Abstract
Background:
Chronic plaque psoriasis is a common papulosquamous skin disorder, for which a number of topical agents are being used including coal tar, topical steroids and more recently topical calcipotriol/betamethasone dipropionate. There is no study comparing purified coal tar preparation with calcipotriol/betamethasone dipropionate ointment in limited chronic plaque psoriasis.
Aims and Objectives:
A prospective randomized open label controlled trial to compare the efficacy and safety of topical application of coal tar-salicylic acid ointment with calcipotriol/betamethasone dipropionate ointment applied once at night for 12 weeks for the treatment of limited chronic plaque psoriasis.
Materials and Methods:
A total of 62 patients of limited chronic plaque psoriasis (body surface area <10%) were randomized into two treatment groups: Group A received topical application of 6% coal tar with 3% salicylic acid ointment and Group B received calcipotriol/betamethasone dipropionate, once at night for 12 weeks. Results were assessed based on psoriasis area severity index (PASI) scores and patient global assessment (PGA) at each visit.
Results:
Mean PASI was significantly lower at week 2 (P = 0.01) and week 4 follow-up (P = 0.05) and the mean reduction in PASI was significantly higher at week 2 (P = 0.02) with calcipotriol/betamethasone than coal tar-salicylic acid, but this difference was not sustained at subsequent follow-up visits. Similarly, PGA scores at weeks 2 and 4 were significantly lower with calcipotriol/betamethasone dipropionate ointment (P = 0.003 and P = 0.007 respectively). There was no significant difference in any parameter during subsequent follow-up visits or at the end of the treatment phase (12 weeks).
Conclusion:
Topical nightly application of calcipotriol/betamethasone dipropionate ointment leads to an initial, more rapid reduction in disease severity, but the overall outcome parameters are comparable in the two treatment groups.
Keywords: Calcipotriol/betamethasone, coal tar, psoriasis
Introduction
What was known?
Both coal tar-salicylic and calcipotriol/betamethasone ointment are topical agents for the treatment of limited chronic plaque psoriasis. Coal tar-salicylic acid is a conventional and inexpensive agent while calcipotriol/betamethasone is a new and relatively expensive topical modality.
Psoriasis is a common papulosquamous skin disorder affecting 1-2% of the world's population. In the majority of cases, the disease is mild, affecting limited body surface. Several topical modalities have been found effective in limited chronic plaque psoriasis including, topical steroids, coal tar, anthralin, tazarotene and the vitamin D analogue calcipotriol (calcipotriene) alone or in combination with betamethasone dipropionate. Coal tar is one of the oldest topical preparations for psoriasis. Purified coal tar has been found to have high efficacy and good safety profile. In a study by Kumar et al., 5% crude coal tar ointment was shown to induce 75% reduction in erythema severity index (ESI) in 59% of patients at 12 weeks.[1] Calcipotriol/betamethasone dipropionate is a relatively new topical agent and there are numerous studies to document its efficacy in psoriasis.[2] It has been shown to produce significantly greater reduction in psoriasis area severity index (PASI) of 68.6% at week 4 compared with placebo (26.6%).[2] It has also been found to be superior to formulations containing calcipotriol alone or tacalcitol.[2,3]
To the best of our knowledge there is no study comparing the efficacy and safety of conventional coal tar application with calcipotriol/betamethasone dipropionate ointment in limited chronic plaque psoriasis.
Materials and Methods
A prospective open label randomized trial was conducted in patients of limited chronic plaque psoriasis (i.e. involving body surface area <10%), who were off all topical medications in last 15 days and oral medications in last 1 month. A written informed consent was taken from all patients. Patients were randomized into two groups using a computer-generated random number table from the website http://www.randomizer.org. Patients in Group A received topical application of 6% coal tar-3% salicylic acid ointment (Salytar-WS® Shalaks/Invida Pharmaceuticals Pvt. Ltd) every night and Group B received calcipotriol/betamethasone dipropionate ointment (Daivobet ointment®, Win Medicare) every night for 12 weeks. No sun-exposure was advised in both groups. Use of emollients was allowed during the day in both groups. The endpoint was 12 weeks of treatment or attainment of PASI score 0, whichever was earlier. Patients were withdrawn from the study if there was <25% reduction in PASI at week 8, deterioration or serious side-effects warranting withdrawal.
Patients were followed-up at weeks 2, 4, 8 and 12 and assessed for disease severity by PASI score, patient's perception of disease severity (patient global assessment [PGA]) on a scale of 0-10 with “10” being baseline disease and “0” being no disease and adverse effects. The following definitions were used for assessing treatment outcome
Complete clearance-attainment of PASI score 0 at any point of time during the treatment period.
Responders-attainment of PASI ≥ 50 at the end point of treatment (‘complete clearance’ cases + cases achieving 50 ≤ PASI < 100).
Poor responders-attainment of PASI < 50 at the end point of treatment.
Deterioration-increase in PASI from baseline any time during 12 weeks of treatment.
Relapse-appearance of disease in the treatment phase after attaining PASI score 0 and stopping treatment.
Loss to follow-up: Any patient who did not return for the follow-up visits after initiation of treatment.
The primary efficacy parameter was a comparison of reduction in PASI from baseline to endpoint of treatment or week 12. Secondary efficacy parameters were mean reduction in PASI at each follow-up visit, proportion of patients achieving PASI 75 and PASI 50 at week 12 or end point of treatment whichever, earlier and PGA score. Other treatment outcomes such as complete clearance, responders, poor responders, deterioration and relapse were also compared between the two groups.
Statistical analysis
Two-sample Wilcoxon rank-sum (Mann-Whitney) test was used to assess statistical significance of difference in PASI, PGA, mean reduction and mean percentage reduction in PASI at each visit between two groups. Chi-square test was used to assess statistical significance of achievement of PASI 75 and PASI 50 in two groups. A P < 0.05 was considered significant.
Results
A total of 62 patients were randomized into two groups: 33 patients in Group A (coal tar-salicylic acid group) and 29 patients in Group B (calcipotriol/betamethasone dipropionate group). Of these 13 patients in Group A and 8 in Group B were lost to follow-up. Hence 20 cases in Group A and 21 in Group B completed the study period [Figure 1]. The two groups were comparable with respect to demographic and clinical profile. Mean PASI score at baseline in Group A (mean psoriasis area and severity index [mPASI] =5.2) was comparable with Group B (mPASI = 4.4).
Figure 1.
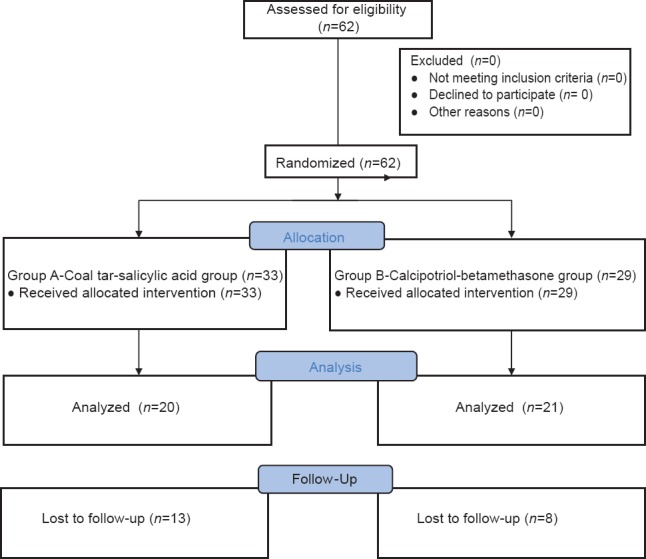
Enrolment of cases in the study
After treatment, mean PASI at week 2 was significantly lower in Group B (2.7) compared with Group A (4.4) and the results were statistically significant (P = 0.01; z = 2.55). Mean PASI at week 4 was also significantly lower in Group B (2.3) compared with Group A (3.5) (P = 0.05; z = 1.96), but there was no significant difference in mean PASI at week 8 (mPASI A = 2.9 and mPASI B = 2.6; P = 0.39; z = 86) and week 12 (mPASI A = 2 and mPASI B = 2.59; P=0.17; z = −1.36) [Figure 2]. Mean percentage reduction in PASI was significantly higher in Group B compared with Group A at week 2 (15.4% in Group A vs. 38.6% in Group B) (P = 0.02; z = −2.36), but not at subsequent follow-up visits. Mean percentage reduction in PASI at week 12 was 61.5% in Group A versus 40.7% in Group B (P = 0.18; z = 1.34). Hence at treatment completion, there was no statistically significant difference in PASI score or PASI reduction between the two groups.
Figure 2.
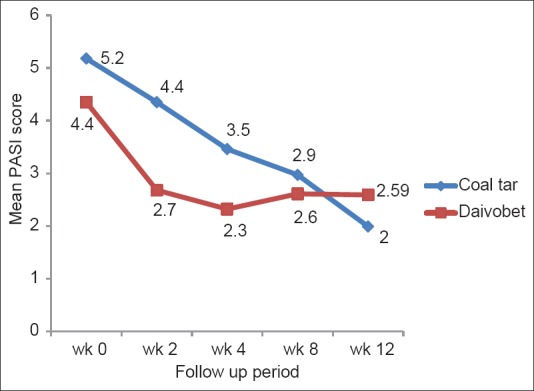
Mean psoriasis area severity index score in two treatment groups at each follow-up visit
PASI 50 was attained by 8 of 20 cases (40%) patients in Group A compared to 9 of 21 (42.8%) patients in Group B (P = 0.36; χ2 = 1.48; d.f. = 1) at the end of treatment. PASI ≥ 75 was achieved by 2 of 20 (10%) patients in Group A compared to 6 out of 21 (28.5%) patients in Group B (P = 0.92; χ2 = 0.33; d.f. = 1) [Table 1].
Table 1.
Comparison of treatment outcomes in the two groups
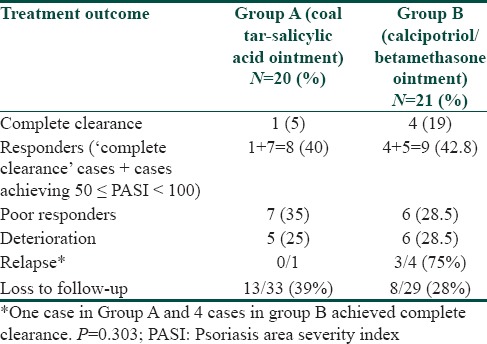
The difference in disease outcome at the end of treatment between the two groups was not statistically significant (P = 0.303; χ2 = 5.07; d.f. = 4) [Table 1]. One case (5%) in coal tar group and 4 (19%) in calcipotriol/betamethasone group had complete clearance of the disease during the treatment phase. Of the four cases in Group B who achieved complete clearance, 3 (75%) cases relapsed on stopping treatment. The only case who cleared completely in Group A, did not relapse during 12 weeks of the study. PGA scores were significantly lower in Group B compared to Group A at week 2 (6.75 and 8.24 respectively) and week 4 (5.41 and 7.92 respectively); (P = 0.003; z = 2.95 and P = 0.007; z = 2.70 respectively). In subsequent follow-up visits, PGA scores were comparable and not statistically significant in the two groups [Figure 3].
Figure 3.
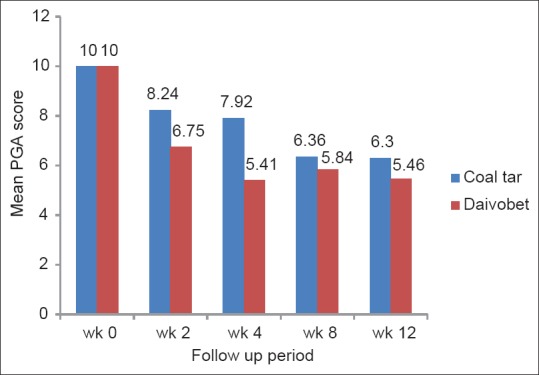
Mean patient global assessment scores in two treatment groups at each follow-up visit
No side-effects were observed in the 2 groups. Unpleasant odor and staining of clothes was not reported by any patient in both groups.
Discussion
Topical therapies are the mainstay of treatment for limited chronic plaque psoriasis.[4,5] These include keratolytics, coal tar, corticosteroids, anthralin, topical PUVA, calcipotriol alone and in combination with topical steroids (betamethasone dipropionate or mometasone) and tazarotene.[6,7,8,9,10,11] The antipsoriatic efficacy of coal tar and calcipotriol/betamethasone has been individually reported in a number of studies. Coal tar is one of the most commonly used, cheap, safe and effective treatment for psoriasis and its utility, either alone or in combination with phototherapy has been proved in many studies.[1,6,7] Salicylic acid present in commercially available coal tar ointments reduces the thickness and scaling of psoriatic plaques, thereby enhancing the penetration of the active medication. In the study by Kumar et al., all patients reported at least 50% reduction and 59.3% had greater than 75% reduction in ESI score with 5% crude coal tar.[1] Its clinical efficacy was found comparable with 0.1% tazarotene gel.[1] In our study, PASI 50 was achieved in 40% cases and PASI 75 in 10% cases after 12 weeks of treatment with coal tar-salicylic acid ointment.
Calcipotriol, a vitamin D analogue, has proven to be highly efficacious in limited chronic plaque psoriasis. There are few trials comparing its efficacy with other topical agents such as steroids and coal tar, but have shown conflicting results. Sharma et al. reported >50% reduction in ESI score at week 4 in 60% of lesions treated with calcipotriol compared to 23.3% of lesions treated with coal tar (P < 0.01); however, there was no significant difference observed for the same parameter at week 12.[12] Pinheiro et al. reported greater reduction in disease severity with twice daily calcipotriol application group compared to coal tar-allantoin-hydrocortisone group at week 8.[13] Tham et al. found faster reduction in PASI score with calcipotriol twice a day compared to coal tar, 15% w/v cream applied once a day and also found the difference to be significant at all follow-up visits (weeks 2, 4 and 6). They reported that with calcipotriol, rapid improvement was seen in 1st 2 weeks of treatment while significant response was seen with coal tar only after 4 weeks of treatment.[14] Alora-Palli et al. on comparing liquor carbonis detergens (LCD) 15% coal tar solution with calcipotriene 0.005% cream twice daily for 12 weeks, reported significantly greater mean reductions in PASI scores at week 12 with LCD (58% vs. 37%).[15] In addition, significantly higher number of patients in LCD group had absent or minimal psoriasis on the PGA scale than the calcipotriene group and improvement was better maintained on 6 weeks follow-up in the same group.
The two-compound formulation of calcipotriol with betamethasone has been found to be superior to either component used alone in a large trial on 1605 patients, in which end point of absent-to-mild disease was achieved in 48% patients treated with a calcipotriene-betamethasone combination compared to only 16.5% patients treated with calcipotriene alone and 26.3% patients treated with betamethasone alone.[11] Another study found the efficacy of calcipotriol/betamethasone formulation to be better than calcipotriol alone at 2 and 4 weeks follow-up and showed a greater reduction in mean PASI in the combined formulation group (68.6% in once daily, 73.8% in twice daily group) than in the twice daily calcipotriol alone group (58.8%) and the vehicle group (26.6%).[2] PubMed search did not yield any literature on studies comparing coal tar with a calcipotriol-betamethasone combination our study showed that though a calcipotriol-betamethasone combination resulted in an initial more rapid reduction in PASI (weeks 2 and 4), but this difference was not sustained at later follow-up visits. At the end of the treatment phase, greater mean reduction in PASI was achieved with coal tar-salicylic acid combination. Greater though not significant number of cases achieved complete clearance, PASI 50 and 75 with a calcipotriol-betamethasone combination. The other outcome parameters were also comparable at the end of the treatment phase. No side-effect was observed with both medications. There was no staining of clothes or irritation with coal tar possibly because a refined commercially available tar formulation was used instead of crude coal tar.
Conclusion
Calcipotriol/betamethasone two compound formulation leads to significantly faster clinical improvement and patient satisfaction than coal tar ointment applied once daily, but this effect is not sustained and the results are comparable after 12 weeks of treatment. A limitation of our study was high drop-out rate of cases in both groups. Hence further studies with larger sample size are required to compare calcipotriol/betamethasone ointment with conventional topical agents.
What is new?
There are few comparative studies on topical therapies for management of limited chronic plaque psoriasis and none between the conventional coal tar-salicylic acid and. calcipotriol/betamethasone ointments. This study compares the efficacy of these two agents in the management of limited chronic plaque psoriasis and finds them to have similar efficacy at the end of the treatment phase, but an initial more rapid decline in PASI score with calcipotriol/betamethasone compared with coal tar.
Acknowledgment
We would like to thank Mrs. M. Kalaivani, Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, for helping with the statistical analysis of the paper.
Footnotes
Source of Support: Calcipotriol/betamethasone ointment (Daivobet) was provided by Win Medicare Pvt. Ltd. However, they had no interference in the design, conduct and analysis of the study
Conflict of Interest: Calcipotriol/betamethasone ointment (Daivobet) was provided by Win Medicare Pvt. Ltd. However, they had no interference in the design, conduct and analysis of the study.
References
- 1.Kumar U, Kaur I, Dogra S, De D, Kumar B. Topical tazarotene vs. coal tar in stable plaque psoriasis. Clin Exp Dermatol. 2010;35:482–6. doi: 10.1111/j.1365-2230.2009.03610.x. [DOI] [PubMed] [Google Scholar]
- 2.Guenther L, Van de Kerkhof PC, Snellman E, Kragballe K, Chu AC, Tegner E, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: A randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147:316–23. doi: 10.1046/j.1365-2133.2002.04967.x. [DOI] [PubMed] [Google Scholar]
- 3.Langley RG, Gupta A, Papp K, Wexler D, Østerdal ML, Curčić D. Calcipotriol plus betamethasone dipropionate gel compared with tacalcitol ointment and the gel vehicle alone in patients with psoriasis vulgaris: A randomized, controlled clinical trial. Dermatology. 2011;222:148–56. doi: 10.1159/000323408. [DOI] [PubMed] [Google Scholar]
- 4.Zeichner JA, Lebwohl MG, Menter A, Bagel J, Del Rosso JQ, Elewski BE, et al. Optimizing topical therapies for treating psoriasis: A consensus conference. Cutis. 2010;86:5–31. [PubMed] [Google Scholar]
- 5.Albrecht L, Bourcier M, Ashkenas J, Papp K, Shear N, Toole J, et al. Topical psoriasis therapy in the age of biologics: Evidence-based treatment recommendations. J Cutan Med Surg. 2011;15:309–21. doi: 10.2310/7750.2011.10080. [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl M. The role of salicylic acid in the treatment of psoriasis. Int J Dermatol. 1999;38:16–24. doi: 10.1046/j.1365-4362.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- 7.Paghdal KV, Schwartz RA. Topical tar: Back to the future. J Am Acad Dermatol. 2009;61:294–302. doi: 10.1016/j.jaad.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Koo J, Behnam SE, Behnam SM. The efficacy of topical tazarotene monotherapy and combination therapies in psoriasis. Expert Opin Pharmacother. 2003;4:2347–54. doi: 10.1517/14656566.4.12.2347. [DOI] [PubMed] [Google Scholar]
- 9.Segaert S, Duvold LB. Calcipotriol cream: A review of its use in the management of psoriasis. J Dermatolog Treat. 2006;17:327–37. doi: 10.1080/09546630600999219. [DOI] [PubMed] [Google Scholar]
- 10.Scott LJ, Dunn CJ, Goa KL. Calcipotriol ointment. A review of its use in the management of psoriasis. Am J Clin Dermatol. 2001;2:95–120. doi: 10.2165/00128071-200102020-00008. [DOI] [PubMed] [Google Scholar]
- 11.McCormack PL. Spotlight on calcipotriene/betamethasone dipropionate in psoriasis vulgaris of the trunk, limbs, and scalp. Am J Clin Dermatol. 2011;12:421–4. doi: 10.2165/11207670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: A prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42:834–8. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro N. Comparative effects of calcipotriol ointment (50 micrograms/g) and 5% coal tar/2% allantoin/0.5% hydrocortisone cream in treating plaque psoriasis. Br J Clin Pract. 1997;51:16–9. [PubMed] [Google Scholar]
- 14.Tham SN, Lun KC, Cheong WK. A comparative study of calcipotriol ointment and tar in chronic plaque psoriasis. Br J Dermatol. 1994;131:673–7. doi: 10.1111/j.1365-2133.1994.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 15.Alora-Palli MB, Perkins AC, Van Cott A, Kimball AB. Efficacy and tolerability of a cosmetically acceptable coal tar solution in the treatment of moderate plaque psoriasis: A controlled comparison with calcipotriene (calcipotriol) cream. Am J Clin Dermatol. 2010;11:275–83. doi: 10.2165/11530380-000000000-00000. [DOI] [PubMed] [Google Scholar]


