Abstract
Introduction:
Alopecia areata (AA) is a common type of hair loss with an autoimmune basis. As the role of homocysteine (Hcys), folate, and CRP has been considered in some autoimmune diseases.
Objectives:
To evaluate homocysteine, folate and CRP level in AA.
Methods:
This study was performed on 29 patients who had AA for at least 6 months affecting more than 20% of scalp, and 32 healthy controls. Levels of serum Hcys, blood high-sensitivity CRP, and RBC folate were measured in all subjects.
Results:
The mean level of RBC folate was significantly lower in the patient group than that in controls (P < 0.001). Also, the level of RBC folate was significantly lower in patients with extensive forms of disease (alopecia totalis/alopecia universalis) in comparison with more localized form (patchy hair loss) (P < 0.05). Patients with higher “Severity of Alopecia Total” (SALT) score had lower RBC folate, as well. Serum Hcys and blood high-sensitivity CRP levels did not show a significant difference in two groups.
Conclusion:
Patients with alopecia areata have lower level of RBC folate which is in negative correlation with both severity and extension of AA.
Keywords: Alopecia areata, C-reactive protein, folate, homocysteine
Introduction
What was known?
Alopecia areata is a common type of hair loss with an autoimmune pathogenesis. Role of homo-cystein, C-reactive protein and folic acid has been proved in some autoimmune disorders.
Alopecia areata (AA) is a common type of hair loss, with 1.7% lifetime risk.[1] Its exact pathogenesis is still unknown, but recent studies emphasize on immunologic basis of the disease.[2] This fact, in addition to the association of AA with some autoimmune conditions, convinces authors toclassify it as an autoimmune disease.[1,3]
The role of homocystein (Hcys) and C-reactive protein (CRP) in pathogenesis of some conditions such as cardiovascular, thyroid, and autoimmune diseases has been recently considered.[4,5] Several studies have proved importance of Hcys in some autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.[6,7] Molecular investigations showed that Hcys causes some alterations in proteins that result in production of new autoantigens, and autoimmunity consequently.[8] Furthermore, increased levels of some cytokines involving in autoimmune pathogenesis have been observed in association with hyperhomocysteinemia.[9] On the other hand, Hcys level is in close association with level of folic acid.[10] Thus, we decided to evaluate serum Hcys, high-sensitivity CRP, and RBC folate levels in AA as an immunologic condition.
Materials and Methods
This study was carried out on 29 patients (12 males and 17 females) with alopecia areata (AA) who referred to our dermatology clinic from August 2011 to 2012 and 32 sex- and age-matched healthy persons. The inclusion criteria were having AA for at least 6 months, affecting more than 20% of the scalp. The diagnosis of AA was made clinically by an expert dermatologist. The controls were selected from patient's family members to exclude confounding effect of nutritional factor. All conditions which may alter serum Hcys, RBC folate, or blood high sensitive CRP level were considered as exclusion criteria including smoking, hypo- or very hyperactive life style, body mass index <20 or >27, recent history of significant weight loss, hypertension, chronic renal or liver disease, diabetes mellitus, metabolic syndrome, polycystic ovary disease, hypothyroidism, using any immunosuppressant during last 3 months, cancers, chronic infections, sleep disorders, and having any drug which may affect Hcys level (OCP, methotrexate, phenytoin, carbamazepine, metformin, theophylline, thiazide diuretics, nitric oxide, dopa, retinoids, statins, fibrate, niacin derivates). The study had been assessed and approved by the institutional ethics committee. All subjects received comprehensive information about the study and signed the informed consent. Patients’ essential information was recorded according to recommended investigative guidelines for AA.[11] This information included age, sex, age at onset of disease, duration of disease, pattern and severity of disease, concomitant nail pitting, and medical and drug history. Severity of AA was scored by an expert dermatologist according to the “Severity of Alopecia Total score” (SALT score). The SALT score ranges from 0 to 100, with 0 indicating no alopecia areata and 100 indicating severe disease. Scalp is divided into four areas: Vertex –40% of scalp surface area; right side and left side of scalp –36% of scalp surface area (18% and 18%) and Posterior aspect of scalp –24% of that.[12] The pattern of hair loss was divided into three groups as follows: Patchy hair loss, alopecia totalis (AT: Loss of whole scalp hair), and alopecia universalis (AU: Loss of whole body hair).
Ten milliliter of fasting peripheral venous blood was taken from patients. Venous blood samples were drawn into sterile tubes containing Na2EDTA, which were instantly put into ice. Serum was then recovered within 10 minutes by low-speed centrifugation (2000 g). All samples were coded and stored at –70°C. The samples used to evaluate serum Hcys, blood high-sensitivity CRP, and RBC folate levels. Also, levels of high-density lipoprotein and triglyceride were evaluated in all cases and controls in order to exclude of effect of these factors on the serum Hcys level.
The serum Hcys level was assayed by the HPLC method (homocysteine HPLC Kit, Immunodiagnostik AG Bensheim Company, Germany). Levels more than 15 μmol/L were considered abnormal. Coefficient of variation of intraassay and interassay was 2.5% and 5.2%, respectively. The detection limit was 0.6 μmol/L.
RBC folate was determined by the radioimmunoassay method. Low RBC folate is defined as values less than 150 ng/mL/cells for adults and less than 160 ng/mL/cells for children. High sensitive blood CRP level was quantified by the turbidimetery method.
Levels of serum Hcys, blood high-sensitivity CRP, and RBC folate were compared with those of controls. Also, different patterns and severities of AA were compared with each other.
Statistical analysis
For continuous variables, values were reported as median and interquartile range (25th-75th percentiles) and categorical data were expressed as the number of subjects (percentage). The normality assumption of the continuous variables was assessed by the Shapiro-Wilk test. To compare the continuous variables between the patient and control groups, the nonparametric the Mann-Whitney U-test was applied. Fisher's exact test was carried out wherever appropriate. A Spearman correlation test was used to detect the association between two variables.
Statistical analysis was performed using the statistical software SPSS 16.0.0. (SPSS Inc. Chicago, IL, USA). P values less than 0.05 were considered statistically significant and two-sided P values were reported for all comparisons.
Results
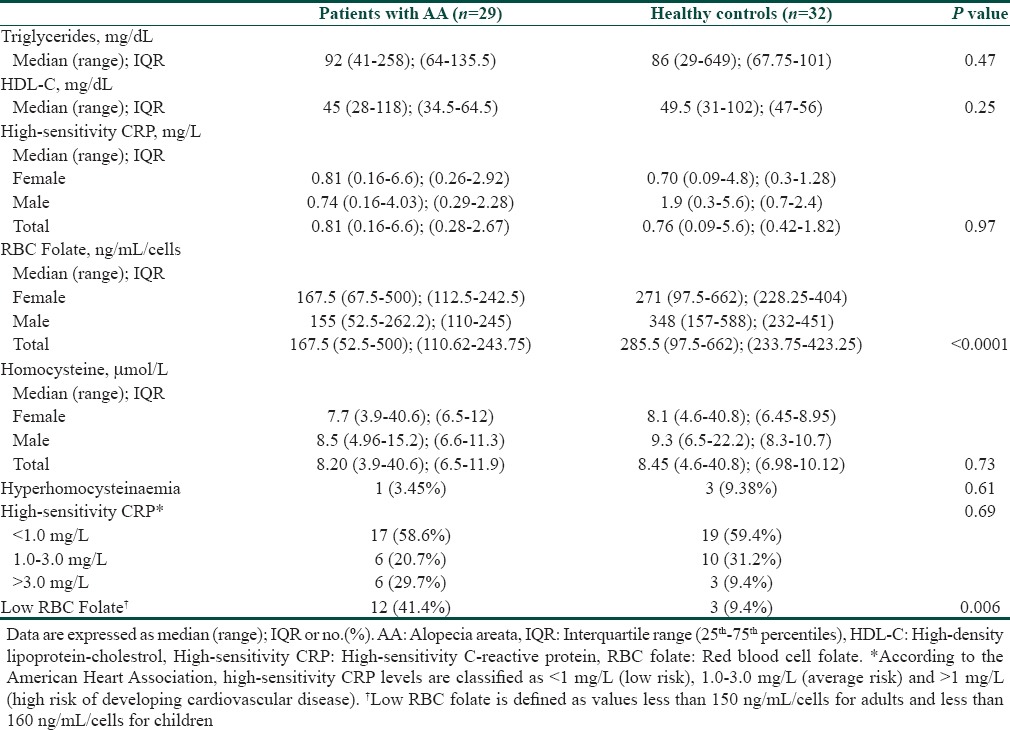
This study comprises 29 patients with alopecia areata (17 women and 12 men) and 32 healthy controls (21 women and 11 men). The mean age ofpatients with alopecia areata were 24.79 ± 11.93 and healthy controls were 26.06 ± 11.53. Other baseline demographics and clinical characteristics of patients and controls are presented in Table 1. There were no significant differences between these two groups due to demographic variables. Furthermore, there was no significant difference between serum TG and HDL levels in two groups [Table 2].
Table 1.
Baseline demographics and clinical characteristics of patients with alopecia areata and healthy controls

Table 2.
Laboratory results of patients with alopecia areata and healthy controls
Age at onset of disease was between 2 and 65.5 years old (median = 14 years old). Duration of disease varied 0.5-18 years (median = 6 years); most of our patients (62.5%) had AA for 5 years or more. Fifty-five percent of our cases had the most severe form of the disease (SALT score = 100) [Table 3]. The pattern of hair loss was patchy in 13 patients (45%), AT in another 13 patients (45%), and AU in 3 patients (10%). Nail pitting was recorded in 14 patients (48%). Five patients (17%) had history of atopic dermatitis and one patient (3.5%) had history of other autoimmune disease. Family history of AA was positive in 7 (24%) of cases.
Table 3.
SALT score in patients with alopecia areata

Patients with alopecia areata had significantly lower RBC folate concentrations compared to healthy controls (P < 0001, Table 2). Low RBC folate level was observed in 12 (41.4%) cases of AA and three (9.4%) subjects of the control group (P = 0.006, Fisher's exact test).
Our findings demonstrated significantly lower levels of RBC folate in alopecia totalis/alopecia universalis patients compared to the patients with patchy hair loss (median (IQR): 117.5 (102.5-170) vs. 202.5 (166.25-247.5), P = 0.047, Figure 1). There was a negative correlation between SALT the score and levels of RBC folate, as well (Spearsman rho = –0.41 and P = 0.03)
Figure 1.
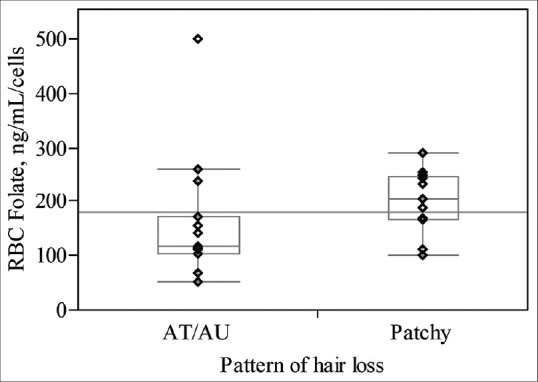
Pattern of hair loss
There was no statistical significant difference between the two groups according to the levels of high-sensitivity CRP and serum homocysteine (Mann-Whitney U-test, Table 3). Hyperhomocysteinemia was detected in one (3.4%) out of 29 cases of AA and 3 (9.4%) of 32 healthy controls with no statistical significant difference (P = 0.61, Fisher's exact test).
Also, we found no significant differences between AT/AU patients and cases with patchy hair loss in the serum levels of high-sensitivity CRP and homocysteine (Mann-Whitney U-test, P = 0.33, P = 0.29, respectively).
Approximately, half of the patients experienced nail pitting. The levels of homocysteine, RBC folate and high-sensitivity CRP did not differ significantly between the patients with and without nail involvement (P = 0.44, P = 0.33, and P = 0.18, respectively) Also, the duration of disease was not associated with these serum levels (P values at least 0.36).
Discussion
The aetiopathogenesis and mechanisms of AA are not fully understood, although many theories have been suggested including infectious, neural, genetic, and organ specific autoimmune hypotheses.[13] One of the probable mechanisms is oxidative stress characterized by an increase in free radical production exceeding the intracellular antioxidant defense. Akar et al. suggested that lipid peroxidation and antioxidant enzymes might be related to the pathogenesis of AA.[14] Homocysteine is a sulfur-containing amino acid derived from methionine and is metabolized by one of two pathways: Remethylation or transsulfuration. Hyperhomocysteinemia is observed in almost 5% of the general population which is developed by genetic enzymatic abnormalities or nutritional deficiencies that interfere with the appropriate metabolism of methionine or Hcy. The increase in extracellular Hcy is toxic to cells[15] and is associated with an increased risk of vascular dysfunction, neurodegenerative diseases, neuropsychiatric disorders, autoimmune diseases, neonatal abnormalities, diabetes, renal disease, osteoporosis, and cancer.[5,16] The oxidation of Hcy produces reactive oxygen species which causes oxidative stress.[17] In this study we found no significant difference between serumthe Hcys level in the study and control groups. Ertugrul et al. reported similar results in their study.[18]
In the present study, the RBC folate level was significantly lower in the alopecia areata group compared to control. In contrast to our result, other few studies which have evaluated the folate level in AA reported no significant difference between cases and controls.[10,13] However, in these studies the folate level has been measured in serum, not in RBC.[18] Serum folate may not be a true indicator of the folate status in the body as it can be impaired by exogenous factors such as drugs and diet. Folate concentrations in RBC are regarded as indicators of the long-term folate status and are not affected by exogenous factors. While the commonly determined folate concentrations in plasma or serum represent the circulating folate, their determination in RBC assesses the intracellular folate status and may reflect the folate status during the past 120 days.[19] In other words, the RBC folate level is a more accurate determinant of folate status in the body which has been evaluated in this study in patients with AA for the first time. Another difference of our study and these reports was that our study included more patients with extensive AA; 55% of our patients had AT/AU while only 4% of the patients had AU in the report of Gonul et al.[10] This difference may be due to performance of this study in an academic referral center to which more severe forms of diseases were referred. Furthermore, in our study RBC folate was significantly lower in extensive forms of disease (AT or AU) in comparison with limited form (patchy hair loss). On the other hand, patients with higher SALT score had significantly lower RBC folate, as well. Thus, the RBC folate level was in negative correlation with both severity and extension of AA.
CRP is a member of the pentraxin family of oligomeric proteins involved in innate immunity. Reported immunoregulatory functions of CRP include enhancement of leukocyte activity, complement fixation, modulation of platelet activation, and clearance of cellular debris from sites of active inflammation.[20] CRP is a sensitive marker of systemic inflammation and promotes secretion of inflammatory mediators.[21] In addition, CRP has been reported to have an important predictor value in cardiovascular diseases.[4] Thus, we decided to investigate the role of CRP in AA. In our study, serum levels of high-sensitivity CRP in patients with AA were not different from that in the control group. To our knowledge, our study is the first study evaluating this parameter in the patients with AA.
In conclusion, the present study showed significantly lower RBC folate levels in AA patients than in controls with no significant difference in the level of serum Hcys and high-sensitivity CRP. The current study demonstrated significant lower RBC folate in extensive AA. As the small number of patients was the limitation of our study, further studies with larger sample size are recommended to evaluate the exact role of these factors in AA.
What is new?
RBC folate is significantly lower in patients with alopecia areata. Also, level of RBC folate is lower in patients with more extensive and more severe forms of disease. Serum Hcys and blood high-sensitivity CRP levels are normal in alopecia areata.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–33. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 2.Alexis AF, Dudda-Subramanya R, Sinha AA. Alopecia areata: Autoimmune basis of hair loss. Eur J Dermatol. 2004;14:364–70. [PubMed] [Google Scholar]
- 3.Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflamm 2010. 2010:928030. doi: 10.1155/2010/928030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 5.Brustolin S, Giugliani R, Félix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43:1–7. doi: 10.1590/s0100-879x2009007500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroecksnadel K, Frick B, Kaser S, Wirleitner B, Ledochowski M, Mur E, et al. Moderate hyperhomocysteinaemia and immune activation in patients with rheumatoid arthritis. Clin Chim Acta. 2003;338:157–64. doi: 10.1016/j.cccn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Jas A, Undas A, Swadzba J, Musial J. Antibodies to N-homocysteinylated albumin in patients with systemic lupus erythematosus. Pol Arch Med Wewn. 2007;117:20–5. [PubMed] [Google Scholar]
- 8.Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, et al. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. 2007;6:503–9. doi: 10.1016/j.autrev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Schroecksnadel K, Frick B, Wirleitner B, Winkler C, Schennach H, Fuchs D. Moderate hyperhomocysteinaemia and immune activation. Cur Pharma Biotechnol. 2004;5:107–18. doi: 10.2174/1389201043489657. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Singh U, Pandey SS. Serum folic acid, vitamin B12 and homocysteine levels in Indian vitiligo patients. Egypt Dermatol Online J. 2012;8:1–7. [Google Scholar]
- 11.Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther. 2011;24:311–9. doi: 10.1111/j.1529-8019.2011.01415.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006;72:315–21. doi: 10.4103/0378-6323.26722. [DOI] [PubMed] [Google Scholar]
- 13.Gonul M, Cakmak SK, Soylu S, Kilic A, Gul U. Serum vitamin B12, folate, ferritin, and iron levels in Turkish patients with alopecia areata. Indian J Dermatol Venereol Leprol. 2009;75:552. doi: 10.4103/0378-6323.55430. [DOI] [PubMed] [Google Scholar]
- 14.Akar A, Arca E, Erbil H, Akay C, Sayal A, Gür AR. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85–90. doi: 10.1016/s0923-1811(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 15.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15:1927–43. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schalinske KL, Smazal AL. Homocysteine imbalance: A pathological metabolic marker. Adv Nutr. 2012;3:755–62. doi: 10.3945/an.112.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilland JC, Favier A, Potier de Courcy G, Galan P, Hercberg S. Hyperhomocysteinemia: An independent risk factor or a simple marker of vascular disease? 1. Basic data. Pathol Biol (Paris) 2003;51:101–10. doi: 10.1016/s0369-8114(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 18.Ertugrul DT, Karadag AS, Takci Z, Bilgili SG, Ozkol HU, Tutal E, et al. Serum holotranscobalamine, vitamin B12, folic acid and homocysteine levels in alopecia areata patients. Cutan Ocul Toxicol. 2013;32:1–3. doi: 10.3109/15569527.2012.683499. [DOI] [PubMed] [Google Scholar]
- 19.Herbert V. Development of human folate deficiency. In: Picano MF, Stockstad EL, Gregory JF, editors. Folic Acid Metabolism in Health and Disease. New York: Wiley-Liss; 1990. pp. 195–210. [Google Scholar]
- 20.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-Reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]


