Abstract
A 56-year-old male developed an ulcer on his glans penis and mucosae of upper and lower lips 3 days after taking ofloxacin, cephalexin, and ornidazole. Clinically, a provisional diagnosis of fixed drug eruption was made. The causative drug was confirmed by an oral provocation test which triggered a reactivation of all lesions only with ornidazole.
Keywords: Fixed drug eruption, ornidazole, provocation test
Introduction
What was known?
Fixed drug eruption to ornidazole has been infrequently reported.
Fixed drug eruption (FDE) is a special variety of allergic drug reaction where the reaction areas become reactivated with well-demarcated erythematous area whenever the same drug is administered. The lesions may be multiple and may occasionally be surmounted by a bulla in the center. In the majority of cases, it heals in 7-10 days leaving behind hyperpigmentation. The lesions are rarely pruritic. It has been seen at any age[1] and due to any drug. Brocq[2] in 1894 was the first to coin the term “Fixed eruption” to describe a pattern of skin eruption due to antipyrine. Since then, numerous drugs including pseudoephedrine, trimethoprim, tetracycline, barbiturates, salicylates, phenolphthalein, ibuprofen oxyphenbutazone, fluconazole, azithromycin, levofloxacin, levamisole, and colchicine have been implicated among many others as a causative agent.[3,4,5,6,7,8,9] FDE due to ornidazole, a relatively new 5 nitro-imidazole derivative has been very infrequently reported,[10,11,12,13] though it is very commonly used for amoebic dysentery in developing countries.
Case Report
A 56-year-old male developed pain, redness, and tenderness of his right index finger since 2 days for which he had been treated elsewhere with cephalexin 500 mg twice daily; ofloxacin 200 mg twice daily; and ornidazole 500 mg twice daily orally. By third day, when I first saw, the infection in the index finger improved but he developed an ulcer on his glans penis [Figure 1] and the mucosae of his upper and lower lips. At this juncture, all the medicines were discontinued and he was started on dexamethasone 2 mg daily orally which led to the clearance of all the lesions during the next 3 days. Dexamethasone was reduced and stopped after 2 days.
Figure 1.
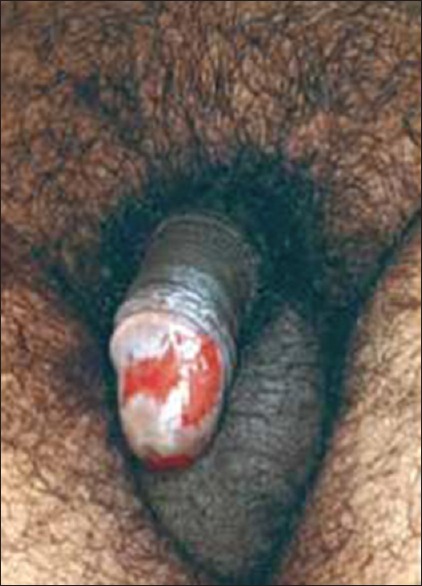
Superficial ulcer of fixed drug eruption on glans penis
One week later, oral provocation test was started after obtaining informed consent by giving one drug each day in full therapeutic dose. There was no reactivation of the lesions with cephalexin, ofloxacin, ciprofloxacin, tinidazole, paracetamol, diclofenac sodium, amoxicillin and clavulanic acid. However, he developed reactivation of itching, redness and occasional erosions on his glans penis, upper and lower lips 4 hours after taking ornidazole 500 mg. He was immediately started dexamethasone 2 mg orally twice daily with clearance of all the symptoms and lesions during the next 24 hours.
Discussion
Recurrence of pruritus, erythema and occasional erosions on the lips and glans penis 4 hours after administration of 500 mg ornidazole confirm that the reaction was due to ornidazole and not due to the other medicines used in the provocation test. Among the nitro-imidazoles, fixed drug reaction to tinidazole and metronidazole is well documented.[14] FDE due to ornidazole is not very frequent. Only very few of FDE due to ornidazole have been reported till now.[10,11,12,13]
FDE seems to be a form of delayed hypersensitivity reaction mediated by CD8+ T cell. The causative drug is supposed to act as hapten which binds to basal keratinocytes resulting in the release of lymphokines and antibodies which damage the basal cell layers. On drug intake, CD8+ cells are reactivated to release interferon and cytotoxic granules into the local micro-environment. Mast cells are also believed to contribute to the activation of intraepidermal CD8+ cells through the induction of cell adhesion molecules.[15]
Provocation tests are still the only reliable method to find the causative agents and are often essential or even mandatory in the patient's interest. Though provocation is very safe especially in fixed eruption, some workers are unwilling to attempt it. If history suggest severe reaction, then it may be safer to start with half the dose on first day, followed by full therapeutic dose on the next day if there is no reaction to the first dose.
What is new?
First case in Indian Literature.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Gupta R. Fixed drug eruptions in a 9-month old child. Indian J Dermatol Venereol Leprol. 1989;55:181–2. [PubMed] [Google Scholar]
- 2.Savin JA. Current causes of fixed drug eruptions. Br J Dermatol. 1970;83:546–9. doi: 10.1111/j.1365-2133.1970.tb15739.x. [DOI] [PubMed] [Google Scholar]
- 3.Korkij W, Soltani K. Fixed drug eruption. A brief review. Arch Dermatol. 1984;120:520–4. [PubMed] [Google Scholar]
- 4.Gupta R. Drugs causing fixed drug eruptions: Confirmed by provocation tests. Indian J Dermatol Venereol Leprol. 2003;69:120–1. [PubMed] [Google Scholar]
- 5.Shukla P, Prabhudesai R. Fixed drug eruption to fluconazole. Indian J Dermatol. 2005;50:236–7. [Google Scholar]
- 6.Nath AK, Adityan B, Thappa DM. Multifocal bullous fixed drug eruption due to fluconazole. Indian J Dermatol. 2008;53:156–7. doi: 10.4103/0019-5154.43212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambooken B, Asokan N. Regularity of recurrence of fixed drug eruption: A pointer to the cause. Indian J Dermatol. 2009;54:188. doi: 10.4103/0019-5154.53176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahalaut P, Alexander E. Azithromycin in acne: A protagonist for fixed drug reaction? Indian J Dermatol. 2008;53:100–1. doi: 10.4103/0019-5154.41661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetrichewel TP, Sudha R, Shobana S, Anandan S. Zosteriform fixed drug eruption to levofloxacin. Indian J Dermatol. 2012;57:327–8. doi: 10.4103/0019-5154.97688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Jain VK, Aggarwal K, Gupta S, Mahendra A. Fixed drug eruption caused by ornidazole. Contact Dermatitis. 2005;53:300–1. doi: 10.1111/j.0105-1873.2005.0654b.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanmukhani J, Shah V, Baxi S, Tripathi C. Fixed drug eruption with ornidazole having cross-sensitivity to secnidazole but not to other nitro-imidazole compounds: A case report. Br J Clin Pharmacol. 2010;69:703–4. doi: 10.1111/j.1365-2125.2010.03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Mahendra A, Gupta S, Kaur S. Multiple fixed drug eruption caused by ornidazole. Dermatitis. 2010;21:330–3. [PubMed] [Google Scholar]
- 13.Marya CM, Sharma G, Parashar VP, Dahiya V. Mucosal fixed drug eruption in a patient treated with ornidazole. J Dermatol Case Rep. 2012;6:21–4. doi: 10.3315/jdcr.2012.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004;15:221–39. doi: 10.1177/154411130401500405. [DOI] [PubMed] [Google Scholar]
- 15.Shiohara T. Fixed drug eruption: Pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316–21. doi: 10.1097/ACI.0b013e32832cda4c. [DOI] [PubMed] [Google Scholar]


