Abstract
Objective:
To determine whether early environmental factors, such as cesarean delivery, breastfeeding, and exposure to smoking or herpes viruses, are associated with neuromyelitis optica (NMO) risk in children.
Methods:
This is a case-control study of pediatric NMO, multiple sclerosis (MS), and healthy subjects. Early-life exposures were obtained by standardized questionnaire. Epstein-Barr virus, cytomegalovirus, and herpes simplex virus 1 antibody responses were determined by ELISA. Multivariate logistic regression models were used to adjust for age at sampling, sex, race, and ethnicity.
Results:
Early-life exposures were obtained from 36 pediatric subjects with NMO, 491 with MS, and 224 healthy controls. Daycare (odds ratio [OR] 0.33, 95% confidence interval [CI] 0.14, 0.78; p < 0.01) and breastfeeding (OR 0.42, 95% CI 0.18, 0.99; p = 0.05) were associated with lower odds of having NMO compared with healthy subjects. Cesarean delivery tended to be associated with 2-fold-higher odds of NMO compared with having MS/clinically isolated syndrome (OR 1.98, 95% CI 0.88, 4.59; p = 0.12) or with being healthy (OR 1.95, 95% CI 0.81, 4.71; p = 0.14). Sera and DNA were available for 31 subjects with NMO, 189 with MS, and 94 healthy controls. Epstein-Barr virus, herpes simplex virus 1, cytomegalovirus exposure, and being HLA-DRB1*15 positive were not associated with odds of having NMO compared with healthy subjects.
Conclusions:
Exposure to other young children may be an early protective factor against the development of NMO, as previously reported for MS, consistent with the hypothesis that infections contribute to disease risk modification. Unlike MS, pediatric NMO does not appear to be associated with exposures to common herpes viruses.
Neuromyelitis optica (NMO) is characterized by optic neuritis, brainstem deficits, and transverse myelitis and is associated with aquaporin-4 (AQP4) antibodies.1 Adult studies suggest that genetic susceptibility to NMO is likely present, but most compatible with a complex disorder with an important component of nongenetic factors.2,3 Environmental risk factors for NMO are thus likely critical to disease onset, but these are largely unknown. In children, cases may follow a viral prodrome whereas in adults molecular mimicry involving T-cell responses against a Clostridium species may be important for disease initiation.4,5 In Japanese adults, exposure to the organisms Helicobacter pylori or Chlamydophila pneumonia may be associated with AQP4-immunoglobulin G (IgG)-positive NMO.6
In contrast with NMO, a number of environmental risk factors have been consistently reported for multiple sclerosis (MS) in adults and children. Epstein-Barr virus (EBV) antibody response is associated with increased risk of MS.7–10 Early exposure to cytomegalovirus (CMV) may be protective, while HLA-DRB1*15:01 status modifies the association of herpes simplex virus 1 (HSV-1) antibodies with MS.10,11 Early-life events such as breastfeeding and exposure to other young children may affect MS risk as well.12–15 The effect of these factors on NMO risk is not known.
The study of environmental factors in pediatric patients offers many advantages. Less bias in the recollection of early childhood events may occur and data are collected during the time of primary exposure to many factors including herpes virus infections. We sought to determine whether early-life events influence the odds of having NMO in children.
METHODS
Standard protocol approvals, registrations, and patient consents.
The project was approved by the local institutional review boards at all participating Pediatric MS Centers (University of California, San Francisco; SUNY at Stony Brook; SUNY at Buffalo; University of Alabama, Birmingham; Boston Children's Hospital; Partners Pediatric MS Center; Mayo Clinic; Children's Hospital Loma Linda; Texas Children's Hospital). When required by the local institutional review board, informed consent and assent were obtained from all parents and patients.
Patients.
For environmental questionnaire data, subjects with NMO, MS, and clinically isolated syndrome (CIS) with high risk of MS were identified through the Pediatric MS Network from May 2011 to June 2013. NMO cases were reviewed by a panel of 4 pediatric demyelinating disease specialists to confirm diagnosis. For serum and DNA studies, MS cases were identified from the aforementioned centers and NMO cases were identified from Stony Brook, University of California, San Francisco, and from the Accelerated Cure Project/Guthy Jackson Foundation. Subjects met published criteria for pediatric NMO and pediatric MS and had disease onset before age 18 years.16–18 All patients with NMO had their sera tested for AQP4 antibody status (Mayo Clinic) as part of their care. Healthy controls were identified at well visits to general pediatric clinics of the same aforementioned institutions from which cases were enrolled and were part of an ongoing case-control study led by the Pediatric MS Network (R01NS071463, principal investigator Waubant). Healthy controls had no history of autoimmune disease. Race and ethnicity were self-reported according to current NIH guidelines.
Environmental exposures.
For all subjects seen by the Pediatric MS Network, parents completed standardized questionnaires regarding pregnancy exposures and complications, delivery method, and early childhood exposures. Data extracted from these questionnaires are prospectively entered into a Web-based data capture system, as part of the Pediatric Multiple Sclerosis and Other Demyelinating Diseases database. The data include history of birth by cesarean delivery, breastfeeding and length of breastfeeding (months), exposure to active or passive cigarette smoking for 6 months or more after birth, attendance at daycare, number of siblings and order of birth, type of insurance (private vs other), and level of education of the parents (the latter 2 as surrogates of socioeconomic status).
Herpes virus assays.
Sera were collected from subjects at the time of enrollment. Batched EBV viral capsid antigen (VCA), Epstein Barr nuclear antigen-1 (EBNA1), CMV, and HSV-1 IgG antibody responses were measured blindly at the Oklahoma Medical Research Foundation. Results for normalized ELISAs (Wampole Laboratories, Princeton, NJ) for EBV-VCA, CMV, and HSV-1 are presented as international standardized ratios.19 EBNA1 ELISA (BioDesign, Carmel, NY) results are reported as relative concentration as previously described.20,21 Positive anti-EBNA1 responses were ≥4 SDs above a panel of controls known to be EBV seronegative.
Genotyping.
Because the effect of viral exposures on MS risk may depend on HLA-DRB1 status, we measured this risk factor in subjects tested for viral responses.19 DNA samples of MS, NMO, and control patients were typed in a blinded manner by a gene-specific Taqman assay for single nucleotide polymorphisms associated with HLA-DRB1*1501/1503 alleles, as previously published.19
Statistical methods.
Statistical analyses were performed using Stata 12.0 statistical software (StataCorp, College Station, TX) and SAS/STAT software (version 9.3; SAS Institute, Inc., Cary, NC). Median [interquartile range] or n (%) was used to describe patient characteristics across the different diagnostic groups. Interbirth interval was defined as the difference in ages between the patient and the nearest younger sibling. Patients with no younger siblings were assigned the highest ranking for interbirth interval. Infant-years exposure was defined as the total amount of time before age 6 that the patient was exposed to a younger sibling or twin younger than 2 years of age. Comparisons were made using χ2 test for binary variables and Kruskal-Wallis test for numeric variables. For each risk factor of interest, analysis was performed using multivariable logistic regression models adjusting for sex, race, and ethnicity for environmental questionnaire data and age at blood draw, sex, race, and ethnicity for sera measured variables. A 2-sided p value <0.05 was considered significant.
RESULTS
Demographic features and early environmental exposures.
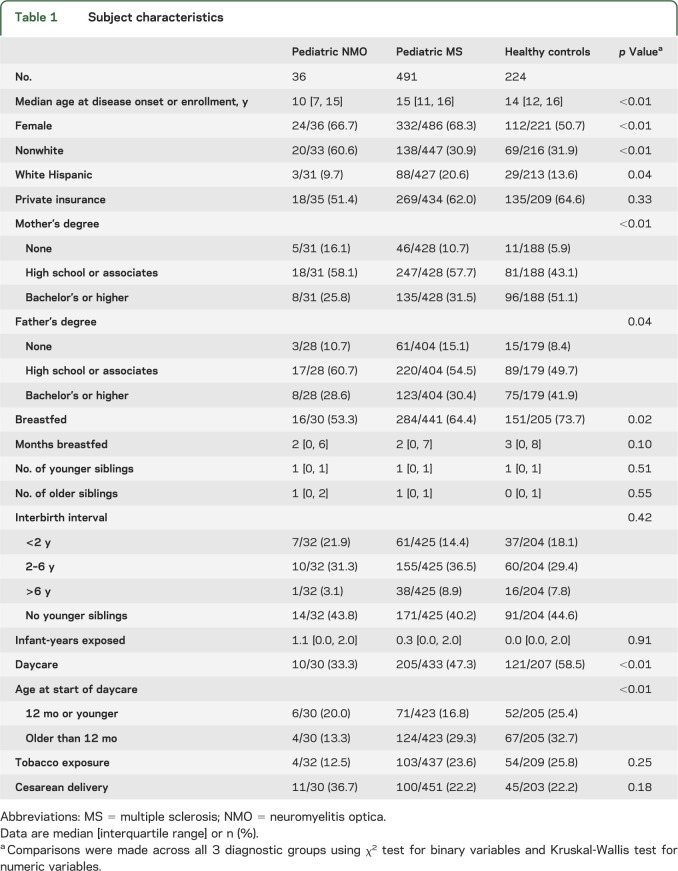
Early environmental exposure questionnaires were completed by the parents of 36 subjects with NMO, 491 with MS, and 224 healthy controls (table 1). Pediatric subjects with NMO were younger at onset than subjects with MS/CIS (p < 0.01). There were also racial differences across the diagnostic groups (p < 0.01) with more African Americans in the NMO group. NMO cases had 58% frequency of positive results for AQP4-IgG. While AQP4-IgG-positive and -negative NMO cases were combined for the primary analyses, we did observe some demographic differences, with AQP4-IgG-positive individuals more likely to be female and of nonwhite descent than AQP4-IgG-negative subjects with NMO (table e-1 on the Neurology® Web site at Neurology.org).
Table 1.
Subject characteristics
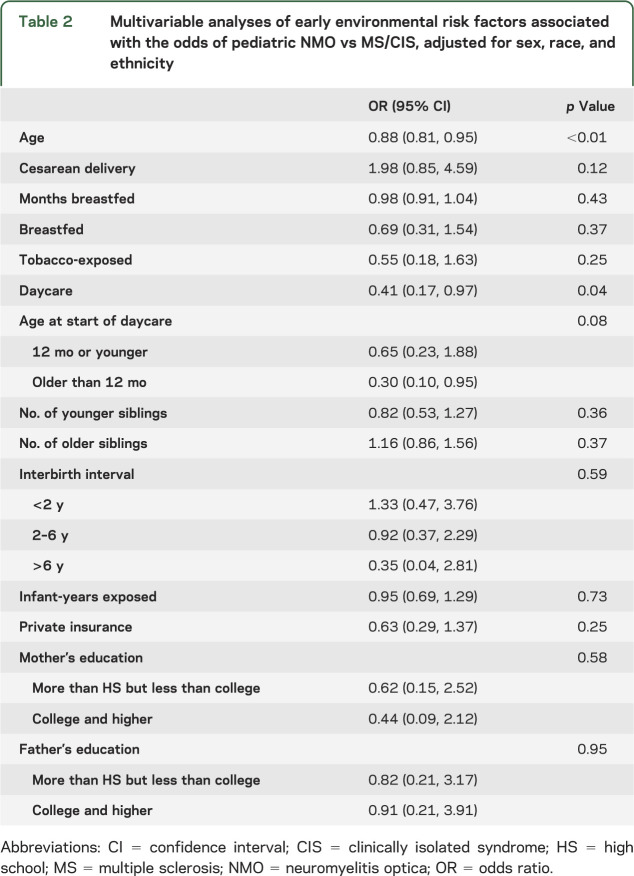
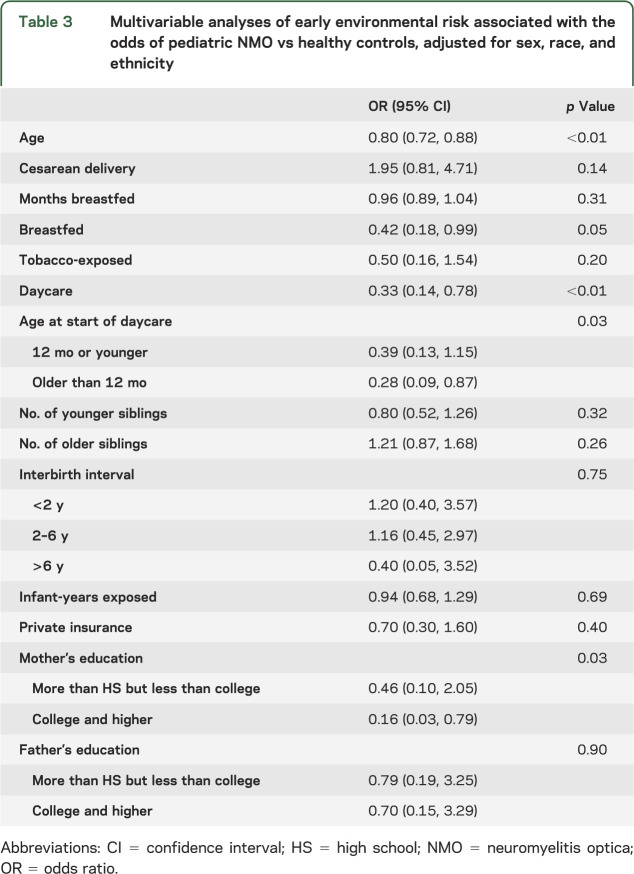
In multivariable analyses adjusted for sex, race, and ethnicity (tables 2 and 3), exposure to daycare was associated with substantially reduced odds of having NMO compared with having MS/CIS (odds ratio [OR] 0.41, 95% confidence interval [CI] 0.17, 0.97; p = 0.04) or with being a healthy control (OR 0.33, 95% CI 0.14, 0.78; p < 0.01). Breastfeeding was also associated with lower odds of having NMO vs being a healthy control (OR 0.42, 95% CI 0.18, 0.99; p = 0.05). Delivery by cesarean section tended to be associated with 2-fold-higher odds of NMO compared with having MS/CIS (OR 1.98, 95% CI 0.88, 4.59; p = 0.12) or with being healthy (OR 1.95, 95% CI 0.81, 4.71; p = 0.14).
Table 2.
Multivariable analyses of early environmental risk factors associated with the odds of pediatric NMO vs MS/CIS, adjusted for sex, race, and ethnicity
Table 3.
Multivariable analyses of early environmental risk associated with the odds of pediatric NMO vs healthy controls, adjusted for sex, race, and ethnicity
Maternal education level was associated with risk of NMO (p = 0.03). College-level education or higher, attained by the mother, reduced the odds of NMO by 84% as opposed to not completing high school (OR 0.16, 95% CI 0.03, 0.79) compared with healthy controls. Other markers of socioeconomic status, insurance type and father's education, as well as exposure to cigarette smoking were not associated with having NMO.
Herpes virus exposures.
For the evaluation of viral susceptibility factors, DNA and serum were available for 31 pediatric NMO cases, 186 pediatric MS/CIS cases, and 94 healthy controls (table e-2). Differences across groups included proportions of patients of nonwhite descent (48.3% for NMO vs 27.2% and 25.3% for MS and healthy subjects, respectively). Sex, age at blood draw, race, and ethnicity were adjusted for in the multivariable analyses.
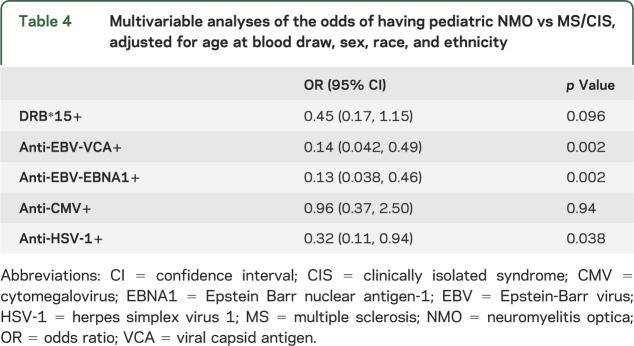
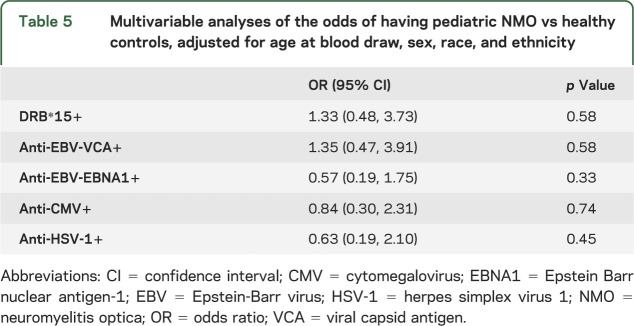
Compared to patients with MS (table 4), EBV antibody responses were associated with lower odds of NMO (VCA OR 0.14, 95% CI 0.042, 0.49; p = 0.002; EBNA1 OR 0.13, 95% CI 0.038, 0.46; p = 0.002). HSV-1 exposure was also associated with lower odds of having NMO compared to subjects with MS (OR 0.32, 95% CI 0.11, 0.94; p = 0.038). There were no differences for viral risk factors between patients with NMO and healthy controls (table 5).
Table 4.
Multivariable analyses of the odds of having pediatric NMO vs MS/CIS, adjusted for age at blood draw, sex, race, and ethnicity
Table 5.
Multivariable analyses of the odds of having pediatric NMO vs healthy controls, adjusted for age at blood draw, sex, race, and ethnicity
HLA-DRB1*15.
Multivariable analyses demonstrated that having the HLA-DRB1*1501 or 1503 allele tended to be associated with lower odds of NMO (OR 0.45, 95% CI 0.17, 1.15; p = 0.096) compared to patients with MS/CIS but not compared to healthy controls (OR 1.33, 95% CI 0.48, 3.73; p = 0.58).
DISCUSSION
In this study of early environmental risk factors of NMO, we have demonstrated that breastfeeding and daycare exposure may be protective. The immune benefits of breastfeeding are well recognized. For autoimmune diseases, the benefit may be related to transfer of maternal antigens and immune cells, resulting in improved immunity or tolerance.22 Protective effects have been demonstrated for atopic allergies, type 1 diabetes, Crohn disease, MS, and rheumatoid arthritis, although some of these findings remain controversial.13,22–30 Early exposure to other children has been consistently associated with decreased MS risk, implicating that viral infections early in life are protective.15,31 Daycare provides one of the largest exposures to pathogens for young children. Our findings suggest that attending daycare also likely protects against the development of NMO. We did not find that exposure to younger or older siblings increased the odds of NMO, but the subjects we evaluated had few siblings and the overall number of included patients with NMO was small, such that an association cannot be excluded.
In MS, while early viral exposures may be protective, EBV infection and, in particular, late EBV infection, resulting in mononucleosis, have been strongly associated with MS risk.7,32 We found no association of EBV exposure as measured by standard VCA or EBNA1 ELISA and NMO compared with healthy subjects. This was despite the older age of blood draw in the subjects with NMO. These data suggest that EBV infection likely does not increase risk of NMO.
Whereas passive smoking has been implicated in increasing risk of some autoimmune diseases including MS, we did not find evidence of an association of early exposure to cigarette smoke with odds of NMO, although our sample size of subjects with NMO was limited.33,34 We found delivery by cesarean section tended to be associated with a 2-fold-greater odds of NMO, compared with either healthy controls or subjects with MS. Even though the observation did not reach statistical significance, the magnitude of the point estimate was the same for analyses with both control groups and warrants further investigation in a larger study. The significance of a nonvaginal birth and autoimmune disease has been previously postulated to relate to early differences in infant gut flora.35,36
Similar to recent findings in adults, we observed demographic differences in AQP4-IgG-positive and -negative subjects with NMO.37,38 Subjects testing positive were predominantly female and nonwhite whereas the sex ratio in AQP4-IgG-negative subjects with NMO neared 1 and the majority were of white ancestry. Because of limited number of NMO cases, we were not able to further stratify our multivariable analyses by AQP4-IgG status, but this would be of interest in future, larger studies of NMO risk factors.
The strengths of our study include the careful case ascertainment and the use of 2 types of controls coming from the same centers. Nevertheless, we acknowledge some limitations of this study. The number of pediatric subjects with NMO was small. The variables most vulnerable to sample-size limitations in our study may be insurance, smoking, and cesarean delivery, because these show plausible patterns isolated to the NMO group, that if present in a larger sample size may have been significant. Recall bias is of concern in case-control studies, but this may be less of a limitation in the pediatric setting where the early environmental risk factor data are ascertained from parents and at time points closer to exposure than in adult studies. Recall bias is also likely to be similar and less influential in comparison of 2 disease states—MS and NMO. Furthermore, the older age and longer disease duration at serum collection for the NMO group means that viral exposures for the NMO group at time of onset may have been different compared with time of serum testing, although analyses were adjusted for both. However, the older age could not explain a lower frequency of seropositivity for viruses studied.
Pathologic and biological evidence now demonstrate that NMO and MS are separate entities. Our study suggests that risk factors for both disorders may also differ. Larger prospective case-control studies will further identify genetic and environmental risk factors specific to NMO. These may in turn help define more in-depth disease processes at play.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Jorge Oksenberg and Stacy Caillier of UCSF for the assistance with sample preparation and DNA typing and Jourdan Anderson of the Oklahoma Medical Research Foundation for assistance with viral antibody testing. The authors also acknowledge Dr. Brian Weinshenker of the Mayo Clinic in his editing of the manuscript, and greatly appreciate the support of the Accelerated Cure Project.
GLOSSARY
- AQP4
aquaporin-4
- CI
confidence interval
- CIS
clinically isolated syndrome
- CMV
cytomegalovirus
- EBNA1
Epstein Barr nuclear antigen-1
- EBV
Epstein-Barr virus
- HSV-1
herpes simplex virus 1
- IgG
immunoglobulin G
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- OR
odds ratio
- VCA
viral capsid antigen
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Graves: design of study, data collection, analysis and interpretation of data, drafting and revising the manuscript. Siri Grandhe: data collection and analysis, revising manuscript. Kelley Weinfurtner: data collection and analysis, revising manuscript. Drs. Krupp and Belman: provided samples, interpretation of data, revising the manuscript. Dr. Ness: provided samples, interpretation of data, revising the manuscript. Dr. Gorman: provided samples, interpretation of data, revising the manuscript. Dr. Weinstock-Guttman: data collection and revising the manuscript. Dr. Patterson and Rodriguez: provided samples, interpretation of data, revising the manuscript. Dr. Chitnis: provided samples, interpretation of data, revising the manuscript. Dr. Aaen: provided samples, interpretation of data, revising the manuscript. Dr. Lotze: provided samples, interpretation of data, revising the manuscript. Dr. Mowry: data collection and analysis, revising the manuscript. Dr. Rose: data collection, revising the manuscript. Timothy Simmons: analysis and interpretation of data, revising the manuscript. Dr. Casper: design of study, data collection, analysis and interpretation of data, revising the manuscript. Dr. James: serotyping, interpretation of data, revising the manuscript. Dr. Waubant: design of study, interpretation of data, revising the manuscript.
STUDY FUNDING
This work was supported by the National MS Society (HC 0165) and NIH (R01NS071463). In addition, work in Dr. James' laboratory was supported by NIH grants U19 AI082714 and U01 AI101934. Dr. Graves' NIH support was through the BIRCWH K12 program (5K12HD052163).
DISCLOSURE
J. Graves was supported by the Foundation for Consortium of Multiple Sclerosis Centers and the NIH Bridging Interdisciplinary Research Careers in Women's Health programs (5K12HD052163) during this work. She has been a one-time advisory board participant for EMD Serono. S. Grandhe and K. Weinfurtner report no disclosures relevant to the manuscript. L. Krupp is supported by the National MS Society, NIH, Robert and Lisa Lourie Foundation, Department of Defense. She has received honoraria, consulting payments, grant support, or royalties from Biogen, MedImmune, Novartis, Teva Neuroscience, Sanofi-Aventis, and EMD Serono. A. Belman reports no disclosures relevant to the manuscript. T. Chitnis has served as a consultant for Biogen Idec, Teva Neurosciences, Novartis, Sanofi-Aventis, and has received grant support from NIH, National MS Society, Guthy-Jackson Charitable Foundation, CMSC and Merck-Serono, and Novartis. J. Ness reports no disclosures relevant to the manuscript. B. Weinstock-Guttman received honoraria for serving in advisory boards and educational programs from Teva Pharmaceuticals, Biogen Idec, Novartis, Acorda EMD Serono, Novartis, Genzyme, and Sanofi. She also received support for research activities from the NIH, National Multiple Sclerosis Society, National Science Foundation, Department of Defense, EMD Serono, Biogen Idec, Teva Neuroscience, Novartis, Acorda, Genzyme, and the Jog for the Jake Foundation. M. Gorman, M. Patterson, M. Rodriguez, T. Lotze, and G. Aaen report no disclosures relevant to the manuscript. E. Mowry is supported by the National MS Society and the NIH. She receives free medication for a randomized control trial from Teva Neuroscience. J. Rose has research funding from Teva Neuroscience and Biogen. He is a member of the Medical Advisory Board for the DECIDE trial, which is funded by Biogen and AbbVie. T. Simmons reports no disclosures relevant to the manuscript. T. Casper has been supported by the National MS Society and the NIH (R01NS071463). He is an ad hoc consultant for Biovest International, Inc. J. James reports no disclosures relevant to the manuscript. E. Waubant is funded by the National MS Society, the NIH, and the Race to Erase MS. She has received honorarium from Teva Neuroscience and Genzyme for 2 educational lectures, and is an ad hoc consultant for Actelion, Sanofi-Aventis, and Roche. She is on an advisory board for a clinical trial of Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 2.Matiello M, Kim HJ, Kim W, et al. Familial neuromyelitis optica. Neurology 2010;75:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matiello M, Schaefer-Klein JL, Hebrink DD, Kingsbury DJ, Atkinson EJ, Weinshenker BG. Genetic analysis of aquaporin-4 in neuromyelitis optica. Neurology 2011;77:1149–1155. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery AR, Buncic JR. Pediatric Devic's neuromyelitis optica. J Pediatr Ophthalmol Strabismus 1996;33:223–229. [DOI] [PubMed] [Google Scholar]
- 5.Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize clostridium ABC transporter. Ann Neurol 2012;72:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimura S, Isobe N, Matsushita T, et al. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry 2013;84:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis: part I: the role of infection. Ann Neurol 2007;61:288–299. [DOI] [PubMed] [Google Scholar]
- 8.Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol 2007;6:773–781. [DOI] [PubMed] [Google Scholar]
- 9.Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 2010;5:e12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowry EM, James JA, Krupp LB, Waubant E. Vitamin D status and antibody levels to common viruses in pediatric-onset multiple sclerosis. Mult Scler 2011;17:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundqvist E, Bergstrom T, Daialhosein H, et al. Cytomegalovirus seropositivity is negatively associated with multiple sclerosis. Mult Scler 2014;20:165–173. [DOI] [PubMed] [Google Scholar]
- 12.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis: part II: noninfectious factors. Ann Neurol 2007;61:504–513. [DOI] [PubMed] [Google Scholar]
- 13.Conradi S, Malzahn U, Paul F, et al. Breastfeeding is associated with lower risk for multiple sclerosis. Mult Scler 2013;19:553–558. [DOI] [PubMed] [Google Scholar]
- 14.van der Mei IA, Ponsonby AL, Taylor BV, et al. Human leukocyte antigen-DR15, low infant sibling exposure and multiple sclerosis: gene-environment interaction. Ann Neurol 2010;67:261–265. [DOI] [PubMed] [Google Scholar]
- 15.Ponsonby AL, van der Mei I, Dwyer T, et al. Exposure to infant siblings during early life and risk of multiple sclerosis. JAMA 2005;293:463–469. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007;68:S7–S12. [DOI] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–1267. [DOI] [PubMed] [Google Scholar]
- 19.Waubant E, Mowry EM, Krupp L, et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology 2011;76:1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James JA, Neas BR, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum 2001;44:1122–1126. [DOI] [PubMed] [Google Scholar]
- 21.McClain MT, Poole BD, Bruner BF, Kaufman KM, Harley JB, James JA. An altered immune response to Epstein-Barr nuclear antigen 1 in pediatric systemic lupus erythematosus. Arthritis Rheum 2006;54:360–368. [DOI] [PubMed] [Google Scholar]
- 22.Jackson KM, Nazar AM. Breastfeeding, the immune response, and long-term health. J Am Osteopath Assoc 2006;106:203–207. [PubMed] [Google Scholar]
- 23.Colebatch AN, Edwards CJ. The influence of early life factors on the risk of developing rheumatoid arthritis. Clin Exp Immunol 2011;163:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003;58:833–843. [DOI] [PubMed] [Google Scholar]
- 25.Koletzko S, Sherman P, Corey M, Griffiths A, Smith C. Role of infant feeding practices in development of Crohn's disease in childhood. BMJ 1989;298:1617–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrao G, Tragnone A, Caprilli R, et al. Risk of inflammatory bowel disease attributable to smoking, oral contraception and breastfeeding in Italy: a nationwide case-control study. Cooperative Investigators of the Italian Group for the Study of the Colon and the Rectum (GISC). Int J Epidemiol 1998;27:397–404. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 2003;290:1721–1728. [DOI] [PubMed] [Google Scholar]
- 28.Simard JF, Costenbader KH, Hernan MA, Liang MH, Mittleman MA, Karlson EW. Early life factors and adult-onset rheumatoid arthritis. J Rheumatol 2010;37:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardwell CR, Stene LC, Ludvigsson J, et al. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care 2012;35:2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisacane A, Impagliazzo N, Russo M, et al. Breast feeding and multiple sclerosis. BMJ 1994;308:1411–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conradi S, Malzahn U, Schroter F, et al. Environmental factors in early childhood are associated with multiple sclerosis: a case-control study. BMC Neurol 2011;11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen TR, Rostgaard K, Nielsen NM, et al. Multiple sclerosis after infectious mononucleosis. Arch Neurol 2007;64:72–75. [DOI] [PubMed] [Google Scholar]
- 33.Ramagopalan SV, Lee JD, Yee IM, et al. Association of smoking with risk of multiple sclerosis: a population-based study. J Neurol 2013;260:1778–1781. [DOI] [PubMed] [Google Scholar]
- 34.Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Exposure to environmental tobacco smoke is associated with increased risk for multiple sclerosis. Mult Scler 2011;17:788–793. [DOI] [PubMed] [Google Scholar]
- 35.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol 2011;38:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol 2013;208:249–254. [DOI] [PubMed] [Google Scholar]
- 37.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology 2013;81:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.