Abstract
The aim of this study was to examine the relationship between the risk of amyotrophic lateral sclerosis (ALS) and exposure to rural environments. Studies were identified through OVID MEDLINE and EMBASE search up to September 2013 using as keywords rural residence, farmers, and pesticide exposure. Twenty-two studies were included for this meta-analysis. Summary odds ratios (ORs) were calculated using random effect model by type of exposure index, and subgroup analyses were conducted according to study design, gender, region, case ascertainment, and exposure assessment. The risk of ALS was significantly increased with pesticide exposure (OR, 1.44; 95% CI, 1.22-1.70) and with farmers (OR, 1.42; 95% CI, 1.17-1.73), but was not significant with rural residence (OR, 1.25; 95% CI, 0.84-1.87). The risk estimates for subgroup analysis between pesticide exposure and ALS indicated a significant positive association with men (OR, 1.96), and in studies using El Escorial criteria for ALS definition (OR, 1.63) and expert judgment for pesticide exposure (OR, 2.04) as well. No significant publication bias was observed. Our findings support the association of pesticide exposure and an increased risk for ALS, stressing that the use of more specific exposure information resulted in more significant associations.
Keywords: Agriculture, Environmental Exposure, Farming, Meta-analysis, Neurodegenerative Diseases, Occupations
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive degenerative neurological disease. About 5%-10% of ALS is familial, with the remaining 90% of people diagnosed with ALS being classified as having sporadic disease (1). Although several environmental risk factors have been considered, the causes of ALS are largely unknown. The association between ALS and exposure to neurotoxic chemicals, such as solvents, pesticides and metals, has been investigated in several epidemiologic studies with inconsistent results (2, 3).
Pesticides are known to be important risk factors for ALS and other neurodegenerative diseases such as Parkinson's and Alzheimer's (4). Although the biologic mechanisms contributing to risk of ALS associated with exposure to pesticides are unknown, many pesticides are considered as potential neurotoxins in various ways (5). In addition, an increased ALS risk with pesticide exposure was also reported from alteration of paraoxonase 1 function, which detoxifies organophosphates (6). Recently, a case of ALS was reported in Korea involving a worker at a waste disposal site who had crushed glass pesticide bottles for for 15 yr (7).
Previous epidemiologic studies, however, have produced inconsistent results when examining the association of pesticides and ALS. Exposure to pesticides has been reported to be associated with ALS risk in some investigations (8, 9, 10), but others have found no relationship (3, 11, 12, 13, 14). One possible explanation for these inconsistencies may be low statistical power since the number of cases available for study is typically limited in the case of rare diseases such as ALS. Therefore, two meta-analyses with regard to pesticide exposure and ALS have been conducted to date (15, 16). Kamel et al. (15) showed that occupational exposure to pesticides as a group significantly increased (about two-fold) the risk for ALS. Another study by Malek et al. (16) also reported a roughly two-fold increase in risk of ALS among men, but not among women.
Previous meta-analyses, however, only included studies of pesticide exposure but excluded studies based on job title such as a farmer or those based on living on a farm, both of which would be used as important surrogate indices for pesticide exposure. Since rural residence or aspects of agricultural activity other than pesticide use may also serve as a potential risk factor for ALS (13, 17, 18, 19, 20), it is important to investigate the risk of ALS with overall environments, from residence in rural area to pesticide exposure.
The objectives of this meta-analysis, therefore, were to investigate the overall scope of exposure to pesticides and rural environments with the risk of ALS by including studies for broad categories of exposure assessment categories such as rural residence, farmers, and pesticide exposure.
MATERIALS AND METHODS
Search strategy and selection criteria
We conducted systematic reviews for rural residence, farmers, and pesticide exposure according to the MOOSE guidelines (21). A search was performed in OVID MEDLINE and EMBASE up to September 2013 using the medical subject headings (MeSH). The search terms for ALS included 'motor neurone disease', 'amyotrophic lateral sclerosis', 'Lou Gehrig disease', 'Charcot disease'. These were combined with search terms for the exposure which included 'agrochemicals', 'pesticide', 'organophosphorus compounds', 'insecticides', 'cholinesterase inhibitors', 'herbicides', 'paraquat', 'gramoxone', 'fungicide', 'agriculture', 'occupational exposure', 'farmer', 'farmworker', 'rural residence', 'rural environment'. We also scanned the bibliographies of relevant articles in order to identify additional studies.
Studies included in our analysis were selected based on the following inclusion criteria: 1) peer-reviewed cohort or case-control studies; 2) studies which investigated the association between rural residence, farmers or pesticide exposure and amyotrophic lateral sclerosis; 3) reported outcome measures with odds ratio (OR) or relative risk (RR) for ALS, or that provided the number of individuals; and 4) written in English. Review articles, case reports, case-series, letters to editors, commentaries, proceedings, laboratory science studies, and any non-relevant tudies were excluded from analysis.
Study identification and data extraction
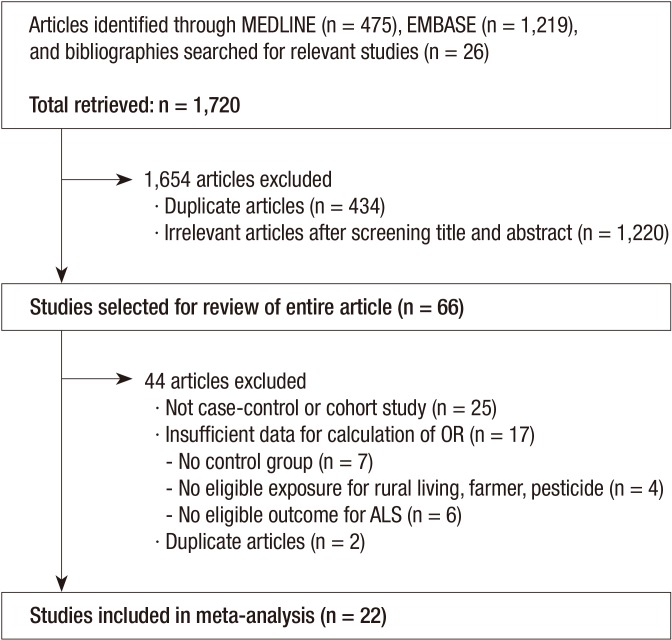
As shown in Fig. 1, a total of 1,720 articles were obtained after searching databases and references and performing a hand-search. After excluding the duplicates (n=434), the remaining articles were reviewed (n=1,286) and 1,220 articles were excluded for not meeting the selection criteria. The remaining 66 articles were selected for review of their entire content. Among them, 44 were excluded for the following reasons: 25 were not case-control or cohort studies, 17 provided insufficient data; no control group (n=7), no eligible exposure for rural living, farmer, pesticide (n=4), and no eligible outcome for ALS (n=6), and two were duplicate articles. Therefore, a total of 22 studies were included in our meta-analysis (3, 8, 9, 11, 12, 13, 14, 15, 17, 18, 19, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31). When the study samples were overlapped in two or more articles, we selected the article with the most comprehensive population. Based on the predetermined selection criteria, two of the authors independently selected all trials retrieved from the databases and bibliographies. Disagreements between evaluators were resolved through discussion or in consultation with a third author. In the case of insufficient or missing data, they were derived either from the text or tables or, when possible, calculated from the relevant data within the study.
Fig. 1.
Identification and selection of studies included in this meta-analyses.
Standardized data extraction forms were used to extract the following data from the studies included in the final analysis: name of the first author with year of publication, journal name, country where the study was conducted, study design, diagnostic criteria, definition of rural residence, farmer, and pesticide exposure, adjusted factors, number of cases/controls or cohort participants, and RR or OR with 95% confidence intervals (CIs).
Case ascertainment was based on using or not using El Escorial criteria for diagnosis of ALS. The World Federation of Neurology developed the El Escorial diagnostic criteria for ALS, which have proven to be accurate in diagnosis of ALS using pathology as a 'gold standard' and represents them as universal guidelines for the diagnosis of ALS (32). Exposure assessment methods were summarized into two categories (i.e., self-reported vs. expert judgement).
Statistical analysis
Overall pooled estimates and their corresponding 95% CI were obtained using DerSimonian and Laird random effects models (33). If the article reported stratified estimates, the strata were combined and the crude OR was recalculated (22, 26, 27, 31). Available raw data were used in a 2×2 table to calculate the OR and 95% CI in a study (20). As the incidence of ALS is low (i.e., 1-3 per 100,000 persons per year), we assumed odds ratio to be equal to relative risk. We conducted meta-analyses stratified by rural residence, farmers, and pesticide exposure separately. Subgroup analyses were performed according to the following characteristics: 1) study design, 2) region (Europe, the USA, and others including Australia and India), 3) gender, 4) case ascertainment (El Escorial criteria or not), 5) exposure assessment (self-reported or expert judgment).
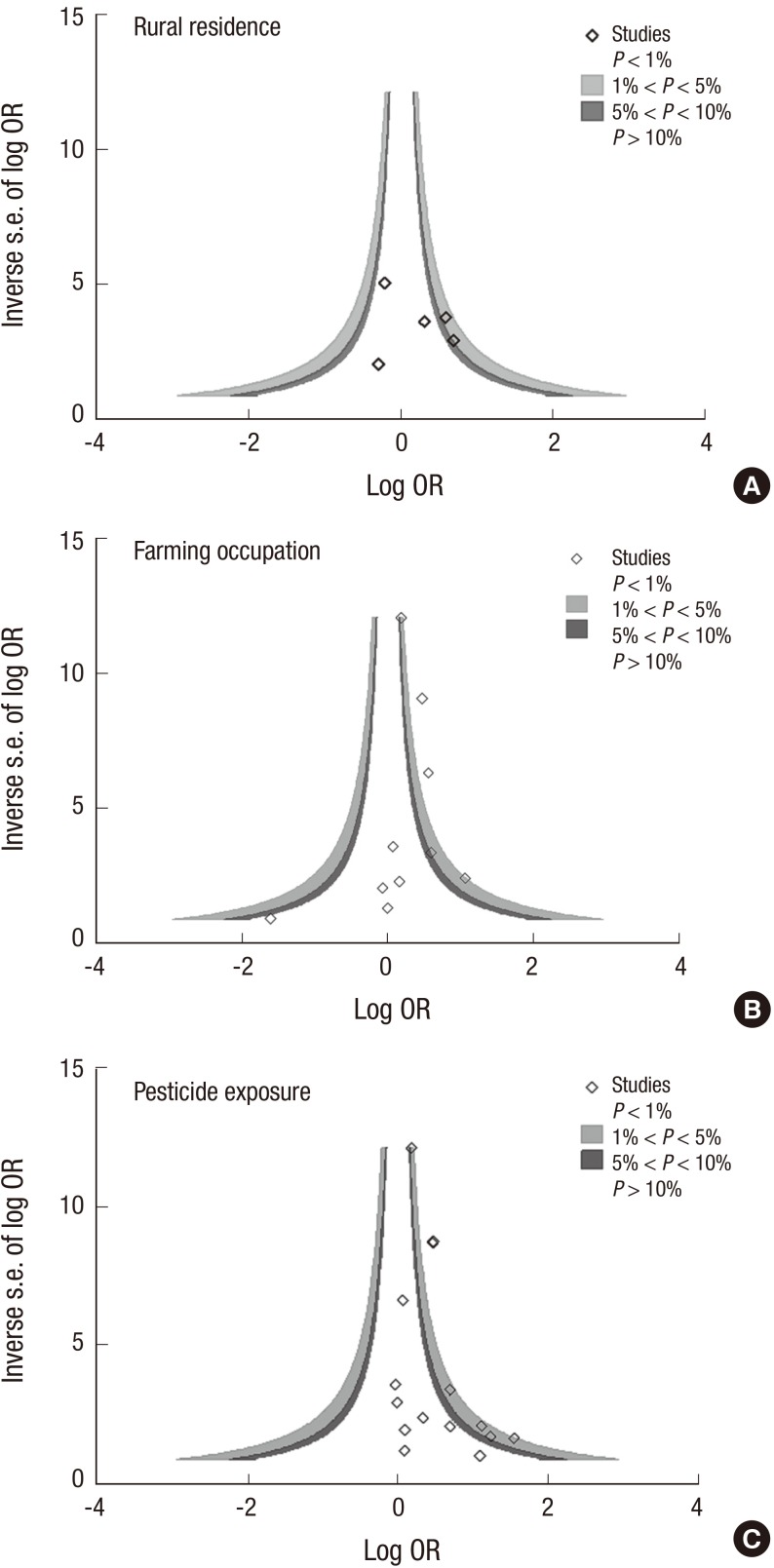
Between-study heterogeneity was assessed using the Cochran's Q and Higgins' I2 statistics. Q-statistic P value of <0.1 was considered statistically significant and I2 of 25, 50, or 75 indicates low, medium, or high heterogeneity, respectively (34). We estimated publication bias by using Begg's funnel plot (35) and Egger's test (36). In addition, contour-enhanced funnel plots were performed in order to aid the interpretation of the funnel plot. Although publication bias for pesticide exposure was not significant for Egger's test (P=0.09), asymmetry in the funnel plot was observed and trim and fill analyses were therefore performed (37). We used the Stata SE version 12.0 software package for statistical analysis (StataCorp, College Station, TX, USA).
Ethics statement
This study analyzed publicly available data, and thus protocol review was unnecessary.
RESULTS
The study included a total of 19 case-control studies (8, 9, 11, 12, 13, 14, 17, 18, 19, 20, 22, 23, 25, 26, 27, 28, 29, 30, 31) and three cohort studies (3, 15, 24) (Table 1). The studies were conducted mainly in Europe or the USA, with the exception of one Indian and one Australian study. Among the total studies, three (13, 17, 18) had data for rural residence, farming occupation, and pesticide exposure and two (19, 26) included data for farming occupation and pesticide exposure. Thus, a total of 30 risk estimates were used for meta-analyses. They include five case-control studies for rural residence (13, 17, 18, 23, 25), ten case-control studies for farming occupation (13, 17, 18, 19, 20, 26, 27, 29, 30, 31), and 15 studies for pesticide exposure; three cohorts (3, 15, 24) and 12 case-control studies (8, 9, 11, 12, 13, 14, 17, 18, 19, 22, 26, 28). El Escorial criteria were used in six studies (8, 17, 18, 22, 26, 27) and pesticide exposure was defined by expert judgment in four studies (8, 9, 19, 24).
Table 1.
Characteristics of studies included in the meta-analysis by type of exposure index
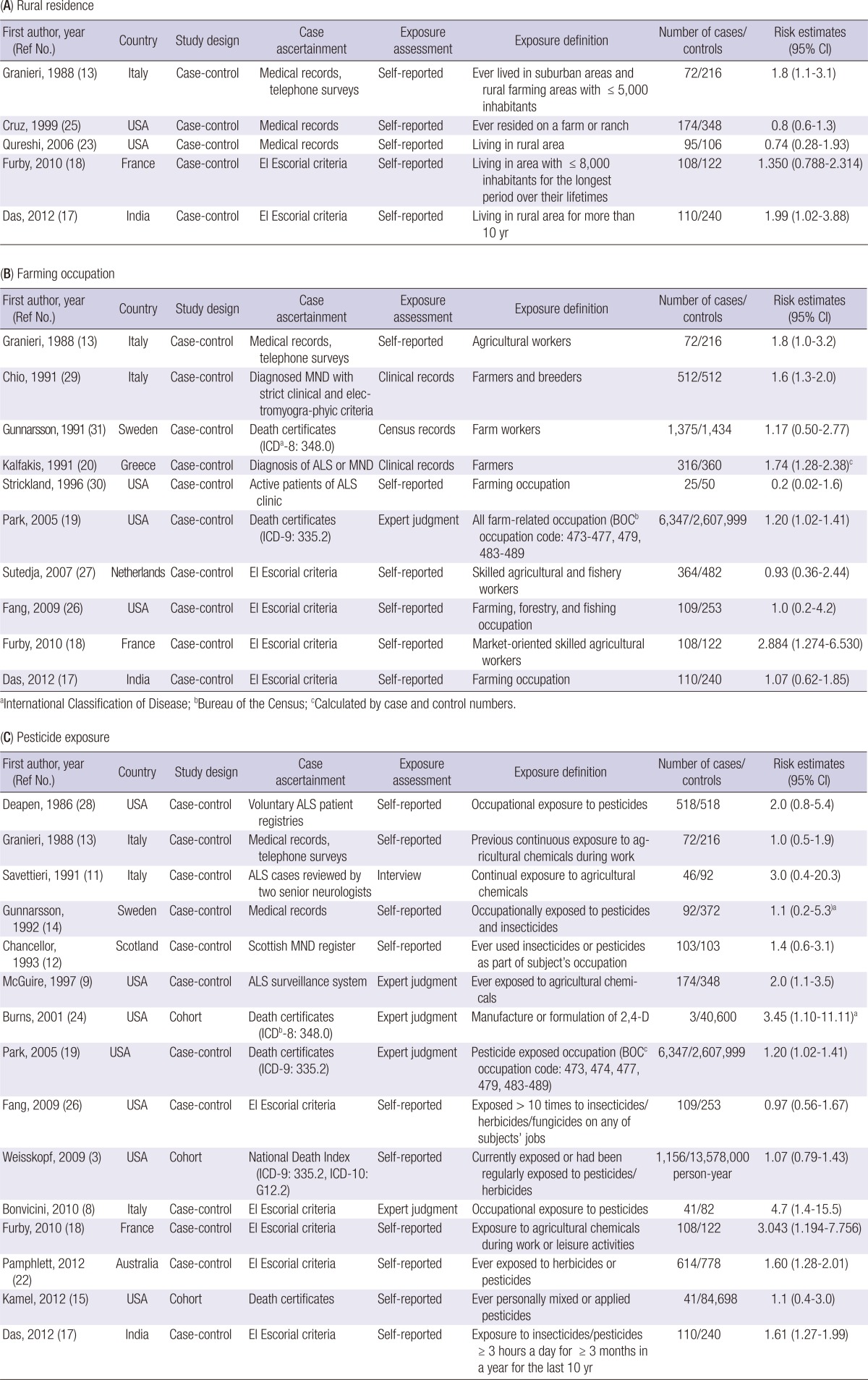
aRisk estimates among males only; bInternational Classification of Disease; cBureau of the Census.
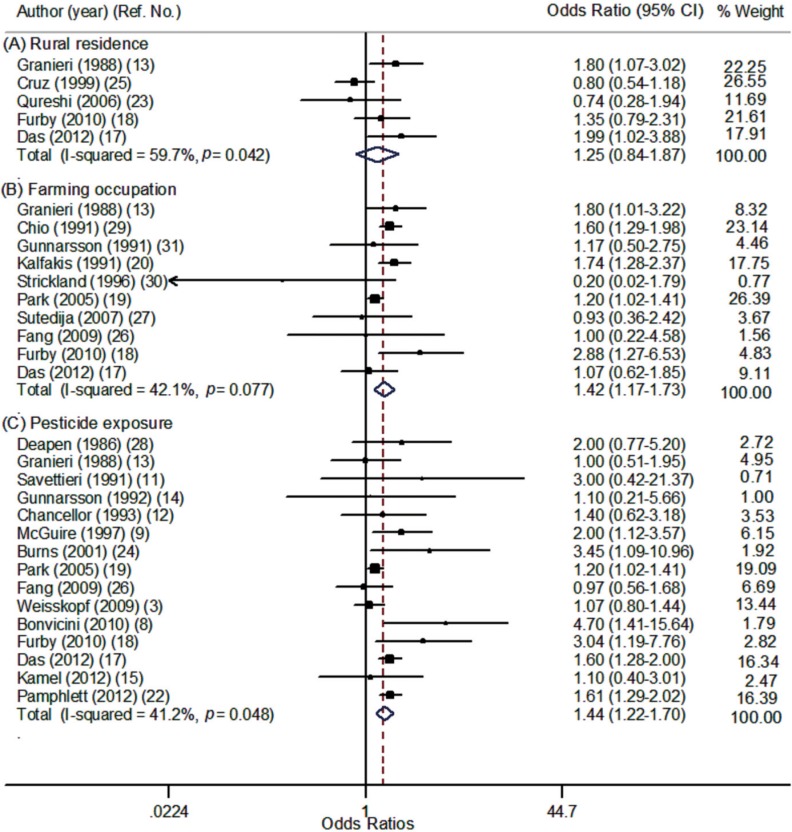
The risk of ALS was significantly increased with pesticide exposure (OR, 1.44; 95% CI, 1.22-1.70) and with farming occupation (OR, 1.42; 95% CI, 1.17-1.73), but was not significant for rural residence (Table 2). Individual estimates from 22 studies and their overall pooled ORs for rural residence, farming occupation, and pesticide exposure are presented in the forest plot separately in Fig. 2. In subgroup analysis, pesticide exposure showed a significantly increased risk of ALS for studies with case-control design (OR, 1.49), among males (OR, 1.96) and with applied expert judgment exposure assessment (OR, 2.04). Results of the Q test and I2 statistics were significantly heterogeneous for total studies but not significant when sub-group analyses were conducted by region or gender. No evidence of publication bias was observed for all three exposure indices, but the plot was a slightly asymmetric in contour-enhanced funnel plot at pesticide exposure index (Fig. 3). After trim and fill analyses, ORs for pesticide exposure were still significant (OR, 1.40; 95% CI, 1.10-1.79) (data not shown).
Table 2.
Summary estimates and heterogeneity for meta-analyses of studies by type of exposure index and subgroups
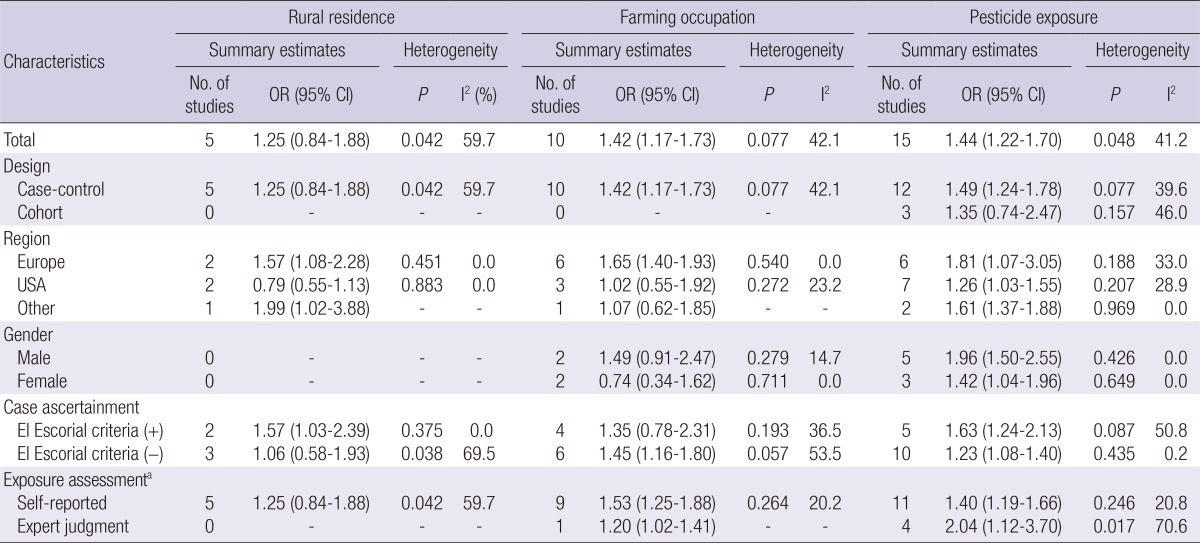
aSelf-reported includes response through interview or questionnaire and records from census or hospital data. Expert judgment means evaluating the status of exposure or subjects' occupation by panel based on subjects' occupational history or census records.
Fig. 2.
Forest plot according to type of exposure index: (A) Rural residence, (B) Farming occupation, (C) Pesticide exposure.
Fig. 3.
Contour-enhanced funnel plot according to type of exposure index: (A) Rural residence, (B) Farming occupation, (C) Pesticide exposure.
DISCUSSION
Our findings from the meta-analysis support an association between pesticide exposure and ALS. The increased risks of ALS were consistent by study deign, country, gender, ALS definition, and type of exposure assessment. However, the risk for ALS was not significantly increased by rural residence. The estimates for ALS had a tendency to be significant as the order of accuracy for pesticide exposure indicators, and the risks were higher in men, in studies using El Escorial criteria and in those using expert judgment, compared to their counterparts. The lack of significant evidence of publication bias supports the robustness of our conclusions.
Our results were consistent with previously published meta-analyses, which reported an association between pesticide exposure and ALS (15, 16). However, our increased risk both men and women were different with a previous study (16) which reported that the significant association was found only among men. In addition, our results showed that the ORs for ALS became significant in the order of rural residence, farmer, and pesticide exposure, which was the order of accuracy for pesticide exposure indicators, and statistical significance was found both for farming occupation and pesticide exposure. The information on rural residence is a crude measure of pesticide exposure because not all rural residents are farmers nor are exposed to farming, and not all farmers actually use pesticides (38). Rural residents may also be exposed to physical, chemical or biological factors other than pesticides. Since rural residence or farmer are a wider category of exposure than pesticide, using rural living or farmer as an indicator for pesticide exposure may underestimate the risk of association with pesticide exposure. Similarly, no significant association between childhood leukemia and parental occupational exposure was observed when farming/agricultural work was used as a surrogate for pesticide exposure, whereas significantly increased risks were observed when specific use of pesticides by the parents was considered (39).
When using El Escorial criteria for case ascertainment, ORs for ALS tend to be higher than when not used. This may be explained by the assertion that clarifying case ascertainment by using El Escorial criteria allows greater precision in the diagnosis of ALS which may impact these effects. Similarly, the OR for ALS was higher when expert judgement was used for exposure assessment compared to self-reported interviews or questionnaires. Although self-reported information may provide detailed data at the individual level, exposure misclassification from recall bias or reliability issues are always of concern. Expert judgment by using job title and occupational history creates greater precision in exposure assessment, despite the fact that it might also result in non-differential exposure misclassification (40). Therefore, our findings may stress the importance of using more objective information for defining disease and exposure in epidemiologic studies.
Men had a higher risk for ALS than women in regard to pesticide exposure, although the confidence intervals were overlapped. This discrepancy by gender was also observed in a previous meta-analysis (16) and a study from India (17). This gender discrepancy included in these studies may partly be explained as men having different features of occupational exposure to pesticides, for example their being exposed to pesticide more frequently or in larger amounts when they use pesticide, or being influenced by sex-related factors.
The possible factors leading to the different risks of ALS and pesticide exposure among countries may include differences in the amounts, pattern, and methods of pesticide use, as well as by genetics. We have considered all pesticides as a single exposure category although pesticides include many different chemicals. However, few studies investigated individual pesticides, their exposure duration or intensity; therefore, we were not able to do subgroup analysis on these issues. Possible interaction among pesticides and genetic factors may also potentiate the different results from countries. Future studies with more detailed information on pesticide use and other potential risk factors are needed to clarify this issue. Further studies for non-occupational pesticide exposure are also needed to describe the full scope of association with pesticide exposure and ALS.
This study has other important limitations. First, the original studies included in this meta-analysis had adjusted for limited potential confounding factors. However, majority of studies included cases and controls of similar demographic characteristics, thus the association between ALS and exposures would not be expected substantially to change by uncontrolled confounding factors. Subgroup analyses by confounding factors were also limited due to few studies adjusted for same factors. Second, publication bias might influence the results of meta-analysis. However, the present analysis did not appear to be hampered by publication bias, since there was no evidence of publication bias as observed by Begg's funnel plot and Egger's test. Duval and Tweedie's trim and fill analyses also provided an adjusted estimate of the effect of pesticide exposure on ALS and revealed that there was still significant risk of ALS with pesticide exposure (37). Heterogeneity may be inevitable in meta-analysis, but sub-group analyses showed consistent positive associations with pesticide exposure and the risk of ALS.
Despite some limitation in terms of detailed information on pesticide exposure, our findings from the meta-analysis of 19 case-control and three cohort studies support an association between pesticide exposure and ALS, but not for rural residence. These meta-analyses present overall scopes of categories for exposure to pesticide and/or rural environment assessment such as rural residence, farming occupation, and pesticide exposure, which help us to more comprehensively understand the relation between ALS and exposure to pesticides. Considering that farmers are commonly frequently exposed to pesticides at a high level, it is important to recommend lowering exposure to pesticides in order to reduce the risk of development of ALS.
Footnotes
All authors declare that they have no conflicts of interest to disclose.
References
- 1.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Wicklund MP. Amyotrophic lateral sclerosis: what role does environment play? Neurol Clin. 2011;29:689–711. doi: 10.1016/j.ncl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Weisskopf MG, Morozova N, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Ascherio A. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:558–561. doi: 10.1136/jnnp.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrón T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol. 2011;256:379–385. doi: 10.1016/j.taap.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Keifer MC, Firestone J. Neurotoxicity of pesticides. J Agromedicine. 2007;12:17–25. doi: 10.1300/J096v12n01_03. [DOI] [PubMed] [Google Scholar]
- 6.Morahan JM, Yu B, Trent RJ, Pamphlett R. A gene-environment study of the paraoxonase 1 gene and pesticides in amyotrophic lateral sclerosis. Neurotoxicology. 2007;28:532–540. doi: 10.1016/j.neuro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Choy S, Kim JW. A case of amyotrophic lateral sclerosis in a worker treating pesticide wastes. Korean J Occup Environ Med. 2011;23:480–487. [Google Scholar]
- 8.Bonvicini F, Marcello N, Mandrioli J, Pietrini V, Vinceti M. Exposure to pesticides and risk of amyotrophic lateral sclerosis: a population-based case-control study. Ann Ist Super Sanita. 2010;46:284–287. doi: 10.4415/ANN_10_03_10. [DOI] [PubMed] [Google Scholar]
- 9.McGuire V, Longstreth WT, Jr, Nelson LM, Koepsell TD, Checkoway H, Morgan MS, van Belle G. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145:1076–1088. doi: 10.1093/oxfordjournals.aje.a009070. [DOI] [PubMed] [Google Scholar]
- 10.Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006;27:130–135. doi: 10.1159/000095552. [DOI] [PubMed] [Google Scholar]
- 11.Savettieri G, Salemi G, Arcara A, Cassata M, Castiglione MG, Fierro B. A case-control study of amyotrophic lateral sclerosis. Neuroepidemiology. 1991;10:242–245. doi: 10.1159/000110279. [DOI] [PubMed] [Google Scholar]
- 12.Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993;56:1200–1206. doi: 10.1136/jnnp.56.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granieri E, Carreras M, Tola R, Paolino E, Tralli G, Eleopra R, Serra G. Motor neuron disease in the province of Ferrara, Italy, in 1964-1982. Neurology. 1988;38:1604–1608. doi: 10.1212/wnl.38.10.1604. [DOI] [PubMed] [Google Scholar]
- 14.Gunnarsson LG, Bodin L, Söderfeldt B, Axelson O. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49:791–798. doi: 10.1136/oem.49.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamel F, Umbach DM, Bedlack RS, Richards M, Watson M, Alavanja MC, Blair A, Hoppin JA, Schmidt S, Sandler DP. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology. 2012;33:457–462. doi: 10.1016/j.neuro.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012;117:112–119. doi: 10.1016/j.envres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Das K, Nag C, Ghosh M. Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012;4:350–355. doi: 10.4103/1947-2714.99517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furby A, Beauvais K, Kolev I, Rivain JG, Sébille V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. J Neurol. 2010;257:792–798. doi: 10.1007/s00415-009-5419-5. [DOI] [PubMed] [Google Scholar]
- 19.Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48:63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- 20.Kalfakis N, Vassilopoulos D, Voumvourakis C, Ndjeveleka M, Papageorgiou C. Amyotrophic lateral sclerosis in southern Greece: an epidemiologic study. Neuroepidemiology. 1991;10:170–173. doi: 10.1159/000110266. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Pamphlett R. Exposure to environmental toxins and the risk of sporadic motor neuron disease: an expanded Australian case-control study. Eur J Neurol. 2012;19:1343–1348. doi: 10.1111/j.1468-1331.2012.03769.x. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi MM, Hayden D, Urbinelli L, Ferrante K, Newhall K, Myers D, Hilgenberg S, Smart R, Brown RH, Cudkowicz ME. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS) Amyotroph Lateral Scler. 2006;7:173–182. doi: 10.1080/14660820600640596. [DOI] [PubMed] [Google Scholar]
- 24.Burns CJ, Beard KK, Cartmill JB. Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945-94: an update. Occup Environ Med. 2001;58:24–30. doi: 10.1136/oem.58.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz DC, Nelson LM, McGuire V, Longstreth WT., Jr Physical trauma and family history of neurodegenerative diseases in amyotrophic lateral sclerosis: a population-based case-control study. Neuroepidemiology. 1999;18:101–110. doi: 10.1159/000069413. [DOI] [PubMed] [Google Scholar]
- 26.Fang F, Quinlan P, Ye W, Barber MK, Umbach DM, Sandler DP, Kamel F. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117:1387–1392. doi: 10.1289/ehp.0900580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutedja NA, Veldink JH, Fischer K, Kromhout H, Wokke JH, Huisman MH, Heederik DJ, Van den Berg LH. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007;69:1508–1514. doi: 10.1212/01.wnl.0000277463.87361.8c. [DOI] [PubMed] [Google Scholar]
- 28.Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986;123:790–799. doi: 10.1093/oxfordjournals.aje.a114308. [DOI] [PubMed] [Google Scholar]
- 29.Chió A, Meineri P, Tribolo A, Schiffer D. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10:174–184. doi: 10.1159/000110267. [DOI] [PubMed] [Google Scholar]
- 30.Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Amyotrophic lateral sclerosis and occupational history. A pilot case-control study. Arch Neurol. 1996;53:730–733. doi: 10.1001/archneur.1996.00550080044011. [DOI] [PubMed] [Google Scholar]
- 31.Gunnarsson LG, Lindberg, Söderfeldt B, Axelson O. Amyotrophic lateral sclerosis in Sweden in relation to occupation. Acta Neurol Scand. 1991;83:394–398. doi: 10.1111/j.1600-0404.1991.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 32.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 36.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 38.MacFarlane E, Glass D, Fritschi L. Is farm-related job title an adequate surrogate for pesticide exposure in occupational cancer epidemiology? Occup Environ Med. 2009;66:497–501. doi: 10.1136/oem.2008.041566. [DOI] [PubMed] [Google Scholar]
- 39.Van Maele-Fabry G, Lantin AC, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- 40.Kauppinen TP, Mutanen PO, Seitsamo JT. Magnitude of misclassification bias when using a job-exposure matrix. Scand J Work Environ Health. 1992;18:105–112. doi: 10.5271/sjweh.1604. [DOI] [PubMed] [Google Scholar]