Abstract
Malignant melanoma poses a remarkable capacity for morphological diversity and often presents as a diagnostic challenge due to its wide clinical presentation. We present a case of a 73-year-old lady, with a large superficial ulcerative nodular mass on the flexor aspect of the right upper arm. On fine needle aspiration poorly differentiated round cell tumor was suggested, with histopathology also supporting the same diagnosis. A final diagnosis of amelanotic melanoma was given following immunohistochemical work-up using a panel of relevant markers. We are presenting this case, not only for its rare clinical presentation, but also for the diagnostic difficulties encountered by us in cytology and histopathology to reach the final diagnosis.
Keywords: Amelanotic melanoma, histopathology, immnohistochemistry, superficial small round cell tumor
Introduction
What was known?
Malignant melanoma of the skin although common, can mimic a wide variety of lesions clinically.
Amelanotic melanoma is a variant of melanoma, which is diagnosed only after a complete work-up, which includes histopathology and IHC.
Melanomas, mainly of the skin are common tumors, but the incidence of amelanotic melanomas has been estimated to be between 1.8% and 8.1% of all melanomas.[1] Amelanotic malignant melanoma is a subtype of cutaneous malignant melanoma that has little or no pigment on visual inspection, as a result of which it can masquerade as a variety of other benign and malignant skin conditions.[2] Herein we present a similar case, in a 73-year-old lady, presenting as a soft-tissue mass with the final diagnosis being reached only after marker studies.
Case Report
A 73-year-old lady, farmer by occupation, presented to the surgical out-patient department with a large ulcerative nodular mass of 3 months duration, in the flexor aspect of the right hand, above the elbow joint, rapidly increasing in size, ulcerated since 2 weeks [Figure 1].
Figure 1.
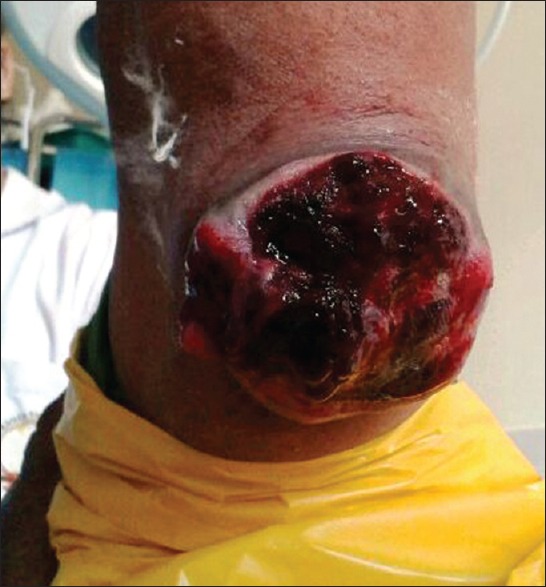
Ulcero-nodular growth on the postero – medial aspect of the right upper arm
Fine needle aspiration was carried out a diagnosis of poorly differentiated round cell tumor was made on cytology [Figure 2].
Figure 2.
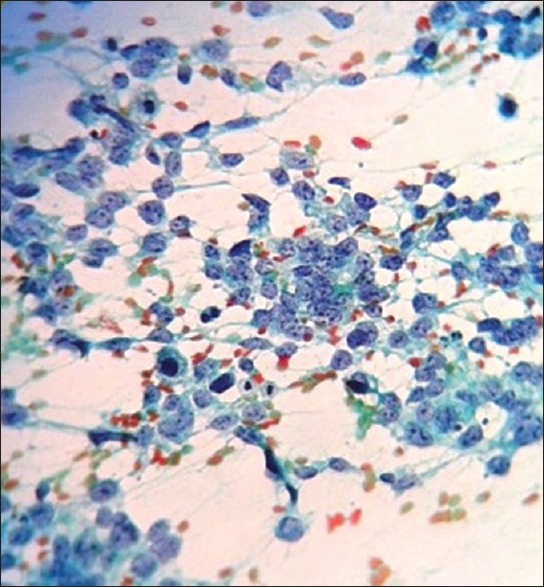
Pap stain of fine needle aspiration cytology – cellular smears with sheets of discohesive round cells
Magnetic resonance imaging showed a soft-tissue mass in the right arm, involving the skin and subcutaneous soft-tissue measuring 7 cm × 6 cm × 5 cm, ulnar nerve sandwiched between the mass and triceps, with no definite infiltration. Wide surgical excision of the mass was performed [Figure 3].
Figure 3.
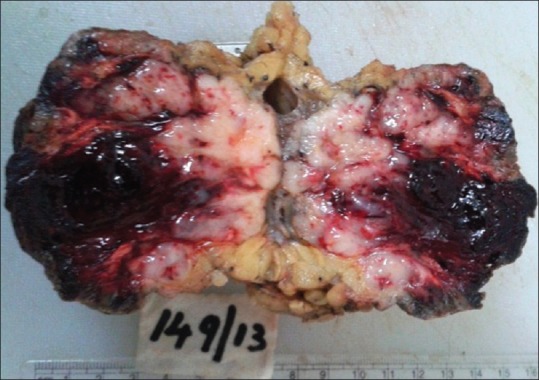
Cut section showing a grey white tumor measuring 7 cm × 5 cm × 4 cm, with hemorrhage
Histopathology showed a dermal neoplasm comprising of small round cells. Mitotic activity was high (2-3/hpf), with areas of necrosis seen. A diagnosis of poorly differentiated round cell tumor was made [Figure 4a–c] and the patient got discharged against medical advice.
Figure 4a.
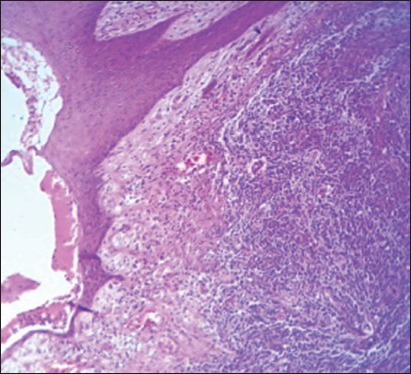
(H and E, ×10) showing the tumor with ulcerated overlying skin
Figure 4c.
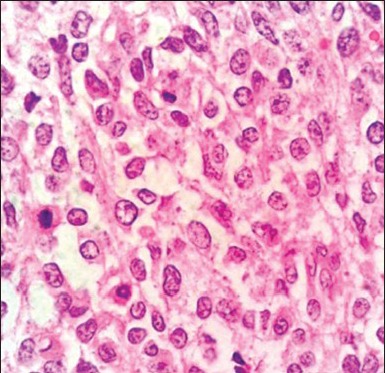
H and E stain showing individual round tumor cells with mitotic figure (arrow) and occasional prominent nucleoli
Figure 4b.
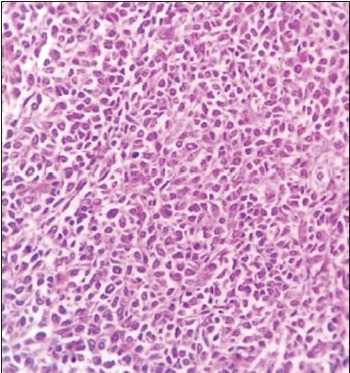
(H and E, ×10) showing the undifferentiated tumor cells in sheets without any definite arrangement
To further categorize the tumor immunohistochemistry (IHC) markers were used comprising Pan cytokeratin (CK), CD45, S-100, CD99, epithelial membrane antigen, CD34, B-cell lymphoma-2 (bcl-2), CK7, CK19, Human Melanoma Black-45 (HMB-45) and CD56. The tumor cells were positive focally for CD99, focally for S-100 and strongly positive for HMB-45.
Fonatana–Masson staining did not reveal any melanin pigment. A final diagnosis of amelanotic melanoma was made, with a Clark's grading of V and Breslow's thickness of 1.5 mm. The patient was contacted to come for further management repeatedly, but has not turned up until date.
Discussion
Malignant melanomas most commonly occur on skin (over 90%) and less commonly on mucosal surfaces (slightly over 1%). It accounts for approximately 4% of all skin cancers, but is responsible for 79% of all skin cancer related deaths.[3] Amelanotic melanoma usually occurs in sun-exposed skin of elderly with photo damage, simulating various skin lesions. It can also be an exophytic nodule, often eroded[2] as in our case. But the tumor size in our patient is one of the largest on presentation, as per PubMed/Medline search.
Cytologically, a high cell yield with a predominant population of dissociated cells, showing nuclear pleomorphism, prominent nucleoli and increased mitotic activity can be seen. However, diagnosis of the amelanotic variant on cytology is challenging because in the absence of pigment, the tumor cells mimic those of carcinoma or sarcoma, like in our case.[4]
Even on histopathology melanoma has attained diagnostic notoriety for its capacity for histomorphological diversity and ability to masquerade as a number of non-melanocytic neoplasms, especially true for the amelanotic variety.[5] Histopathological examination alone, as in our case can leave the pathologist with an extensive list of differential diagnoses as mentioned in Table 1. As each of the tumors express characteristic patterns of markers, IHC is often essential in the formulation of a definitive diagnosis.[6]
Table 1.
Histopathological differentials to be considered for superficial small round cell tumors

With a provisional diagnosis on histopathology as poorly differentiated round cell tumor, which comprise sarcomas, carcinomas, melanomas and lymphomas[7] we ran the initial panel of markers – Pan CK, CD45 and S-100. Pan CK and CD45 were negative, which ruled our carcinomas and lymphomas. S-100 was focally positive. The next panel comprised of desmin, vimentin, CD34, CK7, bcl-2 and CD99. Focal staining for bcl-2 was seen, which still ruled out synovial sarcomas and lymphomas as CK7 and CD45 were negative CD99 was focally positive, but as S-100 was also positive possibilities of primitive neuroectodermal tumor and Ewing's were ruled out. A last panel of CD56 and HMB-45 were done. A strong positivity for HMB-45 was seen, this along with S-100 positivity [Figure 5a–c], absence of pigment on Masson's Fontana stain and histomorphology helped us to a diagnosis of amelanotic melanoma, similar to studies of Limketkai B et al, Roy et al. and Oguri et al.[8,9,10]
Figure 5a.
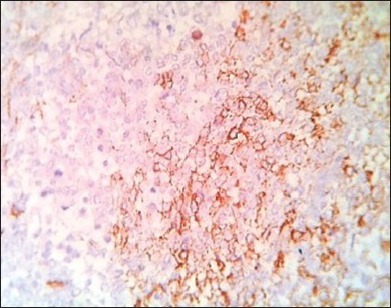
Focal CD99 staining
Figure 5c.
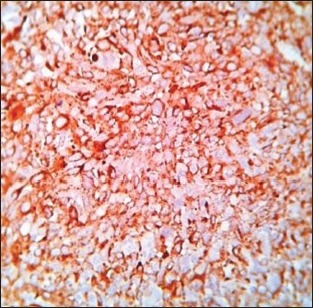
Strong positivity for HMB-45
Figure 5b.
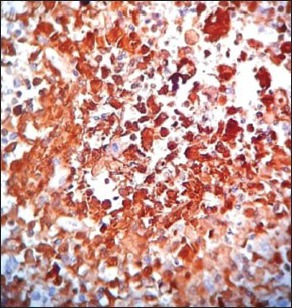
Strong positivity for S-100
A review of our slides did not reveal a few features typical of a melanoma such as pigment, junctional activity, grooves and pseudo inclusions, but cells showing prominent nucleoli, high mitotic activity, infiltration into the surrounding tissues was seen. Malignant melanoma is notorious its great microscopic variability.[11] Many hypothesis have been proposed to explain the lack of pigment in amelanotic melanoma such as lack of tyrosinase, agenesis of melanosomes, undetectable levels of melanin production or abnormal melanogenesis.[1] A study of 36 cases of amelanotic melanoma by Gualandri et al. concluded that amelanotic melanomas have an intrinsic faster speed of growth, worse prognosis, with Breslow's thickness being the most important determinant factor.[12]
Treatment of melanoma, including the amelanotic variant is wide surgical excision in combination with chemotherapy and to a lesser extent immunotherapy and radiation, with a check on nodal status.[3]
Conclusion
Amelanotic melanoma, like in our case can have a very deceptive clinical presentation. The aim of this presentation is to highlight on the fact that when a diagnosis of poorly differentiated round cell tumor is made, possibility of amelanotic melanoma must also be kept in mind, with one melanotic marker being included in the IHC panel, thereby preventing diagnostic delay and aiding in better patient management.
What is new?
Amelanotic melanoma must be considered as one of the differentials in cases show in histopathological features of a superficial poorly differentiated small round cell tumor.
One melanotic marker must be included in the IHC panel, to rule out amelanotic melanoma, thereby preventing diagnostic delay and aiding in better patient management.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Nalamwar R, Kharkar V, Mahajan S, Chikhalkar S, Khopkar U. Nodular amelanotic melanoma. Indian J Dermatol Venereol Leprol. 2010;76:273–5. doi: 10.4103/0378-6323.62972. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Chatterjee M. Amelanotic melanoma with multiple secondaries. Indian J Dermatol Venereol Leprol. 2006;72:252. [Google Scholar]
- 3.Pandiar D, Basheer S, Shameena PM, Sudha S, Dhana LJ. Amelanotic melanoma masquerading as a granular cell lesion. Case Rep Dent. 2013;2013:924573. doi: 10.1155/2013/924573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddaraju N. Ming Yu Cao Dr, editor. Clinical Cytology in the Diagnosis and Management of Melanoma, Current Management of Malignant Melanoma. 2011. [Last accessed on 2014 Sep]. ISBN: 978-953-307-264-7, InTech, DOI: 10.5772/18114. Available from: http://www.intechopen.com/books/current-management-of-malignant-melanoma/clinical-cytology-in-the-diagnosis-and-management-of-melanoma .
- 5.Grayson W. Unusual variants of melanoma. Mini-symposium: Pathology of melanomatous skin tumours. Diagn Pathol. 2010;16:321–9. [Google Scholar]
- 6.Joshi M, Nawrocki P, Balard M. Mini review article. Immunohistochemistry of epithelioid soft tissue sarcomas, literature review based on case studies. Journal of Krishna Institute of Medical Sciences University. 2012;1:16–24. http://www.jkimsu.com . [Google Scholar]
- 7.Machado I, Traves V, Cruz J, Llombart B, Navarro S, Llombart-Bosch A. Superficial small round-cell tumors with special reference to the Ewing's sarcoma family of tumors and the spectrum of differential diagnosis. Semin Diagn Pathol. 2013;30:85–94. doi: 10.1053/j.semdp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Limketkai B, Chandrasekhara V, Milligan F. Primary anorectal amelanotic melanoma presenting as internal hemorrhoids. Gastroenterol Hepatol. 2009;5:516–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S, Dhingra K, Mandal S, Khurana N. Unusual presentation of metastatic amelanotic melanoma of unknown primary origin as a solitary breast lump. Melanoma Res. 2008;18:447–50. doi: 10.1097/CMR.0b013e328317f04b. [DOI] [PubMed] [Google Scholar]
- 10.Oguri H, Izumiya C, Maeda N, Fukaya T, Moriki T. A primary amelanotic melanoma of the vagina, diagnosed by immunohistochemical staining with HMB-45, which recurred as a pigmented melanoma. J Clin Pathol. 2004;57:986–8. doi: 10.1136/jcp.2004.016220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosai J. Melanoma. 9th ed. Ch 4. Vol. 1. Elsevier, Mosby; 2004. Tumour and tumour like conditions of skin; p. 166. [Google Scholar]
- 12.Gualandri L, Betti R, Crosti C. Clinical features of 36 cases of amelanotic melanomas and considerations about the relationship between histologic subtypes and diagnostic delay. J Eur Acad Dermatol Venereol. 2009;23:283–7. doi: 10.1111/j.1468-3083.2008.03041.x. [DOI] [PubMed] [Google Scholar]


