Abstract
Background
Autism Spectrum Disorder (ASD) is a complex neurobehavioral syndrome with no known biomarker so far for early detection. It has been challenging, both to classify typical autism and associate a suitable biomarker with clinical phenotype spectrum. Brain-derived neurotrophic factor (BDNF) has emerged as a key neurotrophin regulating synaptic plasticity, neuronal differentiation and survival.
Purpose
Recently, BDNF depletion is reported in neurodegenerative as well as in psychiatric disorders, associated with severity of neurological dysfunction. Role of BDNF as a biomarker in ASD is gaining significance. Pre-clinical results have linked BDNF depletion in autism and mental retardation, however, with conflicting findings.
Methods
In view of this, a preliminary study was carried out to measure serum BDNF levels in 48 children with ASD and mental retardation, and 29 age-matched controls.
Results
Serum BDNF levels were found significantly higher (p<0.001) in atypical autistic subjects (clinically milder phenotype) as compared to controls, but not in typical ASD cases (clinically severe phenotype). BDNF levels were significantly lower in females with typical/Rett Syndrome (p<0.05), but not in males with typical autism (p>0.1), as compared to controls. Lower BDNF levels indicate impairment in neuroprotective mechanism, while higher levels may imply a manifested protective response.
Conclusion
Our study highlights the differential BDNF response based on the severity of neurobehavioral deficit, indicating a possible neuroprotective role of this molecule and supporting its exploration in targeted therapy in ASD.
Keywords: Disorder, BDNF, Biomarker
Introduction
Autism is a group of complex neurodevelopmental disorders of unknown etiology which manifests with problems with social interaction, language, communication and behavior deficits like stereotype and repetitive activities.1 Though originally described by Kanner2 the etiological factor for autism still remains an enigma. Most cases of classical autism present with failure to develop normal, social or language skills, which is termed early onset autism. However, a group of children with autistic features develop normally until approximately 18 to 24 months of age after which they show neuroregression. This is termed as regressive autism. Symptoms usually start before three years of age and can cause delays or problems in many different skills that develop from infancy to adulthood. However, the average age of diagnosis is about 6 years.3 Centre for Disease Control reports the autism prevalence rate of 1 in 110 children in USA.4,5 However, similar data on prevalence are scarce in India. Autism Spectrum Disorder (ASD) is evaluated by a clinical psychologist using DSM-IV-R criteria which can be quite challenging due to complex phenotypes. Hence, there is need for early diagnosis, and an optimal biomarker for the same is globally being explored. Different people with autism can have varied spectrum of mild to severe autistic features and clinical symptoms. Therefore the term “Autism Spectrum Disorder” is often used by physicians.
The etiology of autism still remains unknown, with many factors implicated in the development of autism phenotype. Chromosomal abnormalities, and Fragile-X have been reported in autistic children.6,7 The central pathology in the brain is arrest of neuronal development. Megalancephalic brain, neuronal disorganization and abnormal migration, cortical heteropia, reduced dendritic spine density, reduction and incursion in purkinje cells and sometimes gliosis are the neuropathological hallmarks of ASD. Several genetic studies link autism with genes that have immunomodulatory functions. However, genetic studies have not yet revealed a specific autism marker and this has led to the hypothesis that an environmental trigger could be associated with certain cases of autism.8 Recent research indicate the role of genetic and environmental factors or an interaction of both, responsible for the clinical symptoms of ASD. A polymorphism G196A which alters the amino acid Valine at the 66th position in the pro-region of BDNF protein to Methionine, is the most studied polymorphism in the BDNF gene. However, it is not reported in all the cases and there has been no studies from India.
Neurotrophins play a pivotal role in the functioning of the nervous system including development, survival, function and plasticity. Brain-Derived Neurotrophic Factor (BDNF) is the most abundant and widely distributed neurotrophin in the mammalian Central Nervous System (CNS). Since the purification of BDNF protein, definitive evidence has emerged for its key role in mammalian brain development, physiology and pathology.9 There is a growing evidence for the role of BDNF in the survival, differentiation and plasticity of neurons throughout the brain and spinal cord. BDNF is thought to participate by inhibiting apoptosis10 and stimulating sprouting and neuronal reorganization.11 The cellular actions of BDNF are believed to be mediated through tyrosine kinase receptor B (TrkB) and by p75NTR (p75neurotrophin receptor), a member of the TNF (tumor necrosis factor) receptor superfamily.12
BDNF is a 119-amino-acid, 13.6-kDa signaling protein13 involved in the central regulation of energy homeostasis. Interestingly, recent research studies have suggested the role of Methyl Cytosine Binding Protein (MeCP2) gene in the epigenetic regulation of gene expression in ASD.14 BDNF gene is one of the target for MeCP2 gene. Decreased expression of BDNF contributes to the cell death and neurodegeneration in Alzheimer, Parkinson and Huntington disease.15 Therefore, animal experiments are aimed at strategies for “BDNF therapies” by targeting BDNF production or increasing BDNF signaling by gene or protein delivery. It is, therefore, logical to estimate the level of BDNF in individuals with definite/typical autism (by DSM-IV criteria) and atypical autism (partly satisfying DSM-IV criteria). Therefore we wanted to examine whether BDNF levels can be correlated to the degree of impairment of neurologic response to brain insult, resulting into clinical spectrum characteristic of autism.
In order to evaluate the relationship between BDNF levels in typical and atypical autism cases, we estimated the serum BDNF levels in children with autism spectrum disorder.
Methods
Subjects
Forty eight children (25 males and 23 females) with autism spectrum disorder, with the mean age of 7.4 years, were enrolled for the study. The children showed varying degree of mental abnormality and were undergoing special training in schools meant for children with mental retardation and autism. These children were examined by the attending pediatrician and evaluated by the clinical psychologist to further categorize into various groups of ASD using the DSM-IV criteria. Detailed clinical history including prenatal, birth and family history was obtained, with emphasis on high risk genetic factors like consanguinity, spontaneous abortions, history of sibling or neonatal death, history of MR and autism in the family. The genetic history was recorded using a pedigree chart. Twenty eight age matched apparently healthy controls were also enrolled in this study.
Ethical Clearance
The study was carried out as per the Ethical Guidelines for Biomedical Research on Human Participants, Indian Council of Medical Research (ICMR), 2006, which is based on the Declaration of Helsinki, 2004.16 Informed written consent was obtained from the parent/guardian of each child enrolled in this study.
Estimation of serum BDNF
Four milliliters of venous blood was collected from each participant. Serum was separated immediately and stored at –20ºC till further use. Serum BDNF concentration was estimated by sandwich ELISA method using the Human BDNF ELISA kit (ELH-BDNF-001) from RayBiotech Inc.
Data Analysis
Serum BDNF concentrations were compared across the control and sample groups using the Students’ t test. The level of significance was set at p<0.05.
Results
Forty eight children with autism and mental retardation were evaluated for serum BDNF levels by comparing them with 29 age matched controls. Serum BDNF levels in children with autism and mental retardation did not differ significantly from the control group (p>0.05). An interesting finding was observed when the autism group was sub-categorized into typical (Group A) and atypical (Group B) forms, based on the severity of the disorder, as per DSM-IV criteria (Table 1). While no significant difference was observed in serum BDNF values of typical autism cases, BDNF levels were significantly higher (p<0.001) in atypical autism cases when compared with controls.
Table 1: Comparison of serum BDNF levels of ASD cases with controls.
| Subjects | Number of subjects (N) | Mean age | Serum BDNF concentration (Mean ± SD) ng/mL | p value as compared to Controls |
|---|---|---|---|---|
| The significance is set at p<0.05, where *** = p<0.001, implying an extremely significant difference. | ||||
| Controls | 25 | 7.44 years | 225.16 ± 79.54 | – |
| Group A (Typical ASD/Definite autism) | 25 | 7.61 years | 198.93 ± 65.32 | 0.19 |
| Group B (Atypical ASD) | 23 | 7.08 years | 306.68 ± 85.93 | 0.0008*** |
Additionally, Rett Syndrome being the most severe, X-linked form of typical autism, comprising of female subjects, the typical autism group was further divided based on the gender of the patient (Table 2). A noteworthy observation was that though both male and female patients were typically autistic, serum BDNF concentration in typical autistic females was significantly lower than that of the control group (p<0.05). Males with typical autism however did not differ extensively in the serum BDNF values in comparison with control subjects.
Table 2: Rett Syndrome being an X-linked disorder manifested in females, we segregated the male and female subjects of the typical ASD group for comparing with controls.
| Subjects | Number of subjects (N) | Serum BDNF concentration (ng/mL) | ||
|---|---|---|---|---|
| Mean | SD (Range) | p value as compared to Controls | ||
| The significance is set at p<0.05. | ||||
| Controls | 25 | 225.16 | ±79.54 (145.63–304.70) | – |
| Typical ASD Males | 14 | 231.02 | ±62.53 (168.49–293.56) | 0.82 |
| Typical ASD Females | 11 | 164.16 | ±47.82 (116.34–211.99) | 0.018* |
Discussion
Autism (OMIM 209850) is a childhood neurodevelopmental disorder that is apparent by 3 years of age and characterized by significant impairment in reciprocal social interaction and communication as well as behavior. The etiology of autism is an enigma, with possible causes ranging from genetic, environment, toxicity, nutritional deficit, to a combination of these factors. In clinical practice, mental retardation (IQ<70) is often a part of autism in 50–70% of cases.17 Despite the efforts of multiple research groups to find out a suitable biomarker for diagnostics and targeted therapy, the genetic and phenotypic heterogeneity associated with autism spectrum disorder makes it the most challenging disorder for diagnosis.18
Both Rett Syndrome in female patients and Rett-Syndrome-like neurodevelopmental disorders in male patients as well as other neurodevelopmental disorders present a wide spectrum of clinical phenotypes that share a combination of symptoms like mental retardation, autism, microcephaly, stereotypic behavior and epilepsy. Recently, mutation in MeCP2 gene is shown not to be a major cause of Rett Syndrome-like or related neurodevelopmental phenotypes in male patients,19 and a need to identify additional novel genes is emphasized by this group. Similarly, Fragile-X Syndrome shares most of behavioral similarities with autism though many patients do not meet the diagnostic criteria for typical autism but still manifest autistic features such as delayed speech development, peculiar speech, gaze aversion, hand beating or flapping, a pervasive lack of responsiveness, bizarre responses to the environment like extreme attachment to animate and inanimate objects and dramatic mood swings related to minor changes in routine.20
Brain-derived neurotrophic factor (BDNF) is emerging as a potential molecule that might help to better understand several neurodevelopmental as well as neurodegenerative disorders. Association of BDNF is reported with changes in behavior, such as hyperactivity, increased depression and psychiatric disorders including schizophrenia and bipolar disorder.21 Multiple studies have shown increase in BDNF in both blood and brain tisssue from autistic subjects.22 Since BDNF readily crosses the Blood-Brain-Barrier, the serum concentrations correlate directly to brain concentration, therefore serum studies of BDNF are thought to accurately reflect BDNF levels in the brain. Specifically elevated BDNF expression has been observed in the brain and blood.1,22
To evaluate the availability of the serum BDNF for diagnosis of patients with neurodevelopmental disorders, we estimated forty eight children with autism spectrum disorder and mental retardation for their serum BDNF concentrations. Based on the severity and presentation of the disease phenotype, we classified autistic children into two sub-groups, viz. Group A (typical autistic cases by DSM-IV criteria) and Group B (cases with atypical autism). Overall, we observed no significant difference in the serum BDNF concentration in autistic children when compared to the age-matched controls (Table 1); the finding which we found different from Miyazaki group.22 Interestingly, when we analyzed the variation in the BDNF concentrations separately in two groups, we could observe significant differences. While the serum BDNF levels in Group A did not differ significantly from controls, BDNF levels in Group B, which comprised of the milder phenotype, were significantly higher than that of normal controls (p<0.001).
In a recent study investigating serum levels of BDNF and SHH (Sonic Hedgehog Protein) in control and autistic children, BDNF serum level were significantly high in mild but not severe autistic children compared to age and sex matched subjects 442 ± 20 (pg/ml), 290 ± 90 (pg/ml), P<0.05), respectively.23
The current knowledge about BDNF in a number of neurodegenerative diseases and feasibility of BDNF therapeutic approach in the clinic has been well reviewed by Zuccato and Cattaneo (2009)15, emphasizing the exogenous restoration of BDNF. Further, a link between BDNF loss and disease progression of Rett syndrome has also been reported.24
The increased BDNF response in some subjects with autism is considered as an immune cell response which may be dysregulated in autism. Among the autism group, the different BDNF response was reported based on patterns of onset with early onset and severe form of autism being on higher trend of BDNF.8 The other research groups are strongly associating the decreased expression of BDNF contributing to the progression of cell death in neurodegenerative disorders like Alzheimer’s Disease.25
Depletion of brain and serum BDNF has also been reported in neurodegenerative disorders like Alzheimer, Parkinson and Huntington diseases. As pre-clinical results have linked BDNF depletion with autism, various laboratories are exploring the therapeutic significance of BDNF in autism and also in neurodegenerative disorders. However, BDNF has been shown to affect angiogenesis pathway, although the procedure for BDNF and VEGF(molecule responsible for vascularisation) interaction is not clear.26 As stated by Muthaian et al the interaction between BDNF and vascularisation factors opens up a window to explore the therapeutic potential of same in disease like Amyotrophic Lateral Sclerosis(ALS). Gupta et al have also shown the role of VEGF in expanding the life span of Indian ALS population.27–29 Moreover, several reviews in last decade have highlighted the studies supporting the role of BDNF in maturation of neural stem cells recruited by body to rescue the site of injury in neurodegenerative disorder.30–34
Implications of serum BDNF levels have been investigated in children with mental retardation and ASD, however, with equivocal findings. Moreover, it has been challenging, both to classify typical autism/ core syndrome (by DSM-IV), and associate a suitable biomarker with clinical phenotype spectrum. BDNF levels are found to be different in the present study on autistic children based on patterns of early onset, and severity of autism behavior. To the best of our knowledge, reports on such studies in Indian subjects are lacking. Role of BDNF as a putative biological marker in autism spectrum disorders (ASD) is gaining significance to evaluate the severity of cognitive and behavioral deficits. The above mentioned research makes BDNF an appropriate candidate for investigation in studies related to the development of the brain dysfunction and autism. The relatively higher level of BDNF in mild phenotypes compared to severe autism in our study (Fig. 1) probably reflected the protective function of this neurotrophic factor and characteristic of clinical heterogeneity.
Fig. 1:
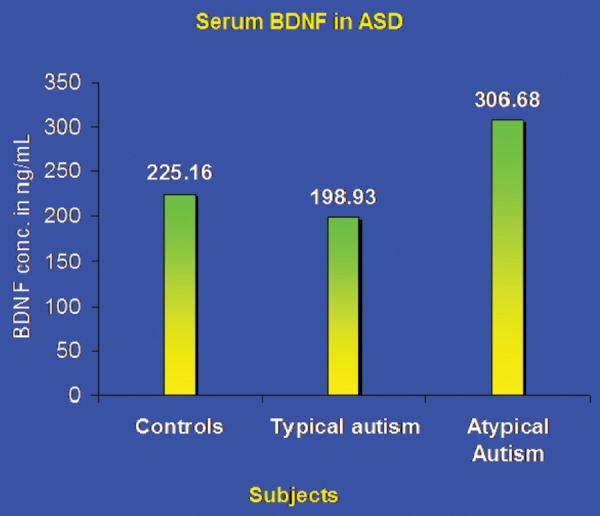
Comparison of BDNF levels between Controls and ASD. As the spectrum of ASD presentation traverses from mild to a severe form, the BDNF response inversely correlates with decreasing values.
With the new research studies on brain and with evidence of inflammation in the cerebellum, autism is more clearly a neurologic rather than a psychiatric or a behavioral disorder.
Multiple lines of evidence suggest that BDNF plays a critical role in serotonergic functions. In rat, brain BDNF has been found to promote the survival and sprouting of serotonergic axons and the axonal growth of injured serotonergic neurons.35,36 In-vitro and in-vivo studies support a regulatory role of BDNF in the survival and maturation of serotonergic neurons.37 BDNF has also been shown to modulate serotonergic neurotransmission in-vitro; in addition, BDNF administration has been found to increase the synthesis and/or turn over of serotonin in-vivo.38 It supports survival and differentiation of certain cholinergic neurons and also some dopaminergic neurons in vitro. BDNF and its receptor, tropomyosin-related kinase B (TrkB), are abundantly expressed in hypothalamic regions.39
Conclusion
Increased understanding of the genetic, molecular and cellular mechanism possibly provides insight into underlying brain damage in autism. Development of targeted and effective treatment strategies and prevention is the acute need in research on autism spectrum disorder. Association of BDNF has been implicated in the pathogenesis of neurodevelopmental disorders like autism. The levels of BDNF have been known to vary in Parkinson’s, Alzheimer’s and autism spectrum disorders. Our study is an attempt to generate data regarding the BDNF levels in ASD in Indian population. Various other studies have been carried out implicating BDNF in the pathology of ASD, however, the controversy still exists and the equivocal findings may be due to clinical phenotypes. The relatively higher level of BDNF in mild phenotypes compared to severe autism probably reflected the protective function of this neurotrophic factor. Our findings indicated the possibility of BDNF and its role as a diagnostic or characteriztion marker in Autism Spectrum Disorders, helping into further differential ASD sub-groups and hence very important for therapy management and rehabilitation in the affected children.
Acknowledgements
The authors are thankful to the parents for their cooperation and greatly acknowledge the help of R and D, SRL, Mumbai to permit the laboratory work.
Footnotes
The article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of Interests: None: Source of funding: None.
References
- 1.Nelson K. Toward a biology of autism: possible role of certain neuropeptides and neurotrophins. Clin Neuro Res. 2001;1(4):300–306. [Google Scholar]
- 2.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 3.Filipek PA, Accardo PJ, Ashwal S et al. Practice parameter: Screening and diagnosis of autism: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Prevalence of — Autism Spectrum Disorders-Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States. 2002 [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Prevalence of —Autism Spectrum Disorders-Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States. 2002;MMWR SS 2007;56(No.SS-01):29–40. [PubMed] [Google Scholar]
- 6.Folstein S, Rosen-Sheidely B. Genetics of autism: Complex aetiology for a heterogenous diosorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 7.Balasubramanian BV, Bhatt CA, Goyel N. Genetic studies in children with intellectual disability and autistic spectrum of disorders. Indian J Hum Genet. 2009;15(3):103–7. doi: 10.4103/0971-6866.60185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enstrom AM, Lit L, Onore CE et al. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23(1):124–33. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaratnasingam S, Janca A. Brain Derived Neurotrophic Factor: a novel neurotrophin involved in psychiatric and neurological disorders. Pharmacol Ther. 2012;134(1):116–24. doi: 10.1016/j.pharmthera.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Riccio A, Ahn S, Davenport CM et al. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999:286. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 11.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 12.Chao MV Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 13.Mowla SJ, Farhadi HF, Pareek S et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276(16):12660–6. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 14.Schanen NC. Epigenetics of autism spectrum disorders Human Molecular Genetics, 2006;Vol. 15(Review Issue No. 2) doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- 15.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Neurology. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 16.De Roy PG. Helsinki and the Declaration of Helsinki. World Med J. 2004;50/1:9–11. [Google Scholar]
- 17.Basel-Vanagaite L. Clinical approaches to genetic mental retardation. IMAJ. 2008;10:821–826. [PubMed] [Google Scholar]
- 18.Szatamari P, Paterson AD, Zwaigenbaum L et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos M, Temudo T, Carriho I et al. Mutations in the MeCP2 gene are not a major cause of Rett Syndrome-Like or related neurodevelopmental phenotype in male patients. J Child Neurol. 2009;24:49–55. doi: 10.1177/0883073808321043. [DOI] [PubMed] [Google Scholar]
- 20.Hagerman RJ, Chudley AE, Knoll JH et al. Autism in Fragile X females. Am J Med Genet. 1986;23(1–2):375–380. doi: 10.1002/ajmg.1320230129. [DOI] [PubMed] [Google Scholar]
- 21.Montegia L, Lukiart B, Barrot M et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression related behaviours. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki K, Narita N, Sakuta R et al. Serum neurotrophin concentrations in autism and mental retardation: A pilot study. Brain Devel. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 23.AL-Ayadhi L. Serum Levels of Brain-Derived Neurotrophic Factor (BDNF) in Autistic Children In Central Saudi Arabia. The Open Conference Proceedings Journal. 2011;2:36–40. [Google Scholar]
- 24.Chang Q, Khare G, Dani V et al. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49(3):341–8. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Phillips HS, Hains JM, Armanini M et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Brain Dev. 2004;26(5):292–295. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 26.Muthaian R, Minhas G, Anand A. Pathophysiology of stroke and stroke induced retinal ischemia: emerging role of stem cells. J. Cell. Physiol. 2012;227:1269–1279. doi: 10.1002/jcp.23048. [DOI] [PubMed] [Google Scholar]
- 27.Gupta PK, Prabhakar S, Abburi C et al. Vascular endothelial growth factor-A and CCL2 genes are upregulated in peripheral blood monocytes in Indian Amyotrophic lateral sclerosis patients. Journal of Neuroinflammation. 2011;8:114. doi: 10.1186/1742-2094-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta PK, Prabhakar S, Sharma S et al. VEGF-a and CCL2 in Indian ALS patients. Journal of Neuroinflammation. 2011;8:47. doi: 10.1186/1742-2094-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand A, Gupta PK, Sharma NK et al. sVEGR1 as a novel marker of ALS in north Indian ALS population. European Journal of Neurology. 2011;19(5):788–792. doi: 10.1111/j.1468-1331.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 30.English D, Sharma NK, Sharma K et al. Neural stem cells- trends and advances. Journal of Cellular Biochemistry. 2013;114:764–772. doi: 10.1002/jcb.24436. [DOI] [PubMed] [Google Scholar]
- 31.Minhas G, Morishita R, Anand A. Preclinical models to investigate retinal ischemia: advances and drawbacks. Frontiers in Neurology. 2012;11(3):75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jindal N, Mukhopadhyay A, Anand A. The emerging role of stem cells in ocular neurodegeneration: hype or hope? Mol Cell Biochem. 2012;365(1–2):65–76. doi: 10.1007/s11010-012-1244-8. [DOI] [PubMed] [Google Scholar]
- 33.Abburi C, Anand A. Ciliary Epithelium: An Underevaluated Target for Therapeutic Regeneration. Critical ReviewsTM in Eukaryotic Gene Expression. 2012;22(2):87–95. doi: 10.1615/.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 34.Minhas G, Anand A. Animal Models of Retinal Ischemia. In-Amit Agrawal, editor. Available from:http://www.intechopen.com Brain Injury–Pathogenesis, Monitoring, Recovery and Management: Intech Press. 2012 [Google Scholar]
- 35.Grider M, Mamounas L, Le W et al. In situ expression of brain derived neurotrophic factor or neurotrophin-3 promotes sprouting of cortical serotonergic axons following a neurotoxic lesion. J. Neurosci Res. 2005;82:404–412. doi: 10.1002/jnr.20635. [DOI] [PubMed] [Google Scholar]
- 36.Mamounas LA, Altar CA, Blue ME et al. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20(2):771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djalili S, Holthe M, Grosse G et al. Effects of Brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during developmen, J Neurochem. 2005;83:1525–1528. doi: 10.1111/j.1471-4159.2004.02911.x. [DOI] [PubMed] [Google Scholar]
- 38.Goggi J, Pullar I, Carney S et al. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro, Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- 39.Tapia-Arancibia L, Rage F, Givalois L et al. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]


