Abstract
Objective
To estimate national hospitalization rates for invasive pneumococcal disease (IPD) in children with sickle cell disease (SCD) before and after the 2000 licensure of the heptavalent pneumococcal conjugate vaccine (PCV7).
Procedure
We performed a retrospective trend analysis of the 1994-2007 Nationwide Inpatient Sample databases. Hospitalizations involving children with SCD and IPD were identified by ICD-9CM code. The primary outcomes, the annual hospitalization rate for IPD in children with SCD and the proportion of hospitalizations for IPD per 100 total SCD hospitalizations, were analyzed using multivariable linear regression and contingency analysis, respectively.
Results
A total of 1,242 hospitalizations for IPD in SCD patients were identified from 1994-2007, with a mortality rate of 2.4%. The national mean annual rate of IPD hospitalization decreased by 65%, from 131.8 cases/year from 1994-2000 to 45.5 cases/year from 2001-2007 (p=0.001). The national proportion of hospitalizations for IPD per 100 total SCD hospitalizations decreased from 0.4 to 0.15 (p<0.0001) over the same interval. Following PCV7 licensure, the mean annual cumulative hospital days and cumulative hospital charges decreased nationally by 53% and 36%, respectively.
Conclusion
In a national sample, PCV7 licensure is temporally associated with a nearly three fold reduction in IPD hospitalizations in children with SCD.
Keywords: sickle cell disease, PCV7, Streptococcus pneumoniae, infection, hospitalization
INTRODUCTION
Children with sickle cell disease (SCD) are at markedly increased risk of infection from Streptococcus pneumoniae, which is estimated to be 300 to 600 times higher than the general population, due to functional hyposplenism.[1-3] Historically, the estimated incidence was six episodes of invasive pneumococcal disease (IPD) per 100 patient years with a 25-35% case fatality rate.[4,5] In the mid-1980's, the polyvalent polysaccharide S. pneumoniae vaccine (PPV23) and prophylactic penicillin were shown to reduce the risk of IPD and were adopted as standard-of-care.[6-8] These interventions decreased, but did not eliminate, the risk of IPD in children with SCD.
In 2000, a heptavalent pneumococcal protein-conjugate vaccine (PCV7) was licensed. Since then, two studies of IPD in children with SCD reported 68%[9] and 90%[10] reductions in IPD. These studies were limited by abbreviated follow-up (two and four years post-PCV7 licensure, respectively), limited geographic representation (the state of Tennessee and metropolitan Atlanta, respectively), and small numbers of cases (50 and 37, respectively). The limited follow-up period is especially important, as recent reports have described a resurgence of non-vaccine pneumococcal serotypes of S. pneumoniae.[11-13]
The aim of this study was to conduct a trend analysis of nationally-representative databases from 1994-2007 for hospitalization due to IPD in children with SCD. Compared to previous reports[9,10], this approach extends the duration of post-PCV7 follow-up by 3 years, includes a nationally representative sample, and includes many more cases. We hypothesized that hospitalization due to IPD would decrease in the years following PCV7 licensure.
METHODS
Overview/Study Design
This study was a retrospective analysis of hospitalization for IPD in children with SCD using the Nationwide Inpatient Sample (NIS) databases from 1994 to 2007. The NIS is a large administrative database collected by multiple state and hospital agencies and assimilated by the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality. It approximates a 20 percent stratified sample of U.S. community hospitals which allows for calculation of national estimates.[14] Because the NIS is a de-identified database without unique patient identifiers, the unit of measure is a discharge, not an individual. The NIS does not include medications (e.g., penicillin), vaccinations, or vaccination history. Approximately 125,000 discharges for persons with SCD are represented in each year's NIS.[15]
Subjects/Discharges
Discharges of interest were < 18 years of age and were identified by ICD-9CM code for any form of SCD, including sickle beta thalassemia without crisis (282.41), sickle beta thalassemia with crisis (282.42), unspecified SCD (282.60), sickle cell anemia without crisis (282.61), sickle cell anemia with crisis (282.62), sickle hemoglobin-C disease without crisis (282.63), sickle hemoglobin-C disease with crisis (282.64), other SCD without crisis (282.68), and other SCD with crisis (282.69). Among discharges with SCD, IPD was identified using ICD-9CM codes for pneumococcal septicemia (038.2), pneumococcal meningitis (320.1), and pneumococcal infection of an unspecified site (041.2). Discharges were included whether the SCD and IPD codes were in the primary or any of 14 secondary discharge ICD-9CM code positions. No changes in ICD-9CM coding practice occurred from 1994-2007 for either SCD or IPD. Our only exclusion criterion was the ICD-9CM code for sickle cell trait (282.5). The Institutional Review Board of the University of Texas Southwestern Medical Center approved this study.
Definitions and Measurements
Variables
In-hospital mortality was coded as a binary variable, as provided by the NIS. Length of stay (LOS) in days was coded as a continuous variable. Hospital charges were obtained from the NIS in whole dollars. To account for rising healthcare costs and inflation from 1994 to 2007, all charges were adjusted to 2007 dollar estimates using the Medical Care Index of the Consumer Price Index. Patient age and gender were obtained from distinct data elements in the NIS. The patients’ insurance coverage was coded using the expected primary payer: Medicare, Medicaid, private insurance, self-pay, no charge, or other. US geographic regions were assigned by the NIS using Northeast, Midwest, South, and West, as defined by the US Census Bureau.
Outcomes
Two primary outcomes were examined in this study. The first was the annual IPD hospitalization rate in children with SCD. The second, IPD hospitalizations per 100 SCD hospitalizations, accounts for year-to-year variability in the SCD hospitalization sampling within the NIS. Secondary outcomes included trends in cumulative hospital days and cumulative hospital charges secondary to IPD. Additionally, we investigated trends in the average age of IPD cases and the frequency of IPD across U.S. geographic regions.
Analyses
Summary statistics were used to describe the SCD discharges with IPD and all non-IPD discharges. National estimates for the annual hospitalization rate for IPD were made using the weights provided by the NIS. Because not all hospitals in the NIS have SCD discharges each year, we created a dummy variable for hospitals without any SCD discharges, a method recommended by the NIS for producing national estimates from a subset of hospitals in the NIS.[14] Standard error calculations for national estimates were made using the method recommended by the NIS.[14] The NIS is a 20% sample of US hospitalizations, so the weighting for each hospitalization is approximately a factor of five. The Wilcoxon rank-sum test was used to analyze annual hospitalization rate for IPD, and χ2 was used to analyze the annual IPD hospitalizations per 100 SCD hospitalizations. Additionally, to analyze the primary outcome of trend in IPD hospitalization rate before and after PCV7 licensure, we used a multivariable linear regression model to make inferences about the pre- and post-PCV7 difference in annual IPD hospitalization rate, controlling for the secular trend. In a sensitivity analysis, the definition for IPD was changed to include the ICD-9CM code for pneumococcal pneumonia (481) in addition to the other IPD codes, and the trend in hospitalization rates was again determined. The Wilcoxon rank-sum test was used to analyze secondary outcomes. P < 0.05 was considered statistically significant with no study-wide correction for multiple comparisons. All analyses were performed using SAS 9.2 (SAS Institute, Gary, North Carolina.)
RESULTS
From 1994-2007, a national weighted estimate of 1,242 discharges were identified with IPD in children with SCD. Among identified discharges, 55% had sickle cell anemia (282.61 or 282.62), and 40% had unspecified SCD (282.60). ICD-9CM coding for IPD among the 1,242 discharges included 64% with pneumococcal septicemia (038.2), 23% with pneumococcal infection of unspecified site (041.2), 7% with pneumococcal meningitis (320.1), and 5% with septicemia and meningitis (038.2 and 320.1.) Among discharges with a pneumococcal infection of an unspecified site (041.2), 82% had an additional ICD-9CM code for infection of a normally-sterile site, including 72% with bacteremia, whereas 12% had no additional ICD-9CM codes for infection, and 7% had infection of non-sterile sites.
Demographic features and outcomes of the 1,242 IPD hospitalizations are compared with all other SCD hospitalizations (n=442,447) in children in the Table. IPD hospitalizations occurred in younger children. No difference between IPD and all other SCD hospitalizations was seen in the gender or the primary payer. IPD hospitalizations demonstrated a higher mortality, LOS, and hospital charges compared with all other SCD hospitalizations.
Table.
Characteristics of IPD and all other hospitalizations for children with SCD (1994-2007)
| Characteristic | IPD (N=1,242) | All Other SCD (N=442, 447) |
|---|---|---|
| Age, years, median (IQR)* | 3 (1 – 6) | 9 (3 – 14) |
| Gender (% female) | 45 | 48 |
| Primary payer (%) | ||
| Medicaid | 66 | 65 |
| Private/HMO | 26 | 28 |
| Self-pay | 3 | 3 |
| In-hospital death (%) | 2.4 | 0.1 |
| Length of stay, days, median (IQR) | 6 (4 – 10) | 3 (2 – 5) |
| Charges, $ × 103, median (IQR) | 11.4 (6.3 – 21.4) | 6.4 (3.8 – 11.7) |
No statistical tests comparing IPD and all other SCD hospitalizations were performed due to the very large sample size of the All Other SCD group.
Abbreviations: IPD, invasive pneumococcal disease; SCD, sickle cell disease; IQR, inter-quartile range
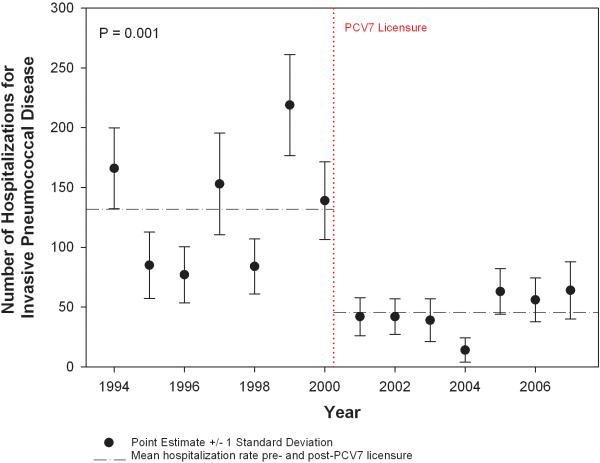
The mean national annual hospitalization rate decreased 65%, from 131.8/year (standard deviation(SD)=12.5) in the seven years before PCV7 licensure to 45.7/year (SD=6.6) in the seven years after PCV7 licensure (p=0.001) (Figure 1). The lowest annual hospitalization rate occurred in 2004 with 14/year (SD=10.2). In multivariable linear regression, the before-after PCV7 term was statistically significant (p=0.012). No significant secular trend in IPD hospitalization was detected. In a sensitivity analysis, we added the ICD-9CM code for pneumococcal pneumonia (481) to the inclusion criteria for IPD. A consistent decrease in the mean national annual hospitalization rate was demonstrated from 242.7/year prior to PCV7 to 83.3/year after PCV7, a 66% decrease. When the year-to-year variability in SCD hospitalization sampling within the NIS was controlled for, the proportion of IPD discharges per 100 total SCD discharges decreased 63%, from a mean of 0.4 before to 0.15 after PCV7 licensure (p<0.0001). Among IPD cases < five years old, the mean annual hospitalization rate decreased 77% from 90.1/year (SD=24.8) to 20.4/year (SD=9.9) post-PCV7 licensure. Among IPD cases ≥ five years old, the mean annual hospitalization rate decreased 40% from 41.6/year (SD=15.5) to 24.9/year (SD=9.9) post-PCV7 licensure.
Figure 1.
National trends in annual hospitalization rates for invasive pneumococcal disease in children with sickle cell disease.
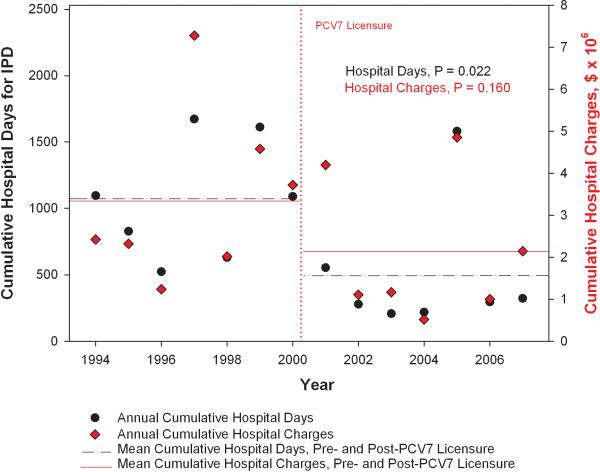
The national trends in cumulative hospital days and hospital charges attributable to IPD in SCD are shown in Figure 2. The mean annual cumulative hospital days attributable to IPD decreased 53% from 1064 days/year [95% Confidence Interval (CI), 768, 1361] prior to licensure of PCV7 to 493 days/year [95% CI, 206, 781] after PCV7 (p=0.022). Similarly, the mean annual cumulative hospital charges attributable to IPD decreased 36% from $3.4 million/year [95% CI, 2.0, 4.7] prior to PCV7 to $2.1 million/year [95% CI, 1.1, 3.2] after PCV7 (p=0.160).
Figure 2.
National trends in annual, cumulative hospital days and hospital charges for invasive pneumococcal disease in children with sickle cell disease.
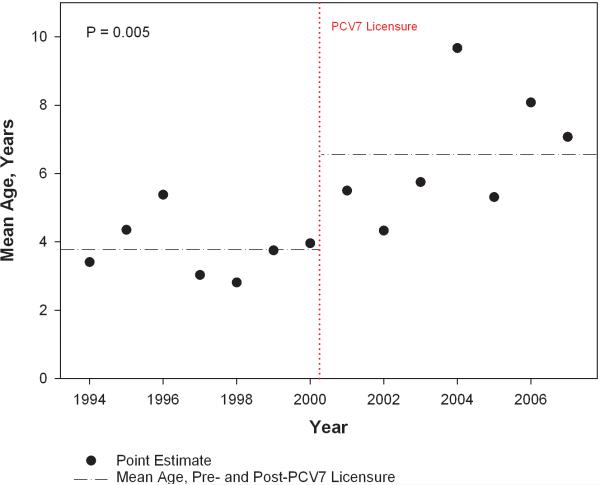
The national trend in average age of infection is shown in Figure 3. The average age of infection increased 76% from 3.7 years [95% CI, 3.0, 4.4] prior to PCV7 to 6.5 years [95% CI, 5.4, 7.8] after PCV7 (p=0.005). We found no significant differences in the change in hospitalization rates for IPD across U.S. geographic regions following PCV7 licensure. The IPD hospitalization rate in the Northeast region decreased 74.5% compared with a 62.8% decrease for the Midwest region, a 63.5% decrease for the South region, and a 61.5% decrease for the West region.
Figure 3.
National trends in mean age of invasive pneumococcal disease in children with sickle cell disease.
DISCUSSION
To our knowledge, these are the first national estimates of IPD in children with SCD before and after PCV7 licensure. A nearly three-fold national reduction was observed in the number of IPD hospitalizations, a reduction that persists even when accounting for year-to-year variability in total SCD hospitalizations within the NIS databases. The national decrease in IPD hospitalization rates was accompanied by a 53% reduction in total hospital days and a 36% reduction in total hospital charges attributable to IPD. A concomitant 71% increase in the average age of IPD cases in SCD was observed. Finally, the case fatality rate for all cases was low at 2.4%.
Prior regional reports of IPD in SCD before and after PCV7 documented corresponding reductions. A 93% reduction in IPD incidence for children with SCD < five years old and a 38% reduction patients ≥ five years old was noted for Tennessee Medicaid patients from 1995 to 2004.[10] A 68% reduction in IPD incidence for SCD patients was observed from 1995 to 2002 in the greater Atlanta area.[9] Similarly, a 75% reduction in the incidence of IPD among the general population of US children < five years old was previously described.[16] We report a comparable reduction in IPD-related hospitalizations in a national sample, although IPD incidence cannot be calculated using NIS databases due to an unknown denominator of children with SCD. This study to documents an increase in the mean age of IPD hospitalizations post-PCV7 licensure. This finding is consistent with the analysis of Tennessee Medicaid data in which children < five years old with SCD were more protected by PCV7 than children ≥ five years old. This trend, in part, may reflect the AAP and CDC recommendations for PCV7 to be administered to children with SCD under five years old.[17,18]
Recent reports of IPD in SCD have estimated case fatality rates of 10% (five deaths/50 cases),[9] 5.4% (two deaths in 37 cases),[10] 12.5% (two deaths/16 cases),[19] and 10.6% (five deaths/47 cases),[20] for an aggregate mortality rate of 9.3% (14 deaths/150 cases). In our study, a low case fatality rate of 2.4% (30 deaths/1242 cases) is observed. It is unclear why the case fatality rate in this study was considerably lower than those described in other contemporary studies. We questioned whether our 12% rate of meningitis was different than previous reports, however, recently-reported meningitis rates for SCD are not different at 15%[9] and 12.5%[19]. We also questioned whether the inclusion of cases identified by the ICD-9CM code for pneumococcal infection of an unspecified site (041.2) may have falsely decreased the case fatality rate by potentially including non-invasive pneumococcal infections. When 041.2 was excluded from the analysis, however, the case fatality rate minimally changed from 2.4% to 3.3%. Serotype 19F was associated with an increased likelihood of death in a prior study of IPD in SCD patients.[21] PCV7 included 19F, which may explain the decreased mortality in the latter half of this study's sample. Other factors, including improvements in the care of sepsis in the ICU setting and improved processes to ensure rapid evaluation and treatment of children with SCD and fever in acute care settings, may also contribute to the lower case fatality rate in this contemporary study.
The temporal association of decreased IPD following PCV7 licensure suggests that it was effective in the SCD population, but a cause-effect relationship cannot be established using these NIS data. Other factors which could have contributed to the observed decrease in IPD include (1) improved adherence to penicillin prophylaxis, which is unlikely given the recent report of poor adherence to penicillin prophylaxis by Sox et al.;[22] (2) a chance decrease in the prevalence of colonization by the pneumococcus or a decrease in pneumococcal virulence which are unlikely to have led to such a large, sustained decrease; or (3) an undetected decline in the national SCD population, which is unlikely given the stable birth rate of SCD confirmed by newborn screening.[23] Hence, the temporal association of decreased IPD following PCV7 licensure suggests that it has been effective in preventing IPD in SCD children, and it supports the need for continued vigilance to ensure that all SCD patients are identified by newborn screening, prescribed prophylactic penicillin, and vaccinated.
Although the decrease in IPD following PCV7 licensure is encouraging, the persistence of IPD hospitalizations is concerning for several reasons. First, since the publication of the Prophylactic Penicillin Study II (PROPS II),[24] many SCD centers, including ours, have routinely offered cessation of daily prophylactic penicillin to SCD patients beginning at five years old. Our finding of an increased average age of IPD hospitalizations following PCV7 might prompt consideration of extending daily prophylactic penicillin past age five years old. Additionally, current CDC recommendations for the recently-licensed 13-valent pneumococcal conjugate vaccine (PCV13) include immunization of SCD patients who have previously received a complete PCV7 series with a single dose of PCV13 up to six years old.[25] Immunization of SCD patients 6-18 years old is left to the discretion of the provider, a recommendation which could be revised to include older children. Finally, in a recent report, we have also shown a shift toward non-vaccine serotypes in IPD in children with SCD vaccinated with PCV7 and PPV23.[13] This persistence of IPD in the post-PCV7 era, especially non-vaccine serotypes, highlights the need for an improved vaccine against the pneumococcus. Current vaccine strategies rely on the polysaccharide capsule of the pneumococcus (responsible for the serotype) to generate an immune response and afford protection. In response to suppression of the prevailing pneumococcal serotypes by PCV7, non-vaccine serotypes, most importantly 19A, have emerged as the predominant serotype responsible for IPD.[11] PCV13 aims to protect against the currently prevailing IPD serotypes, but given pneumococcus's proclivity to adaptation,[26] non-vaccine serotypes may again emerge. Therefore, vaccine development directed toward universal pneumococcal antigens,[27] not the ever-shifting polysaccharide capsules, should be encouraged.
The limitations of this study include potential inaccuracies in ICD-9CM coding for these administrative data. Additionally, we cannot assess the vaccination status or adherence to penicillin prophylaxis for the patients represented in the NIS databases. By investigating inpatient data, we assume that cases of IPD in SCD will lead to hospitalization and would not be treated in an outpatient setting. Another limitation of this study is our inability to account for potential changes in the number of children with sickle cell disease in the US during the study period. US Census data, however, demonstrate only a 2.8% increase in children identified as black or black-in-combination from 2000-2007. The relationship of IPD hospitalization rates with the timing of PCV7 licensure is strictly temporal, so causation cannot be inferred.
In conclusion, this study demonstrates a substantial decrease in IPD hospitalization rates after PCV7 licensure in a national sample of children with SCD. The mean age of IPD cases has increased to 6.5 years after PCV7 licensure, a finding that might prompt reconsideration of routine discontinuation of prophylactic penicillin at five years old and vaccination with PCV13 of SCD patients > six years old. Although the SCD community can celebrate the recent decrease in IPD hospitalizations, the remaining IPD disease burden should support continued efforts to educate SCD patients and families regarding the importance of urgently seeking medical attention for fever, encourage continued adherence to prophylactic penicillin, and underscore the continued need for a better pneumococcal vaccine.
ACKNOLWEDGEMENTS
We thank Dr. George R. Buchanan for his thoughtful review of the earlier drafts of the manuscript.
Grant Support: NIH/NHLBI – U54 HL0705088-06; NIH - UL1 RR024982-03
Footnotes
Disclosure of conflicts of interest: We have no relevant conflicts of interest.
Prior presentations: This work was presented, in part, as a platform presentation at the American Society of Pediatric Hematology-Oncology Annual Meeting, Montreal, Quebec, CA, April 8, 2010.
REFERENCES
- 1.Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971;50(2):97–112. [PubMed] [Google Scholar]
- 2.Powars DR. Natural history of sickle cell disease--the first ten years. Semin Hematol. 1975;12(3):267–285. [PubMed] [Google Scholar]
- 3.Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969;281(17):923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- 4.Overturf GD, Powars D, Baraff LJ. Bacterial meningitis and septicemia in sickle cell disease. Am J Dis Child. 1977;131(7):784–787. doi: 10.1001/archpedi.1977.02120200066014. [DOI] [PubMed] [Google Scholar]
- 5.Zarkowsky HS, Gallagher D, Gill FM, et al. Bacteremia in sickle hemoglobinopathies. J Pediatr. 1986;109(4):579–585. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]
- 6.Ammann AJ, Addiego J, Wara DW, et al. Polyvalent pneumococcalpolysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med. 1977;297(17):897–900. doi: 10.1056/NEJM197710272971701. [DOI] [PubMed] [Google Scholar]
- 7.Weintrub PS, Schiffman G, Addiego JE, Jr., et al. Long-term follow-up and booster immunization with polyvalent pneumococcal polysaccharide in patients with sickle cell anemia. J Pediatr. 1984;105(2):261–263. doi: 10.1016/s0022-3476(84)80124-9. [DOI] [PubMed] [Google Scholar]
- 8.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 9.Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121(3):562–569. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- 10.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A Is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125(3):429–436. doi: 10.1542/peds.2008-1702. [DOI] [PubMed] [Google Scholar]
- 12.Tyrrell GJ, Lovgren M, Chui N, et al. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000-2006. Vaccine. 200927(27):3553–3560. doi: 10.1016/j.vaccine.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 13.McCavit TL, Quinn CT, Techasaensiri C, et al. Increase in invasive Streptococcus pneumoniae infections in children with sickle cell disease since pneumococcal conjugate vaccine licensure. J Pediatr. 2011;158(3):505–507. doi: 10.1016/j.jpeds.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houchens R, Elixhauser A. Final Report on Calculating National Inpatient Sample Variances, 2001. U.S. Agency for Healthcare Research and Quality; Jun, 2005. [June 6, 2005]. HCUP Methods Series Report #2003-2:ONLINE. [Google Scholar]
- 15.Steiner C, Miller J. Sickle Cell Disease Patients in U.S. Hospitals, 2004. Agency for Healthcare Research and Quality; Rockville, Md: 2006. pp. 1–9. HCUP Statistical Brief #21(December) [PubMed] [Google Scholar]
- 16.Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008;57(6):144–148. [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106(2 Pt 1):362–366. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 18.Recommendations of the Advisory Committee on Immunization Practices (ACIP) Preventing pneumococcal disease among infants and young children. MMWR Recomm Rep. 2000;49(RR-9):1–35. [PubMed] [Google Scholar]
- 19.Wang WC, Wong WY, Rogers ZR, et al. Antibiotic-resistant pneumococcal infection in children with sickle cell disease in the United States. J Pediatr Hematol Oncol. 1996;18(2):140–144. doi: 10.1097/00043426-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hord J, Byrd R, Stowe L, et al. Streptococcus pneumoniae sepsis and meningitis during the penicillin prophylaxis era in children with sickle cell disease. J Pediatr Hematol Oncol. 2002;24(6):470–472. doi: 10.1097/00043426-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr. 2003;143(4):438–444. doi: 10.1067/S0022-3476(03)00331-7. [DOI] [PubMed] [Google Scholar]
- 22.Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057–1061. doi: 10.1001/jama.290.8.1057. [DOI] [PubMed] [Google Scholar]
- 23.National Newborn Screening & Genetics Resource Center Webpage < http://nnsis.uthscsa.edu/xreports.aspx?XREPORTID=5>. Accessed.
- 24.Falletta JM, Woods GM, Verter JI, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995;127(5):685–690. doi: 10.1016/s0022-3476(95)70154-0. [DOI] [PubMed] [Google Scholar]
- 25.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1–18. [PubMed] [Google Scholar]
- 26.Claverys JP, Prudhomme M, Mortier-Barriere I, et al. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol Microbiol. 2000;35(2):251–259. doi: 10.1046/j.1365-2958.2000.01718.x. [DOI] [PubMed] [Google Scholar]
- 27.Daniels CC, Coan P, King J, et al. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun. 2010;78(5):2163–2172. doi: 10.1128/IAI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]