Abstract
Objective:
We examined the influence of clinical, radiologic, and echocardiographic characteristics on antithrombotic choice in patients with cryptogenic stroke (CS) and patent foramen ovale (PFO), hypothesizing that features suggestive of paradoxical embolism might lead to greater use of anticoagulation.
Methods:
The Risk of Paradoxical Embolism Study combined 12 databases to create the largest dataset of patients with CS and known PFO status. We used generalized linear mixed models with a random effect of component study to explore whether anticoagulation was preferentially selected based on the following: (1) younger age and absence of vascular risk factors, (2) “high-risk” echocardiographic features, and (3) neuroradiologic findings.
Results:
A total of 1,132 patients with CS and PFO treated with anticoagulation or antiplatelets were included. Overall, 438 participants (39%) were treated with anticoagulation with a range (by database) of 22% to 54%. Treatment choice was not influenced by age or vascular risk factors. However, neuroradiologic findings (superficial or multiple infarcts) and high-risk echocardiographic features (large shunts, shunt at rest, and septal hypermobility) were predictors of anticoagulation use.
Conclusion:
Both antithrombotic regimens are widely used for secondary stroke prevention in patients with CS and PFO. Radiologic and echocardiographic features were strongly associated with treatment choice, whereas conventional vascular risk factors were not. Prior observational studies are likely to be biased by confounding by indication.
There is no definitive study comparing the efficacy of anticoagulation with antiplatelet therapy in patients with cryptogenic stroke (CS) and patent foramen ovale (PFO) or consensus from guidelines1–3 regarding the optimal antithrombotic regimen for secondary stroke prevention. While a meta-analysis including both randomized and (mostly) observational data suggested benefit from anticoagulation,4 the component studies did not control for confounding by indication. It is possible that patient characteristics might determine both therapy choice and prognosis.
The determinants of antithrombotic use in this population are unknown. We examined whether patients with CS + PFO are nonrandomly assigned to secondary prevention strategies and whether treatment is associated with patient characteristics at the time of the index stroke using the Risk of Paradoxical Embolism (RoPE) Study database.
METHODS
The RoPE Study and methods have been described previously.5,6 In brief, we created a database of 3,674 participants with CS and known PFO status (transesophageal echocardiography or transcranial Doppler) by combining existing cohort studies with protocol-driven follow-up. Index events were strokes or TIAs. The operational definition of “cryptogenic” varied somewhat between databases but generally adhered to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria. For the RoPE Study, participants with medium-risk cardiac sources of embolism, including PFO, were considered cryptogenic. To be included, each cohort study was required to have a minimum of 85% follow-up for 1-year outcome and sufficient clinical, echocardiographic, and neuroradiologic data to permit multivariate modeling. Variable definitions have been described previously.5,6 Variables were selected for analysis based on availability across databases and the plausibility that they might influence therapeutic decision-making.
Participants were classified as being treated with antiplatelets or anticoagulation based on treatment after the index event. Subsequent changes in medication were not analyzed. Participants without recorded discharge medications or discharged on a combination of both treatments were excluded in the primary analysis, but the latter were included within the anticoagulation group in a sensitivity analysis. Information was not available in the RoPE datasets about concurrent indications for anticoagulation. Those who were eventually treated with PFO closure were not excluded but were censored at the time of the procedure. Of the 12 RoPE databases, 4 were excluded because treatment was protocol-dictated (French,7 all treated with antiplatelets, and PICSS [PFO in Cryptogenic Stroke Study],8 randomized assignment), treatment was unknown (Bern unpublished), or the prevalence of one treatment was so low as to make hierarchical modeling impossible (Lausanne, antiplatelet use <5%).
Characteristics were compared between patients treated with anticoagulation and antiplatelets using 2-sample t tests (for age) and χ2 tests for all other categorized variables. Analyses were performed on raw data. Data are summarized as means and SDs and percentages, with the sample size for nonmissing values.
Multiple imputation, with 20 imputed datasets, was used to fill in missing data. Generalized linear models with a logit link function were used to estimate the odds ratios for each variable with the outcome of anticoagulation treatment controlling for a random effect of source database. All variables with a p value of <0.20 from these analyses were entered into a multivariable model, and retained if adjusted effects had a p value <0.05. The SAS procedures PROC MI and PROC GLIMMIX and PROC MIAnalyze were used to impute data, perform generalized linear model analyses, and then combine analysis results across the datasets, respectively (SAS version ≥9.2; SAS Institute Inc., Cary, NC).
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Tufts Medical Center Internal Review Board.
RESULTS
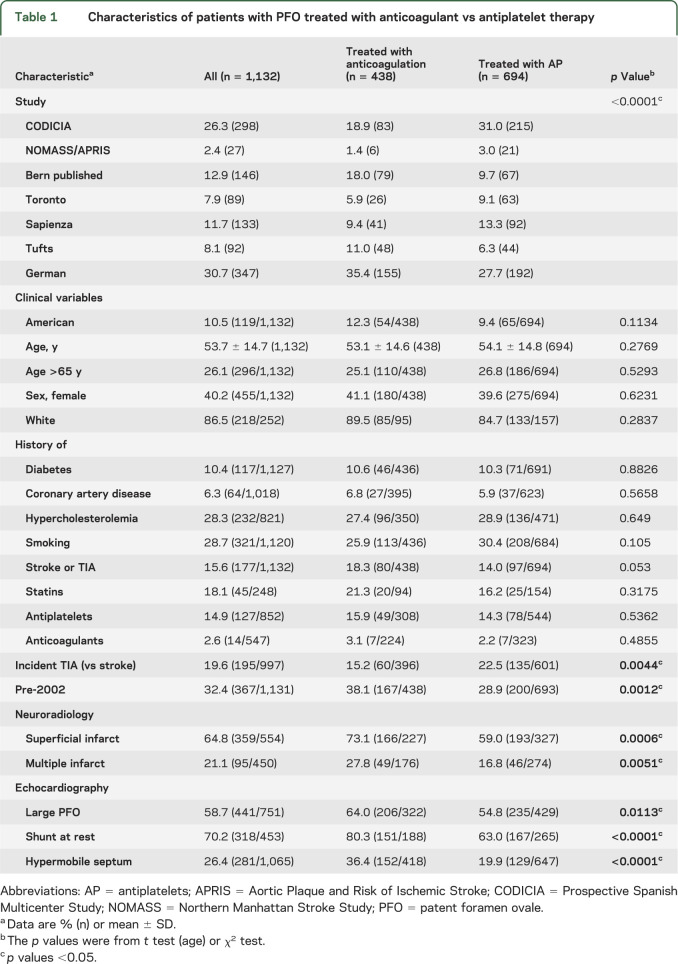
The RoPE databases that met inclusion criteria were CODICIA (Prospective Spanish Multicenter Study), APRIS (Aortic Plaque and Risk of Ischemic Stroke), Bern published, Toronto, Sapienza, Tufts, German, and NOMASS (Northern Manhattan Stroke Study). Participants with CS + PFO treated with anticoagulation or antiplatelets were included (n = 1,132). Overall, 438 participants (39%) were treated with anticoagulation with a range (by database) of 22% to 54%. Table 1 shows the demographics and results overall and by treatment group. The only clinical variables associated with decreased warfarin use were (1) index TIA event, and (2) later participant enrollment. Warfarin use before publication of the PICSS Trial (pre-2002) was 46% and was 35% in 2002 and after (p = 0.001). The radiologic variables associated with increased warfarin use were (1) superficial infarct location (46% vs 31%, p = 0.0001), and (2) multiple acute infarcts on the index imaging (52% vs 31%, p = 0.0027). All 3 echocardiographic variables were associated with treatment choice—anticoagulation was more common in those with large shunts (47% vs 37%, p = 0.0119), shunting at rest (47% vs 27%, p = 0.002), and a hypermobile interatrial septum (54% vs 34%, p < 0.001).
Table 1.
Characteristics of patients with PFO treated with anticoagulant vs antiplatelet therapy
Results from the unadjusted and adjusted generalized linear models controlling for site and using multiple imputation are consistent with those from the raw data. The only exception is that in the multivariable model, once superficial location is controlled for, the p value for multiple infarcts becomes >0.05. Sensitivity analyses that (1) added participants treated with both antithrombotics (n = 51) to the anticoagulation group, and (2) separated component databases into high and low users of anticoagulation yielded the same associations (tables e-1 and e-2 on the Neurology® Web site at Neurology.org).
DISCUSSION
Our data show that echocardiographic and neuroradiologic features putatively indicative of “paradoxical embolism” (i.e., superficial lesion, multiple acute lesions, large shunt, and atrial septal aneurysm) are associated with anticoagulation for secondary stroke prevention in patients with CS + PFO. However, clinical features associated with a probable pathogenic PFO (younger age and the absence of conventional vascular risk factors9) are not. In addition, anticoagulation became less frequently used after publication of PICSS in 2002. We did not investigate outcome rates or the comparative effectiveness of the treatments.
Despite the overall negative results of PICCS, which compared warfarin with aspirin in noncardioembolic stroke, there is some evidence that anticoagulation may be more effective for patients with CS + PFO.2 The pathology in paradoxical embolism (venous thromboembolism) may be analogous to deep vein thrombosis and pulmonary embolism, where warfarin treatment has consistently been superior to antiplatelets. Only a small minority of participants from PICSS had CS + PFO and were randomized to antithrombotic treatment (98/601). A trend in favor of warfarin was observed in this group but did not reach statistical significance (hazard ratio 0.52, 95% confidence interval 0.16–1.67; p = 0.28). A recent meta-analysis4 including mostly observational data suggests a similar—and statistically significant—benefit for anticoagulation over antiplatelet therapy although it was potentially biased by confounding by indication.
Our data, however, suggest that the prior observational studies, and so the meta-analyses,10 are likely to be severely limited by confounding by indication. In the RoPE population, the subgroup treated with anticoagulation and the one treated with antiplatelets are systematically different in ways that may influence the rates of recurrent stroke. Treatment effects measured across these groups are likely to be biased without controlling for confounding variables.
Our study has several limitations. We did not account for the planned sequential use of one antithrombotic and then another, e.g., several months of poststroke warfarin followed by antiplatelets. However, this does not explain the observed associations. We were limited by the variables that were included in the component databases. There may be unmeasured factors that influenced treatment decisions but that were not collected in the component databases. The variable definitions differed by site, potentially leading to patient misclassification. However, effort was made during the creation of the database to harmonize the variables as much as possible.
The RoPE dataset confirms that anticoagulants and antiplatelet drugs are nonrandomly assigned to participants with CS and that there is an association with radiologic and echocardiographic features available at the time of the index event. Conventional vascular risk factors, including age, were not associated with treatment choice. Despite the fact that anticoagulation has not been shown to be beneficial for paradoxical embolism, influential variables are consistent with those often assumed to support an embolic etiology or a perceived “high-risk” PFO.
Supplementary Material
GLOSSARY
- CS
cryptogenic stroke
- PFO
patent foramen ovale
- PICSS
PFO in Cryptogenic Stroke Study
- RoPE
Risk of Paradoxical Embolism
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D.T.: designed and conceptualized the study, analyzed and interpreted data in the study, drafted the manuscript, revised, reviewed, and approved the manuscript for intellectual content. D.K.: designed and conceptualized the study, analyzed and interpreted data in the study, contributed study data, and revised, reviewed, and approved the manuscript for intellectual content. R.R.: analyzed and interpreted data in the study, supervised database management, revised, reviewed, and approved the manuscript for intellectual content. C.W., J.S., H.M., K.N., M.M., E.D., M.E., M.D.: contributed study data, revised, reviewed, and approved the manuscript for intellectual content. S.H., P.M., B.M., A.F., J.L.: revised, reviewed, and approved the manuscript for intellectual content.
STUDY FUNDING
Supported by NIH (UL1 RR025752, R01 NS062153, R21 NS079826).
DISCLOSURE
D. Thaler has consulted for W.L. Gore & Associates. Dr. Thaler is a consultant to AGA Medical Corporation. R. Ruthazer, C. Weimar, J. Serena, H. Mattle, K. Nedeltchev, M. Mono, E. Di Angelantonio, M. Elkind, and M. Di Tullio report no disclosures relevant to the manuscript. S. Homma is a DSMB Member for the St. Jude Medical RESPECT Trial and a consultant for Boehringer Ingelheim Pharma, Pfizer, and BMS Pharmaceutical. P. Michel serves on the DSMB of the SAFE Study. B. Meier is supported by the following research grants to his institution and speakers bureau: AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Pfizer, and St. Jude Medical. A. Furlan is principal investigator of Closure I NMT Medical Boston. J. Lutz reports no disclosures relevant to the manuscript. D. Kent has consulted for W.L. Gore & Associates. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:630S–669S. [DOI] [PubMed] [Google Scholar]
- 2.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276. [DOI] [PubMed] [Google Scholar]
- 3.Messe SR, Silverman IE, Kizer JR, et al. Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2004;62:1042–1050. [DOI] [PubMed] [Google Scholar]
- 4.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012;43:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) Study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials 2011;12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaler DE, Di Angelantonio E, Di Tullio MR, et al. The Risk of Paradoxical Embolism (RoPE) Study: initial description of the completed database. Int J Stroke 2013;8:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740–1746. [DOI] [PubMed] [Google Scholar]
- 8.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625–2631. [DOI] [PubMed] [Google Scholar]
- 9.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013;81:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitsios GD, Thaler DE, Kent DM. Potentially large yet uncertain benefits: a meta-analysis of patent foramen ovale closure trials. Stroke 2013;44:2640–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.