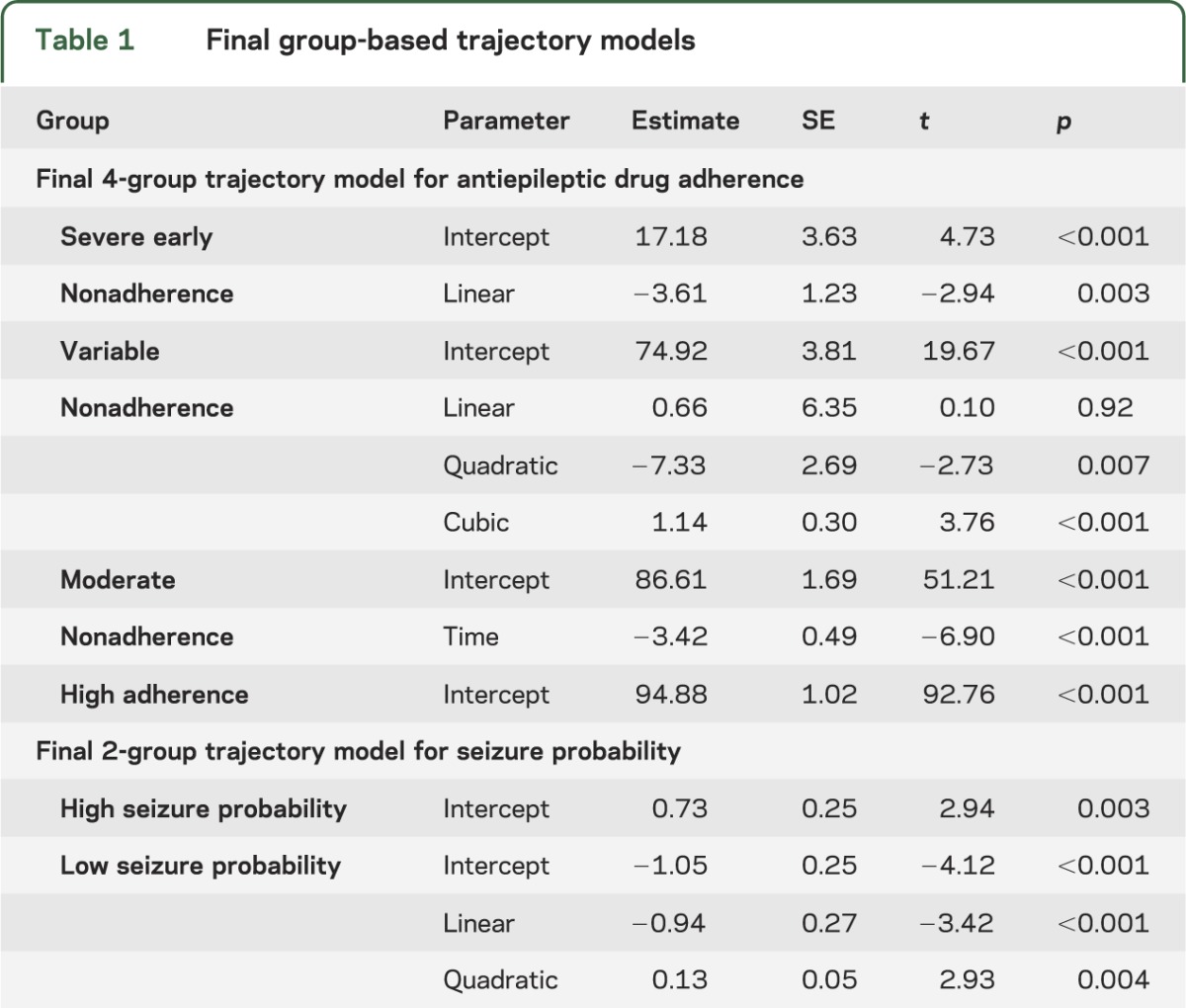
Figure 1. Adherence group trajectories.
Observed data trajectories are represented by dashed lines and model-based trajectories are represented by solid lines.
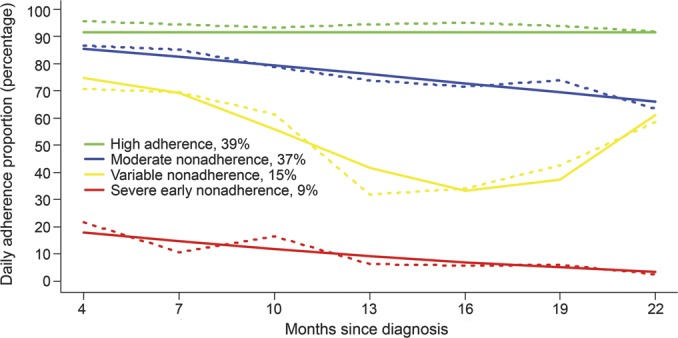
Figure 2. Seizure probability group trajectories.
Observed data trajectories are represented by dashed lines and model-based trajectories are represented by solid lines.
The 4 adherence trajectory groups (figure 1) included a severe early nonadherence group (9%, 95% confidence interval [CI]: 5%–17%), a variable nonadherence group (15%, 95% CI: 9%–24%), a moderate nonadherence group (37%, 95% CI: 27%–48%), and a high adherence group (39%, 95% CI: 28%–49%). The high adherence group reflected stable average adherence rates around 94% for the entire 2-year study period. The remaining 3 groups demonstrated nonadherence patterns that varied in course and level of nonadherence. The moderate and severe early nonadherence groups exhibited declining adherence over 2 years with the moderate group starting at 87% and ending at 64% and the severe early starting at 22% and ending at 3%. The variable nonadherence group demonstrated a significant cubic pattern, in which adherence started at 71%, dipping down to 32% at 1-year postdiagnosis and increasing to 58% by the end of 2 years.
The 2 seizure probability trajectory groups (figure 2) included a high seizure probability group (26%, 95% CI: 23%–29%) and a low seizure probability group (74%, 95% CI: 71%–79%) over the course of the study. The high seizure probability group demonstrated a stable high probability (i.e., 0.70) of seizure recurrence throughout the entire 2-year period. The low seizure probability group's chance of seizure recurrence shortly after diagnosis was 0.30, which declined to 0.09 by 10 months after diagnosis and subsequently remained stable. Notably, participants on sequential monotherapy of 1 to 2 AEDs were more likely to be in the low seizure probability group (78/96 = 81%) while those who tried 3 to 4 AEDs monotherapy were more likely to be in the high seizure probability group (9/13 = 69%; p < 0.001).
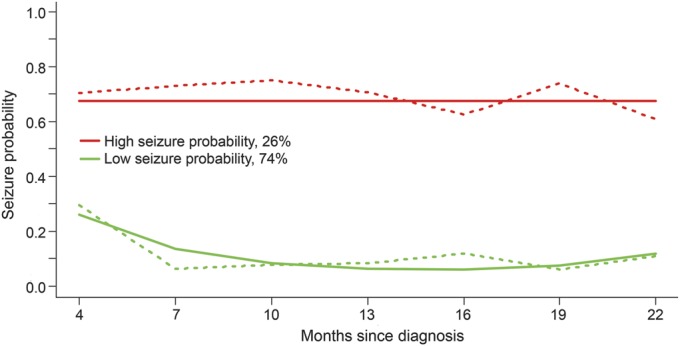
There was an overall association between the adherence and seizure trajectory groups (χ2 = 8.08; df = 3, p < 0.05). A larger relative proportion of patients in the high seizure probability group were found for those in the variable nonadherence trajectory group when compared with the other adherence trajectory groups (figure 3 demonstrates the conditional probability of a particular adherence group given seizure group membership).
Figure 3. Conditional probability of adherence group given seizure group.
The omnibus χ2 null hypothesis test for no association between seizure and adherence trajectories was statistically significant (p < 0.05).
Predictors of seizure probability trajectories.
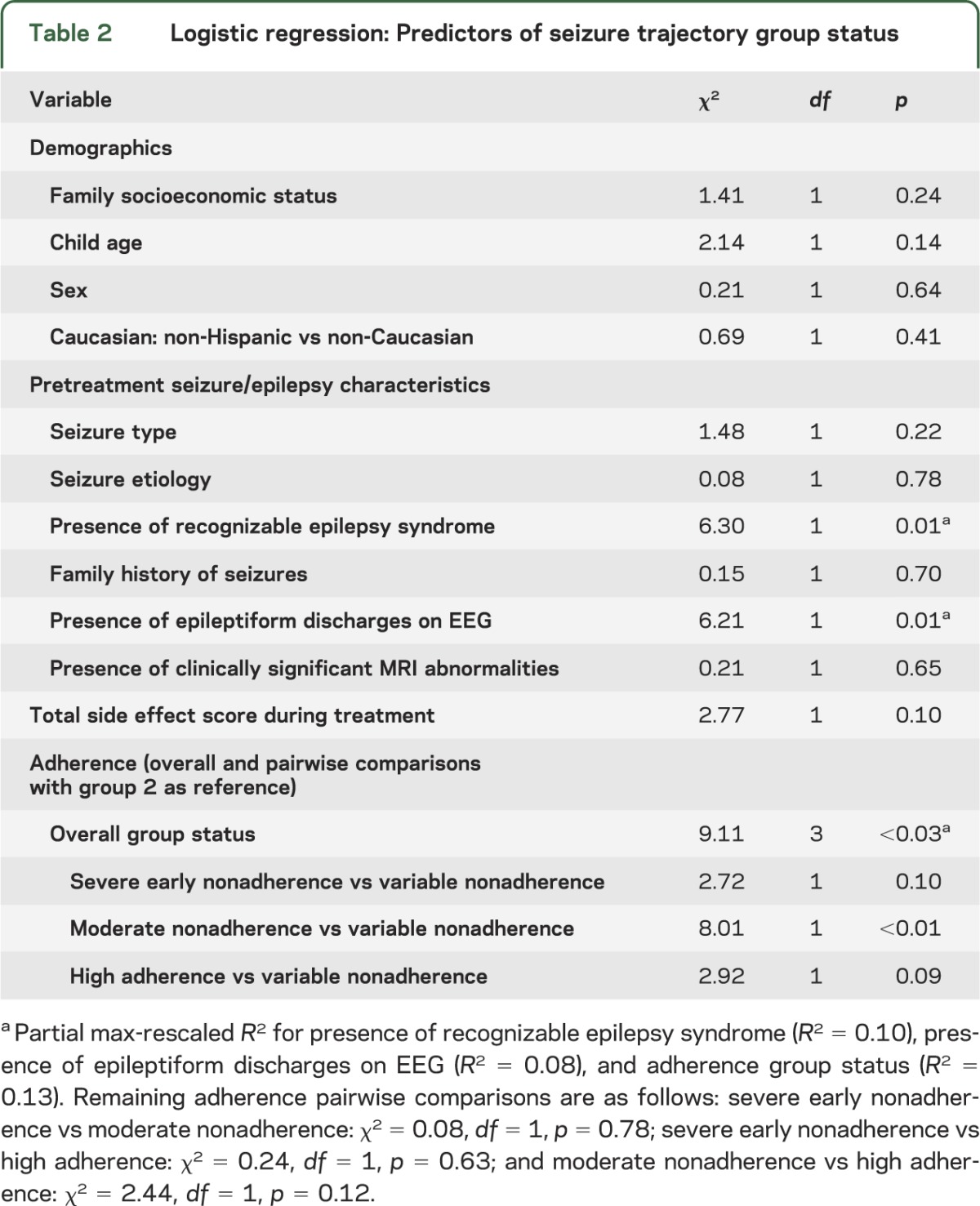
No demographic variables were predictors of seizure trajectory group status (table 2). Partial R2 was calculated using max-rescaled R2 values for statistically significant predictors to account for the fact that standard R2 measures generally have an upper limit of less than 1 for discrete outcome variables.15,16 Two pretreatment seizure/epilepsy characteristics predicted seizure trajectory group status: (1) presence of a recognizable epilepsy syndrome (partial max-rescaled R2 = 0.10), and (2) presence of epileptiform discharges on EEG (partial max-rescaled R2 = 0.08). Participants with a recognizable syndrome were less likely to be a member of the high seizure probability group (b = −2.372; odds ratio = 0.093; 95% CIodds ratio = 0.015, 0.595) while those with the presence of epileptiform discharges on EEG were more likely to be a member of the high activity seizure class (b = 1.649; odds ratio = 5.203; 95% CIodds ratio = 1.422, 19.037). Adherence trajectory group status was a predictor of seizure trajectory group status (partial max-rescaled R2 = 0.13). Specifically, compared with members in the variable nonadherence group, participants in the moderate nonadherence group were less likely to be in the high seizure probability group (b = −2.514; odds ratio = 0.081; 95% CIodds ratio = 0.013, 0.463).
Table 2.
Logistic regression: Predictors of seizure trajectory group status
DISCUSSION
Understanding the complex interplay between biological epilepsy-specific and behavioral factors that underlie the variability in seizure outcomes is a key step toward addressing this persistent problem. This study demonstrated that adherence trajectories and 2 epilepsy-specific biological variables explain a similar proportion of the variability in longitudinal seizure outcomes. Not surprisingly, the presence of a recognizable epilepsy syndrome and epileptiform discharges on EEGs were significant predictors of seizure trajectory group status. Adherence accounted for an additional 13% of variance in predicting seizure outcomes. This is significant because adherence, the only modifiable factor within the logistic regression models, is amenable to clinical adherence promotion interventions.
Adherence itself is a dynamic variable best understood with trajectories rather than cross-sectional point estimates. Four distinct adherence trajectories were identified, including high adherence, moderate nonadherence, variable nonadherence, and severe early nonadherence. Compared with the 5 adherence trajectories found from our prior research of 6-month adherence data,6 2 years of detailed adherence data yielded 4 adherence trajectories including a new unique trajectory, the variable nonadherence group. Several factors may have contributed to these new trajectories, including differing calculations of adherence (e.g., daily adherence vs mean adherence over 7 months), timeframe (e.g., inclusion of the first month of AED therapy vs not), and changes to adherence behaviors after the first 6 months of therapy. An important area for future research is to examine predictors of our nonadherence trajectories, including family psychosocial barriers (e.g., forgetting, executive dysfunction, child behavior) given that caregivers were predominately responsible for AED adherence in this age range.
Seizure recurrence is unpredictable and fluctuates because of complex interactions among biological, environmental, and behavioral factors. A dynamic approach was used (via trajectories) to describe the probability of a child having seizures over a 2-year period rather than using a predefined time threshold (e.g., 1 or 2 years seizure-free). As expected, 2 seizure trajectories were identified. Twenty-six percent of the cohort was in the high seizure probability group, which is consistent with previously reported rates of seizure intractability.1–3 In contrast, three-quarters of the population exhibited a low probability of seizure recurrence over 2 years. This latter group's initial higher probability of seizures soon after diagnosis was likely attributable to the time required to titrate and optimize AED therapy.
There is a complex relationship between adherence and seizure probability trajectories. For the patients and families at the 2 extremes of adherence (i.e., severe early nonadherence group and high adherence), biological variables may have a more dominant role in outcome. It is plausible that the patients in the severe early nonadherence group with low seizure probability either had a spontaneous remission of their epilepsy without the need for AEDs or were misdiagnosed and never had epilepsy. In contrast, the patients in the high adherence group with high seizure probability probably have a more severe underlying biological brain dysfunction that prevented the prescribed AED from adequately preventing seizures regardless of the patient's adherence. Adherence has a dominant role in seizure probability trajectory for the patients with variable nonadherence when compared with those patients with moderate nonadherence.
Several limitations of the current study should be noted. While the current analysis identified several important predictors of seizure outcome, accounting for 31% of the variance, there may be other variables that are important to examine that were not measured in the current study (e.g., genetic biomarkers). The study cohort is a relatively homogeneous population of children with mostly idiopathic epilepsy. This approach optimized our ability to detect the unique effect of adherence by minimizing the confounding impact that the entire spectrum of epilepsy etiologies (e.g., malformations, brain injury) would have exerted on seizure outcome. However, we are unable to assess the role of adherence across the entire epilepsy spectrum. The heterogeneous seizure types allowed us to examine the impact of epilepsy-specific variables (e.g., syndrome vs not) on seizure probability outcomes; however, this mixture of seizure types prevented seizure frequency, both during and before treatment, from being used to develop the seizure trajectories. Another limitation is that daily adherence was averaged across 3-month time intervals. While mean adherence is important for describing adherence levels across a population, daily adherence levels better describe intra- and interpatient adherence variability, which may be more critical for intervention. Future studies could examine the relationship between AED adherence and long-term health outcomes, including successful weaning, rate of relapse, and health-related quality of life. Future work including larger samples could further examine the strength of the relationship between epilepsy-specific biological and behavioral factors with seizure outcomes.
Results from the current study highlight several important and novel findings. First, the relationship between nonadherence to AEDs and seizure outcomes is not linear, as it is with other chronic conditions (e.g., diabetes, hypertension, HIV).17–19 However, these data suggest that patients in the moderate and variable nonadherence groups could benefit from adherence promotion interventions early in the epilepsy course that are geared to the family's specific adherence barriers, which were not examined in this study. Several empirically supported adherence interventions (e.g., multimodal interventions involving education, organization strategies, and problem-solving) have been developed for pediatric chronic conditions.20,21 For example, a recent family-based problem-solving intervention trial demonstrated improvements in adherence for children with new-onset epilepsy.22 Early adherence interventions may help change the course of seizure outcomes, especially if we can reduce the variability of nonadherence.
Supplementary Material
ACKNOWLEDGMENT
The authors especially thank the families from the New Onset Seizure Clinic who participated in this 2-year longitudinal study. The authors also thank the research assistants, undergraduate and graduate students, predoctoral interns, and postdoctoral fellows (Cincinnati Children's Hospital Medical Center) for recruiting study patients and data collection. Finally, the authors appreciate the support of the New Onset Seizure Team at Cincinnati Children's Hospital in conducting this study.
GLOSSARY
- AED
antiepileptic drug
- CI
confidence interval
- LCGM
latent class growth modeling
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Avani Modi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Modi: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, administrative, technical, or material support, study supervision. Wu: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Rausch: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Peugh: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Glauser: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This research was funded by a grant from the NIH awarded to the first author (K23HD057333: Novel Adherence Measurement and Intervention in Children with New-Onset Epilepsy). The study sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
DISCLOSURE
A. Modi reports that she has been a consultant for Novartis Pharmaceuticals Inc., who has an interest in antiepileptic drugs. Y. Wu, J. Rausch, and J. Peugh report no disclosures relevant to the manuscript. T. Glauser reports that he is an advisor to, a speaker for, and has received research grants from companies with interests in antiepileptic drugs, including UCB Pharma, Eisai, Upsher-Smith, Lundbeck, Questcor, Supernus, Sunovion, and AssureRx. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 2.Holland KD, Glauser TA. Response to carbamazepine in children with newly diagnosed partial onset epilepsy. Neurology 2007;69:596–599. [DOI] [PubMed] [Google Scholar]
- 3.Holland KD, Monahan S, Morita D, Vartzelis G, Glauser TA. Valproate in children with newly diagnosed idiopathic generalized epilepsy. Acta Neurol Scand 2010;121:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg AT, Testa FM, Levy SR. Complete remission in nonsyndromic childhood-onset epilepsy. Ann Neurol 2011;70:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geerts A, Brouwer O, Stroink H, et al. Onset of intractability and its course over time: the Dutch Study of Epilepsy in Childhood. Epilepsia 2012;53:741–751. [DOI] [PubMed] [Google Scholar]
- 6.Modi AC, Rausch JR, Glauser TA. Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA 2011;305:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi AC, Rausch JR, Glauser TA. Early pediatric antiepileptic drug nonadherence is related to lower long-term seizure freedom. Neurology 2014;82:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RM, Butler JA, Thomas VA, Peveler RC, Prevett M. Adherence to treatment in patients with epilepsy: associations with seizure control and illness beliefs. Seizure 2006;15:504–508. [DOI] [PubMed] [Google Scholar]
- 9.Cramer JA, Glassman M, Rienzi V. The relationship between poor medication compliance and seizures. Epilepsy Behav 2002;3:338–342. [DOI] [PubMed] [Google Scholar]
- 10.Nakao K, Treas J. The 1989 Socioeconomic Index of Occupations: Construction from the 1989 Occupational Prestige Scores. Chicago: University of Chicago, National Opinion Research Center; 1992. [Google Scholar]
- 11.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League against epilepsy. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 12.Morita DA, Glauser TA, Modi AC. Development and validation of the pediatric epilepsy side effects questionnaire. Neurology 2012;79:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 14.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 2012;129:256–264. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 16.Nagelkerke N. A note on a general definition of the coefficient of determination. Biometrika 1991;78:691–692. [Google Scholar]
- 17.Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med 2008;168:271–276. [DOI] [PubMed] [Google Scholar]
- 18.Rausch JR, Hood KK, Delamater AM, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012;35:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrijens B, Goetghebeur E, de Klerk E, Rode R, Mayer S, Urquhart J. Modelling the association between adherence and viral load in HIV-infected patients. Stat Med 2005;24:2719–2731. [DOI] [PubMed] [Google Scholar]
- 20.Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. J Pediatr Psychol 2010;35:368–382. [DOI] [PubMed] [Google Scholar]
- 21.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol 2008;33:590–611. [DOI] [PubMed] [Google Scholar]
- 22.Modi AC, Guilfoyle SM, Rausch J. Preliminary feasibility, acceptability, and efficacy of an innovative adherence intervention for children with newly diagnosed epilepsy. J Pediatr Psychol 2013;38:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


