Abstract
Kinase inhibitors have revolutionized cancer therapy by becoming the first-line agents for advanced solid malignancies replacing the traditional chemotherapeutic agents. Cutaneous side-effects with these drugs are common, but owing to their infrequent use in Indian patients, our current knowledge of toxicity is scanty and primarily based on the western literature. Cutaneous reactions can adversely affect patients’ quality of life (QoL) and can lead to dose modifications and treatment interruptions. The report discusses concurrent hand-foot skin reaction (HFSR) and hair depigmentation in an Indian patient being treated with sunitinib for advanced renal cell carcinoma. The pathogenesis and treatment strategies for this characteristic phenomenon and other cutaneous toxicities of kinase inhibitors have also been reviewed.
Keywords: Cutaneous toxicity, kinase inhibitors, sunitinib
Introduction
What was known?
Cutaneous toxicities with kinase inhibitors are common, but our knowledge regarding these is scanty and primarily based on western literature.
Sunitinib belongs to a group of novel multitargeted kinase inhibitors (MKIs) affecting tumor cell angiogenesis and proliferation.[1,2] The drug has been recently developed and approved for treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma.[2] We report a case of concurrent hand-foot skin reaction (HFSR) and hair depigmentation in an Indian patient being treated with sunitinib. The pathogenesis of this characteristic phenomenon and other cutaneous side-effects with sunitinib have also been discussed.
Case Report
A 50-year-old male patient was referred to the dermatology department of our tertiary care hospital with complaints of painful reddish, yellow skin lesions over palms and soles and depigmentation of body hair. The patient was following-up in the radiotherapy department for advanced renal cell carcinoma and undergoing chemotherapy with sunitinib in a dose of 50 mg daily for the past 2 months. Six weeks following chemotherapy, the patient developed tingling and burning sensation over his hands and feet followed by appearance of painful reddish lesions interfering with daily activities of patient. A few days later, he also noticed gradual depigmentation of his body hair which started from the beard, followed by the chest, and extremities.
Cutaneous examination revealed multiple erythematous to yellow colored plaques surmounted with scales present over bilateral palms and soles [Figures 1 and 2]. The lesions were located primarily over the pressure points, flexural aspect of the digits, and finger webs. They were tender to touch. Variable degree of hair depigmentation was also appreciated involving the beard area, the chest, and the extremities [Figures 3 and 4]. Rest of the cutaneous examination including the nails and the mucosae was unremarkable.
Figure 1.
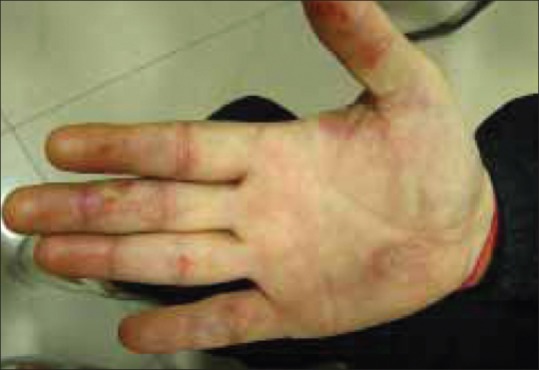
Erythematous to yellow coloured scaly plaques present on the hypothenar eminence of palm, digital creases, and finger webs
Figure 2.
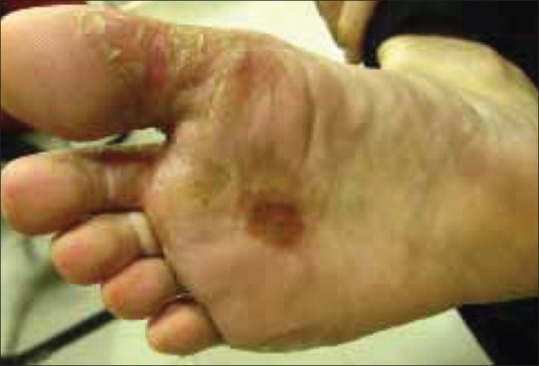
Erythematous plaques surmounted by scales present on the ventral aspect of the toes and adjoining parts of the sole
Figure 3.
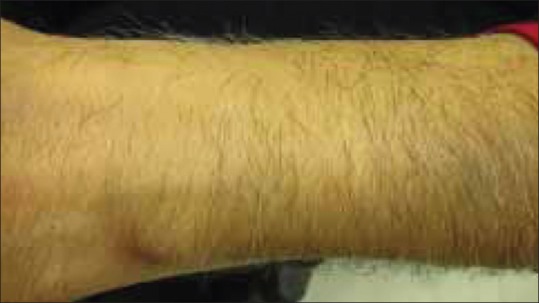
Depigmented hair on the forearm along with normal pigmented hair
Figure 4.
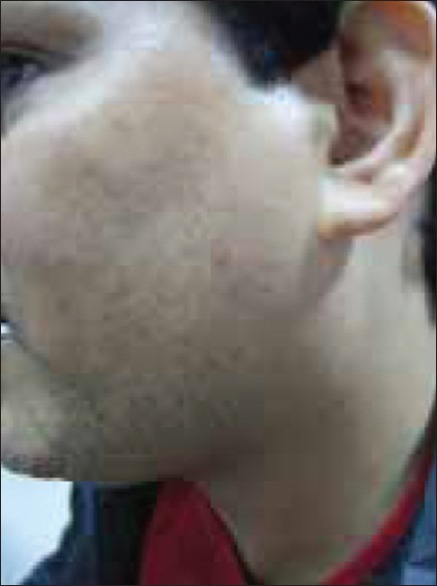
Beard hair showing varying degrees of depigmentation along with normally pigmented hair
Based on the history and clinical examination, a diagnosis of HFSR with hair depigmentation secondary to sunitinib was made. The patient was started on clobetasol 0.05% ointment to be applied twice daily along with moisturizers. He was also advised to avoid hot water and take analgesics as and when required. The patient had to be shifted on a 2 weeks on-1 week off regimen for sunitinib, contrary to the earlier 4 weeks on-2 weeks off regimen. For hair depigmentation, the patient was counseled that the change was reversible and repigmentation would occur once sunitinib is stopped.
Discussion
During the last decade, several novel agents targeting important tumor growth and maintenance pathways have been developed for anticancer therapy [Table 1].[3] The agents offer promising therapeutic effects and are generally well-tolerated. However, like other chemotherapy agents, these new MKIs have a variety of cutaneous side effects that can affect the patient's quality of life (QoL) and also lead to treatment disruptions.[1,2] Sorafenib and sunitinib are two novel MKIs that are increasingly being used for solid malignancies like advanced renal cell carcinoma, gastrointestinal stromal tumor, and hepatocellular carcinoma.[1]
Table 1.
Kinase inhibitors approved for various malignancies
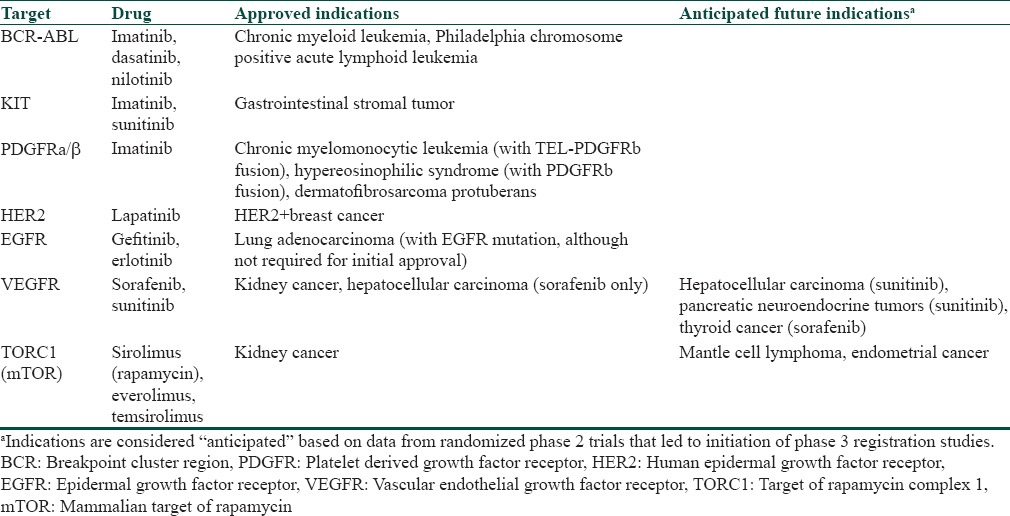
A number of cutaneous reactions have been reported with both sunitinib and sorafenib [Table 2].[4]
Table 2.
An overview of dermatological side effects of kinase inhibitors and blocking antibodies available in
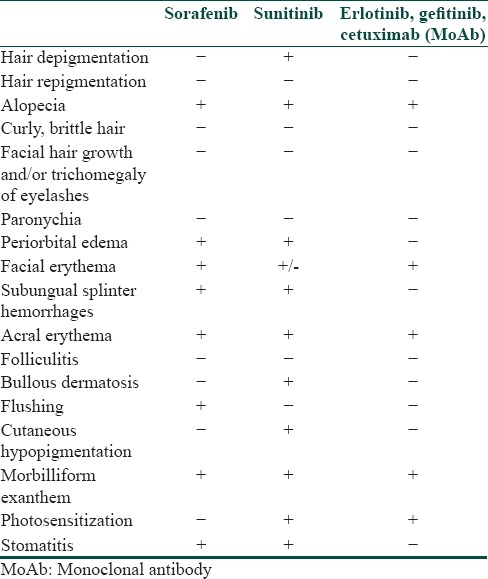
HFSR is one of the commonest cutaneous toxicities observed with sunitinib.[1,2,5] HFSR appears in the first 2-4 weeks of treatment as hyperkeratotic lesions with superficial blistering typically surrounded by a peripheral halo of erythema.[5] The lesions usually affect the flexural surfaces of the digits and the pressure areas of palms and soles.[6,7] The lesions need to be differentiated from the classical chemotherapy-induced hand-foot syndrome (HFS) which is characterized by acute erythema, swelling, and desquamation.[8] The exact mechanism of pathogenesis of HFSR is not known. Inhibition of platelet derived growth factor receptor (PDGFR) and c-KIT receptors on human keratinocytes by MKIs has been postulated to cause HFSR.[1,9] It has been hypothesized that MKIs are excreted in the sweat glands resulting in direct skin toxicity, making palms, and soles most vulnerable for the reaction.
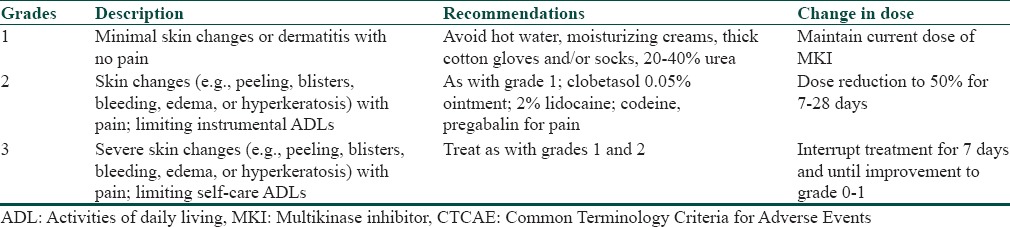
The goal of treatment is to balance the patient's QoL and continue administration of this life-prolonging therapy. The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE v 4.0) grades HFSR on the basis of severity [Table 3].[2,10] The grading system is helpful in guiding therapeutic decisions as the treatment protocol varies depending upon the severity of reaction [Table 3]. Our patient developed grade 2 changes and hence was started on topical steroids along with symptomatic treatment.
Table 3.
CTCAE version 4.0 grades for hand-foot and skin reaction with treatment recommendations for each
Varying degrees of yellowish-to-greyish discoloration of skin and hair has also been reported during sunitinib therapy.[11,12] Depigmentation occurs after 5-6 weeks of treatment and is reversible after 2-3 weeks off treatment.[13] Skin and hair discoloration has been thought to be caused by blockade of c-KIT signaling.[14] C-KIT influences pigmentation by regulating genes encoding the melanogenesis enzymes tyrosinase and tyrosinase-related protein 1. In a sunitinib dosing schedule of 4 weeks on-2 weeks off, patients have developed alternating bands of normal and depigmented hair.[11]
Kinase inhibitors have revolutionized cancer therapy by becoming the first-line agents for advanced solid tumors and have brought hope to patients not responding to more traditional therapies. Awareness of the unique cutaneous toxicities associated with these agents and their appropriate management is extremely important to ensure that these drugs are used to their maximum potential.
What is new?
The report discusses concurrent hand-foot skin reaction and hair depigmentation in an Indian patient on sunitinib and reviews the cutaneous side-effects of kinase inhibitors.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161:1045–51. doi: 10.1111/j.1365-2133.2009.09290.x. [DOI] [PubMed] [Google Scholar]
- 2.McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Ther. 2011;24:396–400. doi: 10.1111/j.1529-8019.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 3.Sawyers CL. Targeted therapy with small molecule kinase inhibitors. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott; 2011. pp. 190–205. [Google Scholar]
- 4.Revuz J, Valeyrie-Allanore L. Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. Edinburgh: Mosby; 2003. pp. 333–53. [Google Scholar]
- 5.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–11. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 6.Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–61. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 7.Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, et al. Hand-foot skin reaction in patients treated with sorafenib: A clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–6. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
- 8.Gordon KB, Tajuddin A, Guitart J, Kuzel TM, Eramo LR, VonRoenn J. Hand-foot syndrome associated with liposome-encapsulated doxorubin therapy. Cancer. 1995;75:2169–73. doi: 10.1002/1097-0142(19950415)75:8<2169::aid-cncr2820750822>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Morita E, Lee DG, Sugiyama M, Yamamoto S. Expression of c-kit ligand in human keratinocytes. Arch Dermatol Res. 1994;286:273–7. doi: 10.1007/BF00387600. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Common terminology criteria for adverse events v4.0 (CTCAE) [Last accessed on 2011 Jan 25]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf .
- 11.Rosenbaum SE, Wu S, Newman MA, West DP, Kuzel T, Lacouture ME. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support Care Cancer. 2008;16:557–66. doi: 10.1007/s00520-008-0409-1. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann JT, Kanz L. Sunitinib and periodic hair depigmentation due to temporary c-KIT inhibition. Arch Dermatol. 2008;144:1525–6. doi: 10.1001/archderm.144.11.1525. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 14.Moss KG, Toner GC, Cherrington JM, Mendel DB, Laird AD. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther. 2003;307:476–80. doi: 10.1124/jpet.103.052530. [DOI] [PubMed] [Google Scholar]


