Abstract
Although the incidence of and mortality following ST-segment elevation myocardial infarction (STEMI) is decreasing, time-trends in anatomical location of STEMI and associated short-term prognosis have not been examined in a population-based community study. We determined 22-year trends in age- and race-adjusted, gender-specific incidence rates and 28-day case fatality of hospitalized STEMI by anatomic infarct location among a stratified random sample of 35-74 year old residents of four communities in the Atherosclerosis Risk in Communities (ARIC) study. STEMI infarct location was assessed by 12-lead electrocardiograms (ECG) from the hospital record, and was coded as anterior, inferior, lateral and multi-location STEMI using the Minnesota Code. Between 1987 and 2008, a total of 4,845 patients had an incident STEMI; 37.2% were inferior STEMI; 32.8% were anterior; 16.8% occurred in multiple infarct locations and 13.2% were lateral STEMI. For inferior, anterior and lateral STEMI in both men and women, significant declines were observed in the age-adjusted annual incidence rate and the associated 28-day case fatality. In contrast, for STEMI in multiple infarct locations, neither the annual incidence rate nor the 28-day case fatality changed over time. The age- and race-adjusted annual incidence rate and associated 28-day case fatality of STEMI in anterior, inferior and lateral infarct locations declined over 22 years of surveillance; however, no decline was observed for STEMI in multiple infarct locations. In conclusion, our findings suggest there is room for improvement in the care of patients with multi-location STEMI.
Keywords: ST segment elevation myocardial infarction, Epidemiology, Trends
Introduction
Detailed evaluation of temporal trends in myocardial infarction (MI) rates is essential to monitor the population burden of cardiovascular disease (CVD), and to understand the determinants of coronary heart disease (CHD) mortality over time.1 During a STEMI, the distribution of ST-elevations on a surface electrocardiogram (ECG) corresponds to anatomic locations of ischemic myocardium 2 and has prognostic importance.3 Recent studies have demonstrated declines in the incidence,1,4,5 severity,6, 7 and mortality after an MI.1,5 Some STEMI consensus treatment guidelines 7 and clinical practice monographs 8 draw from historic patterns in STEMI infarct location, and suggest that STEMI treatment may differ by infarct location.9 However, whether patterns of STEMI infarct location by ECG have changed and apply to STEMI in the contemporary era is unknown. We describe herein twenty-two year trends in rates of incident STEMI by ECG infarct location and associated survival in a community setting.
Methods
Since 1987, the Atherosclerosis Risk in Communities (ARIC) study has conducted surveillance of hospitalized MI and deaths due to CHD among residents 35 to 74 years of age in four communities: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. The combined study population of these four communities was approximately 396,000 persons in 2008. Twenty-four percent of the population in ARIC communities under surveillance were black. Forsyth County, North Carolina and the city of Jackson, Mississippi were 20% and 50% black, respectively, while the remaining two communities in ARIC Community Surveillance are predominantly white.6 Details of the surveillance methods used have been previously reported and are only briefly described here.10,11
A stratified random sample of suspected hospitalized MIs was identified from electronic discharge lists obtained from all hospitals (n=23) serving the four communities.6 Trained ARIC staff abstracted data from medical records, selected on age, community of residence and discharge code (ICD-9-CM codes 402, 410-414, 427, 428, and 518.4). Detailed descriptions of investigation and validation of selected ICD codes used in ARIC surveillance have been provided elsewhere.12,6 Sampling probabilities varied by race, sex, field center and discharge code stratum and were adjusted periodically.13 Medical records were abstracted for presence of chest pain, history of MI or CVD and cardiac biomarkers.7 Copies of up to three ECGs (first, last and third) were obtained from each hospitalization and sent to the University of Minnesota Electrocardiographic Reading Center for classification by the Minnesota Code.14,15 A computerized algorithm was applied to data on chest pain, biomarkers and electrocardiographic findings to classify MI.10 Criteria for each of these three diagnostic elements in the algorithm remained constant over the study period and have been described in detail.5 All eligible hospitalized events were classified as definite, probable, suspect or no MI.10 Definite and probable MI were combined to define MI events for this analysis. Definite and probable MI events with abnormal biomarkers were further classified by Minnesota Code of the ECGs obtained as ST-segment elevation MI (STEMI) or non ST-segment elevation MI (NSTEMI).14 This analysis was restricted to validated STEMI events without a history of prior MI noted in the medical record.
All ECGs were visually coded using Minnesota code categories of ST-segment elevations into one of four anatomic locations based on conventional anatomic grouping of surface ECG leads: V1-V5; II, III, AVF; or I, AVL, V6. Using these three lead groups, four anatomic locations (anterior, inferior, lateral or multiple) were derived for this study. Anterior STEMI was defined as ST-segment elevation ≥ 2.0mm in any of leads V1-V4 or an ST-segment elevation ≥ 1.0mm in V5; inferior STEMI as ST-segment elevation ≥ 1.0mm in any of leads II, III or aVF; and lateral STEMI as ST-segment elevation ≥ 1.0mm in any of leads I, aVL, and V6 alone or in the presence of anterior ST-segment elevations.16,6 ST-elevations in the anterior and lateral ECG lead-groups were combined to increase sensitivity to detect lateral STEMI, as ischemia of the lateral wall may be poorly represented by the lateral leads (I, aVL, V6) alone.17,18 Finally, to represent ischemia in multiple anatomic locations, a multi-location STEMI was defined as ST-segment elevations in 2 or more of anterior, inferior or lateral locations.
Vital status post-hospital discharge was determined through linkage with the National Death Index (NDI). Twenty-eight day case fatality was determined for all incident STEMI cases and, due to limited events by infarct location, we grouped 28-day case-fatality into two time period categories (1987-1996 and 1997- 2007) for comparison. Case fatality results are not reported for 2008 due to a lag in NDI reporting.
All analyses were weighted and standard errors were computed using stratified random sampling methodology to account for the sampling scheme.5,12,21 Descriptive statistics for baseline characteristics were computed by year group and by STEMI infarct locations. The annual percent changes in event rates were computed across the year groups for each baseline characteristic, and the corresponding standard errors were approximated by the delta method. Wald tests were used to compare baseline characteristics between STEMI locations. Age- and race-adjusted, sex-specific, annual incidence rates per 10,000 persons of STEMI by anatomic location were computed based on population denominator estimates using interpolation and extrapolation of 1990 and 2000 U.S. Census population estimates. Age and race adjustment was by the direct method using the 2000 U.S. population estimates as the standard. Twenty-two year trends in incident STEMI are reported by location and gender based on linear or quadratic Poisson regression models. Quadratic trends are reported only when the quadratic term in the model is significant at the 0.05 level. Pairwise comparisons of the bootstrapped average annual percent change estimated from the location-specific linear regression models were made at the 0.05 level with Bonferroni adjustment for multiple comparisons. Logistic regression was used to model age and race-adjusted 28-day case fatality percentages as a function of location, time interval (1987-1996 and 1997-2007), and gender. Contrasts were specified to test differences in case fatality between year-groups for each location. The differences of trends in case fatality trends between genders were tested; gender-specific trends were not reported due to insignificant p-values and small numbers of events. Event-rate trends analysis was conducted in the statistical package SUDAAN Loglink and case fatality analyses were conducted in SUDAAN Logistic.
Results
From 1987 through 2008, there were an estimated 4,845 incident hospitalized STEMIs in the four ARIC study communities among residents 35 to 74 years of age. Over the twenty-two year study period, 31.3% of STEMIs occurred in women. There were statistically significant changes in both the characteristics of STEMI patients and the methods used to treat these patients over the study duration (Table 1).
Table 1. Characteristics of Patients with ST-segment Elevation Myocardial Infarction by Year Groups: The ARIC Study - Community Surveillance, 1987-2008.
| Year Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1987- 1988 (n 420)* |
1989- 1990 (n 474)* |
1991- 1992 (n 548)* |
1993- 1994 (n 630)* |
1995- 1996 (n 599)* |
1997- 1998 (n 516)* |
1999- 2000 (n 437)* |
2001- 2002 (n 290)* |
2003- 2004 (n 240)* |
2005- 2006 (n 334)* |
2007- 2008 (n 357)* |
Annual % change (95% CI) |
| Age (years) | 58.8 | 58.0 | 57.8 | 58.4 | 58.4 | 57.9 | 58.6 | 57.6 | 58.1 | 58.7 | 56.8 | -0.1 (-0.2, 0.1) |
| Women | 30.3% | 30.3% | 31.4% | 29.3% | 32.2% | 30.5% | 33.7% | 30.0% | 35.5% | 26.6% | 36.9% | 0.5 (-0.5, 1.5) |
| Black | 11.8% | 12.0% | 14.2% | 20.8% | 21.7% | 27.4% | 23.6% | 21.5% | 25.1% | 25.5% | 36.3% | 4.6 (3.3, 5.9) |
| Age > 65 years | 27.8% | 25.7% | 30.4% | 30.6% | 32.0% | 28.2% | 33.2% | 27.4% | 30.3% | 25.2% | 19.8% | -0.7 (-1.8, 0.3) |
| History of Hypertension | 48.7% | 50.7% | 48.1% | 53.9% | 51.1% | 56.7% | 58.7% | 53.4% | 59.0% | 54.2% | 62.3% | 1.1 (0.4, 1.7) |
| Diabetes Mellitus† | NA | NA | NA | 21.9% | 25.8% | 26.8% | 23.7% | 24.0% | 25.5% | 26.6% | 26.3% | 0.8 (-1.1, 2.6) |
| PCI within 24hrs† | NA | NA | NA | 15.6% | 20.8% | 23.3% | 31.3% | 52.7% | 63.2% | 59.8% | 53.9% | 9.9 (7.7, 12.1) |
| CABG within 24hrs† | NA | NA | NA | 2.3% | 4.1% | 2.0% | 2.0% | 0.8% | 0.9% | 0.0% | 0.7% | -14.0 (-20.6, -7.3) |
| Thrombolytic therapy | 39.2% | 52.7% | 60.0% | 54.0% | 41.4% | 39.0% | 32.6% | 29.4% | 8.5% | 1.7% | 2.4% | -8.7 (-9.4, -8.0) |
Abbreviations: STEMI, ST-elevation myocardial infarction. ARIC, Atherosclerosis Risk in Communities. CI, confidence interval. PCI, percutaneous intervention. CABG, coronary artery bypass graft
Characteristics presented by mean or percentage
Weighted number of incident, unadjusted hospitalized STEMI per year group
Data on diabetes, PCI or CABG were unavailable prior to 1993.
Overall for all years combined, 37.2% of STEMIs were inferior; 32.8% anterior; 16.8% occurred in multiple infarct locations; and 13.2% were lateral. There were differences in age and race by STEMI infarct location (Table 2).
Table 2. Characteristics by ST-segment Elevation Myocardial Infarct Location The ARIC Study – Community Surveillance, 1987-2008.
| Variable | Inferior (n 1804)* | Anterior (n 1590)* | Lateral (n 640)* | Multiple (n 813)* | P-value† |
|---|---|---|---|---|---|
| Age (years) | 58.6 (0.3) | 58.3 (0.4) | 57.9 (0.5) | 56.9 (0.5) | 0.03 |
| Women | 34.0% (1.3) | 29.8% (1.6) | 28.3% (1.9) | 31.5% (2.3) | 0.06 |
| Black | 12.4% (0.9) | 31.3% (1.8) | 23.7% (2.1) | 17.5% (2.0) | <0.01 |
| Center | |||||
| Jackson, MS | 17.0% (1.1) | 25.5% (1.5) | 21.4% (1.8) | 23.7% (2.3) | <0.01 |
| Forsyth Co., NC | 41.4% (1.4) | 43.3% (1.8) | 40.5% (2.3) | 36.6% (2.4) | |
| Minneapolis, MN | 24.2% (1.5) | 17.4% (1.2) | 21.8% (1.8) | 24.8% (2.0) | |
| Washington Co., MD | 17.5% (1.0) | 13.8% (1.0) | 16.3% (1.5) | 14.9% (1.6) |
Abbreviations: STEMI, ST elevation myocardial infarction.
Numbers in parentheses are standard error for estimates
Weighted number of incident, unadjusted hospitalized STEMI by infarct location.
P-value for differences in characteristics across STEMl locations using Wald test.
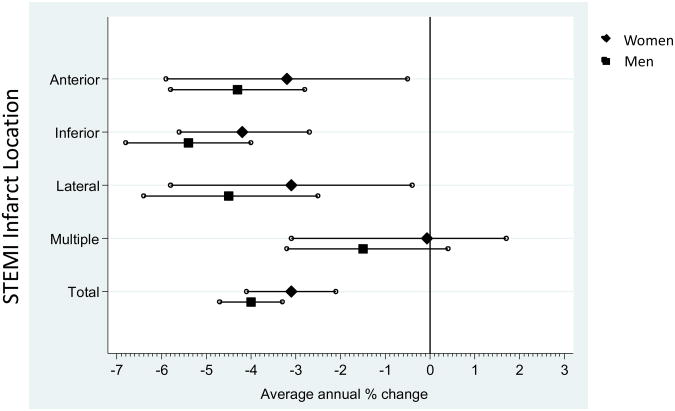
For all STEMI infarct locations combined, from 1987 through 2008 there was an age- and race-adjusted average annual decrease in STEMI incidence rates in both men and women for inferior, anterior and lateral STEMI (Table 3 and Figure 1). The decline was most notable for inferior STEMI in men (Table 3 and Figure 2). In contrast, there was no substantial change in the rates of STEMI in multiple infarct locations for either men or women. A quadratic trend was observed in anterior STEMIs for both genders; trends in the remaining locations and genders were linear with a statistically significant difference between the inferior and multiple location trends in both genders.
Table 3. Average annual percent change (95% confidence interval) in event rates (per 10,000 persons) of STEMI by infarct location, adjusted for age and race. The ARIC Study – Community Surveillance, 1987-2008.
| Women | Men | |||
|---|---|---|---|---|
|
| ||||
| STEMI location | Avg. annual % change (95% C.I.) | P-value† | Avg. annual % change (95% C.I.) | P-value† |
| Anterior | -3.2 (-5.9, -0.5)† | 0.10‡ | -4.3 (-5.8, -2.8)† | <0.001‡ |
| Inferior | -4.2 (-5.6, -2.7) | <0.001 | -5.4 (-6.8, -4.0) | <0.001 |
| Lateral | -3.1 (-5.8, -0.4) | 0.03 | -4.5 (-6.4, -2.5) | <0.001 |
| Multiple | -0.7 (-3.1, 1.7) | 0.55 | -1.5 (-3.2, 0.4) | 0.13 |
|
| ||||
| Total | -3.1 (-4.1, -2.1) | <0.01 | -4.0 (-4.7, -3.3) | <0.01 |
Abbreviations: STEMI, ST elevation myocardial infarction
Negative numbers indicate a decrease in incidence rates
P-value from Wald test of hypothesis that average annual percent change is zero.
Average annual percent change and P-value from a quadratic regression model. All other estimates and P-values from a linear regression model using a Wald test.
Figure 1. Age- and race-adjusted Average Annual Percent Change of STEMI by Infarct Location, stratified by gender: The ARIC Study – Community Surveillance, 1987-2008.
Key:
Abbreviations: STEMI, ST elevation myocardial infarction
Figure 2. Age- and race-adjusted Annual Rates (per 10,000 persons) of STEMI by Infarct Location, stratified by gender: The ARIC Study – Community Surveillance, 1987-2008.
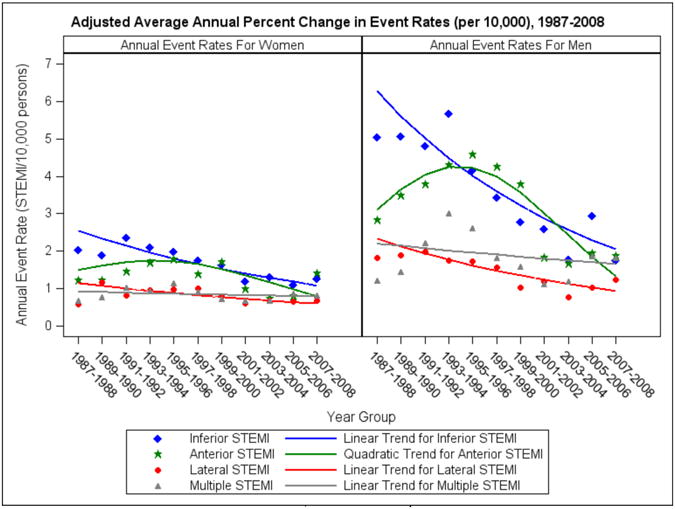
Key:
Abbreviations: STEMI, ST elevation myocardial infarction
Overall, the 28-day age- and gender-adjusted case fatality for STEMI decreased from 8.9% (95% CI 7.5, 10.1) in 1987-1996 to 5.4% (95% CI 4.1, 6.6) in 1997-2007. Changes in age-and gender-adjusted 28-day case fatality from 1987-96 to 1997-2007 differed significantly among STEMI locations (P value = 0.041). The decline in age and gender-adjusted 28-day case fatality from 1987-96 to 1997-2007 was statistically significant for anterior, inferior and lateral STEMI (Table 4). In contrast, no significant change in 28-day case fatality for multi-location STEMI was observed.
Table 4. Age- and gender- adjusted 28-day Case Fatality by STEMI Infarct Location The ARIC Study – Community Surveillance, 1987-2007*.
| Year Group | ||||
|---|---|---|---|---|
|
| ||||
| STEMI infarct location | 1987-1996 | 1997-2007 | All years | P-value† |
| Anterior | 11.0 (7.9, 14.1) | 5.3 (3.1, 7.5) | 8.5 (6.5, 10.5) | <0.01 |
| Inferior | 6.0 (4.5, 7.5) | 3.3 (1.9, 4.8) | 5.0 (3.9, 6.0) | 0.02 |
| Lateral | 14.8 (8.8, 20.7) | 5.9 (2.9, 9.0) | 11.0 (7.2, 14.8) | <0.01 |
| Multiple | 7.7 (5.1, 10.3) | 9.1 (5.3, 12.9) | 8.4 (6.1, 10.6) | 0.54 |
|
| ||||
| Total | 8.9 (7.5, 10.4) | 5.4 (4.2, 6.6) | 7.4 (6.5, 8.5) | <0.001 |
Abbreviations: STEMI, ST elevation myocardial infarction
Models also adjusted for gender. Data was complete through 2007.
P-value for comparison of 1987-1996 to 1997-2007 by Wald test
Discussion
The principle findings of this investigation are that between 1987 and 2008 (1) the rate and 28-day case fatality for inferior, anterior and lateral STEMI declined while (2) the rate and 28-day case fatality for multi-location STEMI did not change. By 2008, multi-location STEMI was the second most common and had the highest 28-day case fatality. The difference in rates and outcomes by anatomic location may relate to the distribution of CAD risk factors in the ARIC study population. Obesity, diabetes and hypertension have remained prevalent in the United States19,20 and the age at first MI has increased.21 Older age, diabetes and hypertension are risk factors for multi-vessel CAD,22,23 and may increase risk for multi-location STEMI.
There are several potential explanations as to why the 28-day case fatality for multi-location STEMI did not improve. First, infarct size is an independent predictor of post-MI prognosis 24 and is likely greater for multi-location versus single anatomic location STEMI. Second, multi-location STEMI has been associated with multi-vessel CAD and poor prognosis after an MI.25 Third, percutaneous revascularization may be more difficult in patients with multi-vessel CAD and multi-location STEMI.26 Lastly, incomplete revascularization has been associated with poor post-MI prognosis 27 and may be more common in patients with multi-location STEMI and multi-vessel CAD.
As in other studies,28 we observed a shift in STEMI revascularization strategies over time from urgent CABG and thrombolytics towards the widespread use of PCI. However, the increase in PCI was not associated with improved 28-day case fatality for multi-location STEMI. This finding may have implications for the revascularization of patients with multi-vessel CAD, including patients with multi-location STEMI. 34
Strengths of our study include the population-based design; the inclusion of African-Americans from multiple communities in the United States; and the standardized validation of events and ECG coding. To our knowledge, no other study has used surveillance ECG data to detail trends in rates of STEMI by anatomic location.
The main imitation of the current study is the imprecise nature of the surface ECG to localize infarct location.30 However, the ECG lead-groups used represent large regions of myocardium with prognostic value,3 and are more accurate than administrative codes of STEMI location.4 While multi-location STEMI may indicate multi-vessel CAD, multi-location STEMI could arise from proximal CAD or from a coronary artery subtending multiple regions of myocardium. Since STEMI diagnosis is much less biomarker dependent than NSTEMI,4,5 the lack of biomarker adjustment in our study is unlikely to have significantly affected our findings. We do not have information on STEMI infarct location for patients who died before reaching a hospital in the ARIC surveillance study. However, sudden cardiac death as an initial manifestation of CAD has decreased over time,29 and is therefore unlikely to have altered our findings.
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
Funding & Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01- HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone PH, Raabe DS, Jaffe AS, Gustafson N, Muller JE, Turi ZG, Rutherford JD, Poole WK, Passamani E, Willerson JT, Sobel BE, Roberston T, Braunwald E. Prognostic significance of location and type of myocardial infarction: independent adverse outcome associated with anterior location. J Am Coll Cardiol. 1988;11:453–463. doi: 10.1016/0735-1097(88)91517-3. [DOI] [PubMed] [Google Scholar]
- 3.Welty FK, Mittleman MA, Lewis SM, Healy RW, Shubrooks SJ, Jr, Muller JE. Significance of location (anterior versus inferior) and type (Q-wave versus non-Q-wave) of acute myocardial infarction in patients undergoing percutaneous transluminal coronary angioplasty for postinfarction ischemia. Am J Cardiol. 1995;76:431–435. doi: 10.1016/s0002-9149(99)80125-8. [DOI] [PubMed] [Google Scholar]
- 4.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-Two Year Trends in Incidence of Myocardial Infarction, CHD Mortality, and Case-Fatality in Four US Communities, 1987 to 2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC., Jr Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119:503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 7.Krumholz HM, Anderson JL, Brooks NH, Fesmire FM, Lambrew CT, Landrum MB, Weaver WD, Whyte J, Bonow RO, Bennett SJ, Burke G, Eagle KA, Linderbaum J, Masoudi FA, Normand SL, Pina IL, Radford MJ, Rumsfeld JS, Ritchie JL, Spertus JA. ACC/AHA clinical performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures on ST-Elevation and Non-ST-Elevation Myocardial Infarction) J Am Coll Cardiol. 2006;47:236–265. doi: 10.1016/j.jacc.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348:933–940. doi: 10.1056/NEJMra022700. [DOI] [PubMed] [Google Scholar]
- 9.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 10.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 11.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Rosamond WD, Chambless LE, Sorlie PD, Bell EM, Weitzman S, Smith JC, Folsom AR. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987-2000. Am J Epidemiol. 2004;160:1137–1146. doi: 10.1093/aje/kwh341. [DOI] [PubMed] [Google Scholar]
- 13.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 14.Edlavitch SA, Crow R, Burke GL, Huber J, Prineas R, Blackburn H. The effect of the number of electrocardiograms analyzed on cardiovascular disease surveillance: the Minnesota Heart Survey (MHS) J Clin Epidemiol. 1990;43:93–99. doi: 10.1016/0895-4356(90)90061-s. [DOI] [PubMed] [Google Scholar]
- 15.Edlavitch SA, Crow R, Burke GL, Baxter J. Secular trends in Q wave and non-Q wave acute myocardial infarction. The Minnesota Heart Survey. Circulation. 1991;83:492–503. doi: 10.1161/01.cir.83.2.492. [DOI] [PubMed] [Google Scholar]
- 16.Wagner GS, Macfarlane P, Wellens H, Josephson M, Gorgels A, Mirvis DM, Pahlm O, Surawicz B, Kligfield P, Childers R, Gettes LS. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:1003–1011. doi: 10.1016/j.jacc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Menown IB, Adgey AA. Improving the ECG classification of inferior and lateral myocardial infarction by inversion of lead aVR. Heart. 2000;83:657–660. doi: 10.1136/heart.83.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sgarbossa EB, Birnbaum Y, Parrillo JE. Electrocardiographic diagnosis of acute myocardial infarction: Current concepts for the clinician. Am Heart J. 2001;141:507–517. doi: 10.1067/mhj.2001.113571. [DOI] [PubMed] [Google Scholar]
- 19.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. Jama. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 20.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg RJ, Spencer FA, Yarzebski J, Lessard D, Gore JM, Alpert JS, Dalen JE. A 25-year perspective into the changing landscape of patients hospitalized with acute myocardial infarction (the Worcester Heart Attack Study) Am J Cardiol. 2004;94:1373–1378. doi: 10.1016/j.amjcard.2004.07.142. [DOI] [PubMed] [Google Scholar]
- 22.Rioufol G, Finet G, Ginon I, Andre-Fouet X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation. 2002;106:804–808. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 23.Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, Pollack CV, Jr, Gore JM, Chandra-Strobos N, Peterson ED, French WJ. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Hands ME, Lloyd BL, Robinson JS, de Klerk N, Thompson PL. Prognostic significance of electrocardiographic site of infarction after correction for enzymatic size of infarction. Circulation. 1986;73:885–891. doi: 10.1161/01.cir.73.5.885. [DOI] [PubMed] [Google Scholar]
- 25.Niles NW, McGrath PD, Malenka D, Quinton H, Wennberg D, Shubrooks SJ, Tryzelaar JF, Clough R, Hearne MJ, Hernandez F, Jr, Watkins MW, O'Connor GT. Survival of patients with diabetes and multivessel coronary artery disease after surgical or percutaneous coronary revascularization: results of a large regional prospective study. Northern New England Cardiovascular Disease Study Group. J Am Coll Cardiol. 2001;37:1008–1015. doi: 10.1016/s0735-1097(00)01205-5. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Lennon RJ, Holmes DR, Jr, Bell MR, Rihal CS. Correlates of procedural complications and a simple integer risk score for percutaneous coronary intervention. J Am Coll Cardiol. 2002;40:387–393. doi: 10.1016/s0735-1097(02)01980-0. [DOI] [PubMed] [Google Scholar]
- 27.Rosner GF, Kirtane AJ, Genereux P, Lansky AJ, Cristea E, Gersh BJ, Weisz G, Parise H, Fahy M, Mehran R, Stone GW. Impact of the Presence and Extent of Incomplete Angiographic Revascularization After Percutaneous Coronary Intervention in Acute Coronary Syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) Trial. Circulation. 2012;125:2613–2620. doi: 10.1161/CIRCULATIONAHA.111.069237. [DOI] [PubMed] [Google Scholar]
- 28.Gerber Y, Rihal CS, Sundt TM, 3rd, Killian JM, Weston SA, Therneau TM, Roger VL. Coronary revascularization in the community. A population-based study, 1990 to 2004. J Am Coll Cardiol. 2007;50:1223–1229. doi: 10.1016/j.jacc.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Ni H, Coady S, Rosamond W, Folsom AR, Chambless L, Russell SD, Sorlie PD. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;157:46–52. doi: 10.1016/j.ahj.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]