Abstract
Objective:
To investigate the prevalence of connective tissue abnormalities in patients with spontaneous cervical artery dissections (sCeAD).
Methods:
We systematically assessed clinically detectable signs of connective tissue aberration in a series of consecutive patients with sCeAD and of age- and sex-matched patients with ischemic stroke unrelated to CeAD (non-CeAD IS) by a standard examination protocol including 68 items, and performed extensive molecular investigation for hereditary connective tissue disorders in all patients with sCeAD.
Results:
The study group included 84 patients with sCeAD (mean age, 44.5 ± 7.8 years; 66.7% men) and 84 patients with non-CeAD IS. None of the patients with sCeAD met clinical or molecular diagnostic criteria for established hereditary connective tissue disorder. Connective tissue abnormalities were detected more frequently in the group of patients with sCeAD than in the group of those with non-CeAD IS (mean number of pathologic findings, 4.5 ± 3.5 vs 1.9 ± 2.3; p < 0.001). Eighty-one patients (96.4%) in the sCeAD group had at least one detectable sign compared with 55 patients (66.7%) in the group with non-CeAD IS (p < 0.001). Skeletal, ocular, and skin abnormalities, as well as craniofacial dysmorphisms, were the clinical signs more strongly associated with sCeAD. Signs suggesting connective tissue abnormality were also more frequently represented in patients with sCeAD than in patients with traumatic CeAD (28.6%, p < 0.001; mean number of pathologic findings, 1.7 ± 3.7, p = 0.045).
Conclusions:
Connective tissue abnormalities are frequent in patients with sCeAD. This reinforces the hypothesis that systemic aberrations of the connective tissue might be implicated in the pathogenesis of the disease.
The pathogenesis of spontaneous cervical artery dissection (sCeAD), the most frequent cause of ischemic stroke in young and middle-aged adults, is still unclear. The finding of composite collagen fibrils and fragmented elastic fibers on electron microscopic examination of skin biopsy specimens in more than half of patients with sCeAD is an argument in favor of the hypothesis that connective tissue aberrations might have a causative role.1,2 However, whether such morphologic alterations represent subclinical phenotypes of a generalized disorder of the connective tissue remains to be determined. Hereditary connective tissue disorders (HCTDs) have been rarely diagnosed among patients with CeAD and external stigmata of connective tissue abnormalities inconsistently detected.3,4 These observations cast doubts on the assumption of a primary disorder of the connective tissue. Therefore, although the hypothesis of an underlying arteriopathy leading to structural instability of the vessel wall is likely and generally accepted, the exact nature of this disorder is still matter of debate.1 To further investigate this issue, we systematically searched for clinical signs of connective tissue anomalies in a cohort of patients with CeAD and control subjects.
METHODS
We undertook a hospital-based case-control study of consecutive patients with CeAD and patients with non-CeAD ischemic stroke (non-CeAD IS) prospectively recruited during a 44-month period (between March 2010 and November 2013). Patients in the CeAD group were those whose diagnosis was confirmed by MRI/magnetic resonance angiography or conventional angiography,5 with or without stroke.
Dissections occurring as an immediate consequence of a major trauma were labeled “traumatic” (for definition, see e-Methods on the Neurology® Web site at Neurology.org). The group of control subjects was selected from a cohort of patients with first-ever cerebral ischemia, after exclusion of the subgroup with CeAD-related infarction. Non-CeAD IS patients were frequency-matched with CeAD patients on sex and age (in 3-year intervals). Clinically detectable signs of connective tissue abnormalities were systematically investigated in each subject by 2 raters, a neurologist (A.G.) and a geneticist (M. Colombi), expert in the assessment of connective tissue signs, to ensure a homogeneous evaluation. This standardized examination included the assessment of 68 signs reflecting the spectrum of the clinical features observed in the Ehlers-Danlos syndrome vascular type, Marfan syndrome, pseudoxanthoma elasticum, osteogenesis imperfecta, arterial tortuosity syndrome, and Loeys-Dietz syndrome (table 1).e3–e13 Each sign was counted as an all-or-none variable (present vs absent), resulting in an individual connective score and a mean number of pathologic findings for each group (mean score). The 2 raters were blinded to the status of the patients. Interrater reliability was assessed by having the 2 examiners categorize the same set of 68 signs in all patients and was evaluated using the κ statistic, according to the method described by Cohen.6 We categorized these signs according to the specific organ or system involved into (1) skin abnormalities, (2) ocular abnormalities, (3) skeletal abnormalities, (4) craniofacial dysmorphisms, and (5) abnormalities involving other organs.
Table 1.
Items included in the standard examination protocol


All patients with CeAD underwent direct sequencing of TGFBR1 and TGFBR2 genes,7 62 of SMAD3 and TGFB2 genes, while only in selected cases, COL3A1 (n = 10), SLC2A10 (n = 3), FBN1 (n = 2), and ACTA2 (n = 2) genes were analyzed, based on individual characteristics. To test the hypothesis that connective tissue disorders have a major role also in the pathogenesis of sporadic CeAD cases, we planned to exclude from the present analysis those patients whose clinical features had eventually fulfilled the diagnostic criteria for one of the monogenic disorders reported above and/or in whom molecular screening revealed causative mutations.
Standard protocol approvals, registrations, and patient consents.
The study was approved by relevant local authorities. Written informed consent was obtained from all patients (or next of kin).
RESULTS
The study group was composed of 84 patients with sCeAD (mean age, 44.5 ± 7.8 years; 66.7% men) and 84 patients with non-CeAD IS (mean age, 44.0 ± 8.7 years; 66.7% men). Compared to patients with non-CeAD IS, patients with sCeAD had a lower prevalence of hypercholesterolemia, a higher prevalence of migraine, particularly without aura, tended to be taller and thinner, and less frequently hypertensive, smokers, and diabetics (table 2).
Table 2.
Demographic and clinical characteristics of the study group

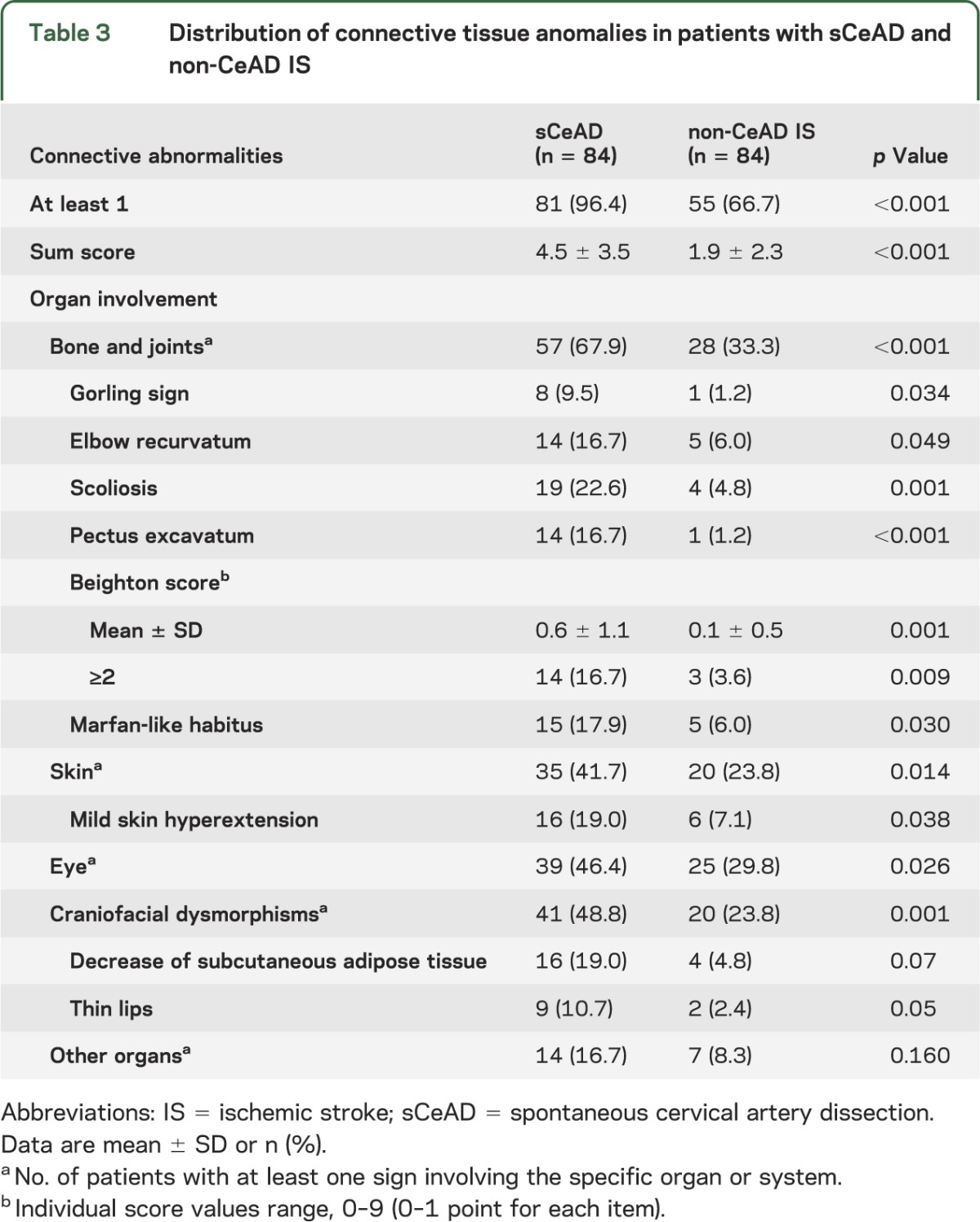
Short-lasting triggering factors preceding the occurrence of the vascular event were also detected more often in the group of patients with sCeAD than in the group of patients with non-CeAD IS (table e-1). None of the patients with sCeAD met clinical diagnostic criteria or carried causal mutations for established HCTD. Interrater agreement for the assessment of connective signs was high with 80 of the 84 patients rated identically by the 2 raters (κ = 0.84). Signs suggesting connective tissue abnormalities were detected more frequently in the group of patients with sCeAD than in the group of those with non-CeAD IS (mean score, 4.5 ± 3.5 vs 1.9 ± 2.3; p < 0.001; figure e-1). Eighty-one patients (96.4%) in the sCeAD group had at least one detectable sign compared with 55 patients (66.7%) in the group with non-CeAD IS (p < 0.001). Overall, skeletal, ocular, and skin abnormalities, as well as craniofacial dysmorphisms, were more frequently observed in the group of patients with sCeAD, as opposed to the abnormalities involving other organs, which were equally distributed in the 2 groups. Facial appearance of patients with sCeAD was more often characterized by tight skin, hollow cheeks, and prominent staring eyes as a consequence of paucity of adipose tissue. The most prominent skeletal features were scoliosis and mild pectus excavatum, while the most prominent skin feature was mild hyperextension. Hypermobility/laxity of some joints was also more frequently observed in the group of patients with sCeAD (Beighton score8 ≥2 in 16.7% vs 3.6% [p = 0.009]; mean score, 0.6 ± 1.1 vs 0.1 ± 0.5 [p = 0.001]; table 3).
Table 3.
Distribution of connective tissue anomalies in patients with sCeAD and non-CeAD IS
We also studied 7 patients whose CeAD was obviously due to a major trauma, during the same study period. Signs suggesting connective tissue abnormality were less frequently represented in this group compared to the group of patients with sCeAD (traumatic CeADs with at least one detectable sign, 28.6%, p < 0.001; mean score, 1.7 ± 3.7, p = 0.045).
DISCUSSION
Our findings indicate that clinically detectable connective tissue abnormalities, frequently observed in patients with HCTD, are also highly prevalent in patients with sCeAD. This provides indirect support to the “connective hypothesis” of the disease. Structural deviations in the main components of connective tissue, collagen and elastic fibers, may lead to functional impairment of the mechanical stability and elasticity of the arterial wall, predisposing to dissection at given points of minor resistance. Similar to other previous studies, we did not diagnose a definite HCTD in any of the patients included in our series, despite the extensive clinical and molecular investigation.9 Whether this implicates the existence of a milder form of connective tissue disorder predisposing to CeAD remains unknown, even though it seems plausible. The low prevalence of clinically recognizable connective signs in patients with traumatic CeAD, as opposed to spontaneous cases, is a further argument in favor of this view. Overall, our findings are in line with the prevailing theory that sCeAD represents a multifactorial disease and the end result of a synergistic interplay between an underlying constitutional arteriopathy and short-lasting environmental factors. These triggering factors, normally insufficient to induce arterial wall rupture alone, could transiently facilitate dissection in a fragile, previously asymptomatic, vessel wall. Obviously, we cannot but speculate on the relation between the clinical signs of connective tissue abnormality we detected and a generalized disorder of the connective tissue. In this regard, our findings need to be validated by a study that correlates patient phenotype with pathologic data. Notably, the only study in which clinical signs of connective tissue abnormalities were systematically assessed by standard examination found no preponderance of these stigmata in patients with CeAD and argued against a connective tissue disorder underlying the disease. However, the 25 clinical items used in that analysis reflect the spectrum of signs mainly found in Marfan and Ehlers-Danlos syndromes, and it is unclear why authors did not include specific features of other HCTDs in their standardized examination protocol.4 Difference in sample size and the lack of patients' molecular characterization should also be considered when comparing the results of that study with ours, because they might contribute to explain some discrepancies.
The most relevant shortcoming of both analyses, as well as of others previously reported,2,3 is that they rely heavily on the identification and subjective interpretation of signs that are qualitative or semiquantitative (i.e., skin extensibility, joint hypermobility, tissue fragility, and bruising), and for which, therefore, normal standards are unavailable. However, the excellent interrater agreement in the assessment of these items makes this drawback unlikely in our analysis. Clinically detectable signs of connective tissue abnormalities are subtle in most patients with sCeAD and, therefore, they might remain undetected because they can only be recognized by experienced examiners. This represents an obvious limitation to the possibility of applying such a standardized approach in everyday clinical practice when trying to detect patients at higher risk of dissection. Nevertheless, in the absence of rigorous histopathologic studies, the demonstration of a distinctive connective tissue phenotype in patients with sCeAD reinforces the hypothesis that the disorder implicated in the pathogenesis of the disease is probably not limited to the vascular bed, and that the connective tissue is the most likely target organ.
Supplementary Material
GLOSSARY
- HCTD
hereditary connective tissue disorder
- IS
ischemic stroke
- sCeAD
spontaneous cervical artery dissection
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Giossi: manuscript drafting/revising, study design, data analysis and interpretation, data acquisition. Marco Ritelli, Paolo Costa, Andrea Morotti, Loris Poli, Elisabetta Del Zotto, Irene Volonghi, Nicola Chiarelli, Massimo Gamba, Paolo Bovi, Giampaolo Tomelleri, Monica Carletti, Nicoletta Checcarelli, Giorgio Meneghetti, Michele Morra, Mauro Chinaglia, Valeria De Giuli, Marina Colombi: manuscript drafting/revising, data acquisition. Alessandro Padovani: manuscript drafting/revising, study supervision. Dr. Pezzini: manuscript drafting/revising, study design, data analysis and interpretation, data acquisition, statistical analysis, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Débette S. Pathophysiology and risk factors for cervical artery dissection: what have we learned from large hospital-based cohorts? Curr Opin Neurol 2014;1:20–28. [DOI] [PubMed] [Google Scholar]
- 2.Brandt T, Orberk E, Weber R, et al. Pathogenesis of cervical artery dissections: association with connective tissue abnormalities. Neurology 2001;57:24–30. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Wijdicks EFM, Michels VV, Vockley J, Godfrey M. Heritable connective tissue disease in cervical artery dissections: a prospective study. Neurology 1998;50:1166–1169. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich R, Heidbreder A, Rohsbach D, et al. Connective tissue and vascular phenotype in patients with cervical artery dissection. Neurology 2007;68:2120–2124. [DOI] [PubMed] [Google Scholar]
- 5.Pezzini A, Del Zotto E, Archetti S, et al. Plasma homocysteine concentration, C677T MTHFR genotype and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke. Stroke 2002;33:664–669. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 7.Pezzini A, Drera B, Del Zotto E, et al. Mutations in TGFBR2 gene cause spontaneous cervical artery dissection. J Neurol Neurosurg Psychiatry 2011;82:1372–1374. [DOI] [PubMed] [Google Scholar]
- 8.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis 1973;32:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grond-Ginsbach C, Pjontek R, Aksay SS, Hyhlik-Durr A, Bockler D, Gross-Weissmann ML. Spontaneous arterial dissection: phenotype and molecular pathogenesis. Cell Mol Life Sci 2010;67:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


