Abstract
We evaluated whether sonographic findings can provide additional diagnostic yield in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), and can more accurately predict nodal metastasis than chest computed tomography (CT) or positron emission tomography (PET)/CT scans. EBUS-TBNA was performed in 146 prospectively recruited patients with suspected thoracic lymph node involvement on chest CT and PET/CT from June 2012 to January 2013. Diagnostic yields of EBUS finding categories as a prediction model for metastasis were evaluated and compared with findings of chest CT, PET/CT, and EBUS-TBNA. In total, 172 lymph nodes were included in the analysis: of them, 120 were malignant and 52 were benign. The following four EBUS findings were predictive of metastasis: nodal size ≥10 mm, round shape, heterogeneous echogenicity, and absence of central hilar structure. A single EBUS finding did not have sufficient diagnostic yield; however, when the lymph node had any one of the predictive factors on EBUS, the diagnostic yields for metastasis were higher than for chest CT and PET/CT, with a sensitivity of 99.1% and negative predictive value of 83.3%. When any one of predictive factors is observed on EBUS, subsequent TBNA should be considered, which may provide a higher diagnostic yield than chest CT or PET/CT.
Graphical Abstract
Keywords: Endoscopic Ultrasound; Needle Aspiration; Lymph Nodes, Lymphatic Metastasis; Prediction
INTRODUCTION
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is widely used for a pathological diagnosis in patients with suspected metastasis to the thoracic lymph nodes. EBUS-TBNA is a minimally invasive technique, performed under local anesthesia, and affords excellent diagnostic yields, with a sensitivity of 69%-99.1% and negative predictive value (NPV) of 11%-98.9% (1, 2, 3, 4, 5, 6). Additionally, it allows access to the hilar and interlobar lymph nodes, which are inaccessible by mediastinoscopy (7).
Recently, to increase the effectiveness of the procedure and the diagnostic yield of TBNA, studies have been performed on the usefulness of sonographic findings during EBUS for the prediction of nodal metastasis in the mediastinum or hilum and have suggested that some sonographic findings can be helpful in predicting metastasis (8, 9, 10, 11). For example, Fujiwara et al. (9) evaluated the association between nodal metastasis and EBUS findings in patients with lung cancer, which included nodal size (≥10 mm vs. <10 mm), shape (round vs. oval), margins (distinct vs. indistinct), echogenicity (heterogeneous vs. homogeneous), central hilar structure (CHS; absence vs. presence), and coagulation necrosis sign (CNS; absence vs. presence). They showed that a round shape, distinct margins, heterogeneous echogenicity, and presence of CNS could be independent predictive factors for nodal metastasis, and that when all four were determined as benign, 96.0% of lymph nodes were proven non-metastatic. Other recent studies using endoscopic ultrasound (EUS) have also shown that sonographic findings of lymph nodes can be useful for predicting nodal metastasis in patients with malignancies, such as lung and esophageal cancer (12, 13, 14, 15, 16, 17, 18, 19).
However, there are no reported data as to whether sonographic findings during EBUS can more accurately predict nodal metastasis than other imaging modalities, such as chest computed tomography (CT) or integrated positron emission tomography (PET)/CT scans, which are routinely performed prior to EBUS-TBNA and can provide additional diagnostic yield in EBUS-TBNA. Thus, in the current study, we prospectively evaluated the EBUS findings of suspected thoracic lymph node involvement as predictive factors for nodal metastasis and compared the diagnostic yield of EBUS finding categories as a prediction model for metastasis with chest CT, integrated PET/CT, and EBUS-TBNA.
MATERIALS AND METHODS
Study patients and data collection
We prospectively recruited adult patients with a pathologically confirmed or suspected malignancy who had suspected thoracic lymph node involvement on chest CT or integrated PET/CT and who were planned to undergo EBUS TBNA, between June 2012 and January 2013 at Samsung Medical Center, a 1,961-bed referral hospital in Seoul, Republic of Korea. All patients underwent a conventional diagnostic work-up including contrast-enhanced chest CT. Most recruited patients underwent integrated PET/CT prior to EBUS-TBNA, depending on the decision of the physician.
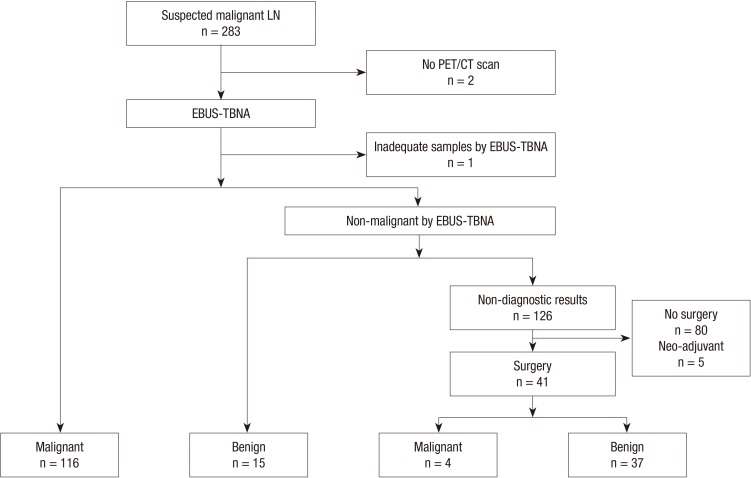
The indications for EBUS-TBNA for thoracic lymph node were: 1) the presence of lymph node with a short axis diameter of ≥10 mm on chest CT or 2) lymph node with increased FDG uptake compared with surrounding tissue on PET/CT scan regardless of size (1). EBUS findings that have been reported as predictive factors for metastasis (9) - size, shape, margin, echogenicity, CHS, and CNS - were evaluated under blinded condition by two bronchoscopists before TBNA, without rapid on-site cytopathological evaluation (ROSE). If non-diagnostic results were revealed by EBUS-TBNA, the lymph nodes were subsequently confirmed by mediastinoscopy or lymph node dissection in surgical candidates. Lymph nodes that did not undergo integrated PET/CT and had inadequate samples by EBUS-TBNA were excluded from the analysis. Lymph nodes that had non-diagnostic EBUS-TBNA results but that were not confirmed by surgery or that underwent neo-adjuvant chemothrapy before surgical confirmation were also excluded (Fig. 1).
Fig. 1.
The results for lymph nodes sampled by EBUS-TBNA; LN, lymph node; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; PET/CT, positron emission tomography/computed tomography.
Lymph nodes identified as malignant by EBUS-TBNA or by surgery were considered malignant. Lymph nodes identified with obvious benign etiologies by EBUS TBNA or confirmed as benign by surgery were considered benign. Diagnostic yields for identifying metastasis were evaluated on a per-nodal station basis.
Chest CT and integrated PET/CT scan
CT scans were obtained with a 64-detector row scanner (Light Speed VCT XT; GE Healthcare, Milwaukee, WI, USA), using the enhanced helical technique (40 mA, 120 kVp, beam width of 10 20 mm, beam pitch of 1.375 1.5). For an enhancement study, 100 mL of contrast medium (Iomeron 300; Bracco, Milan, Italy) were administered intravenously. Lymph nodes with a short-axis diameter of more than 10 mm were considered positive for malignancy. If lymph nodes contained nodular or laminated calcification, they were regarded as benign, regardless of size (20).
Image acquisition of integrated PET/CT was performed using a PET/CT device (Discovery LS, GE Healthcare, Milwaukee, WI, USA) that consisted of an Advance NXi PET scanner and an eight-slice Light Speed Plus CT scanner. After fasting for at least 6 h before the PET/CT examination, patients received an intravenous injection of 370 MBq of 18F FDG and then rested for 45 min before imaging. Lymph nodes were considered positive for malignancy if the maximum standardized uptake value (SUVmax) was more than 2.5 on 18F FDG PET/CT (21).
EBUS-TBNA
EBUS-TBNA was performed using a flexible ultrasonic puncture bronchoscope with a linear scanning transducer (BF-UC-260F-OL8, Olympus, Tokyo, Japan). TBNA biopsies were performed using a dedicated 22-gauge needle (NA-201SX-4022, Olympus). Before EBUS-TBNA, local anesthesia was achieved by nebulization with 4% lidocaine. All procedures were performed under conscious sedation using midazolam. We routinely infused midazolam at 0.06 mg/kg before starting EBUS-TBNA. If sedation was inadequate or the patients were irritable during the procedure, additional 1-mg doses of midazolam were administered. If more than one node was detected, EBUS-TBNA was performed at all accessible node stations. N3 nodes were sampled first, and then N2, and N1 nodes were sampled. We attempted at least three passes at each node as possible (22). All aspirated specimens were sent for cytological and/or histological examination and when tuberculous lymphadenopathy was suspected clinically, microbiological tests were performed (AFB stain, RT-PCR, and culture for Mycobacterium tuberculosis). ROSE was not performed.
All aspirated samples were categorized by pathological report. The presence of frank malignant cells was considered malignant. The presence of obvious evidence of benign etiologies was considered benign. Samples that showed only blood, mucus, benign bronchial epithelial cells, or no lymphoid tissue were considered inadequate samples. Other results of EBUS-TBNA were considered to be non-diagnostic. All specimens were evaluated by an experienced lung pathologist.
EBUS findings
Lymph nodes were categorized based on the EBUS findings (9): 1) short-axis diameter (≥10 mm vs. <10 mm), 2) shape (round vs. oval), 3) margin (distinct vs. indistinct), 4) echogenicity (heterogeneous vs. homogeneous), 5) CHS (absence vs. presence), and 6) CNS (absence vs. presence) (8, 9). Round shape was defined as when the ratio of the short to long-axis diameter of lymph nodes was ≥1.5 and oval shape was defined as when the ratio of the short to long-axis diameter was <1.5. Distinct margin was defined as when the majority of the margin (>50%) was clearly visualized with a high echoic border. Heterogeneous echogenicity was defined as multiple low echoic spots within the lymph node. The presence of CHS was defined as a linear, flat, hyperechoic area in the center of the lymph node. The presence of CNS was defined as a hypoechoic area within the lymph node without blood flow. Short-axis diameter >10 mm, round shape, distinct margin, heterogeneous echogenicity, absence of CHS, and presence of CNS were considered positive for malignancy. Interpretations of sonographic findings were performed under blinded condition by two bronchoscopist, and inconsistencies were re-evaluated and the agreements were recorded.
Statistical analysis
All data are presented as medians (interquartile ranges, IQRs) for continuous variables and as numbers (percentages) for categorical variables. Differences in EBUS findings between malignant and benign nodes were evaluated using the chi-square test or Fisher's exact test. Logistic regression analysis of EBUS findings for prediction of nodal metastasis was performed. The diagnostic sensitivity, specificity, positive predictive value (PPV), NPV, and accuracy were calculated using standard definitions. To compare diagnostic sensitivities and specificities among the diagnostic modalities, McNemar's tests were performed. Values of P<0.05 were considered to indicate statistical significance. All statistical analyses were performed using the PASW 18.0 software (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study was approved by the institutional review board of the Samsung Medical Center (IRB No. 2012-05-081). Informed consent was obtained from all participants.
RESULTS
Characteristics of patients and lymph nodes
EBUS-TBNA was performed in 283 lymph nodes of 146 recruited patients during the study period. The median age of the study patients was 63 (IQR, 54-71) yr, and 98 (67.1%) were men. Previous malignancy (n=28, 19.2%) was the most common underlying condition. Of the 146 patients, 120 (82.2%) had malignancies and 26 (17.8%) had benign diseases (Table 1). During the procedures of EBUS-TBNA, a few (n=5) patients had event of hypoxemia (<90%) but, spontaneously fully recovered, and there was no serious complication associated with sedation and procedures of EBUS-TBNA.
Table 1.
Characteristics of study patients
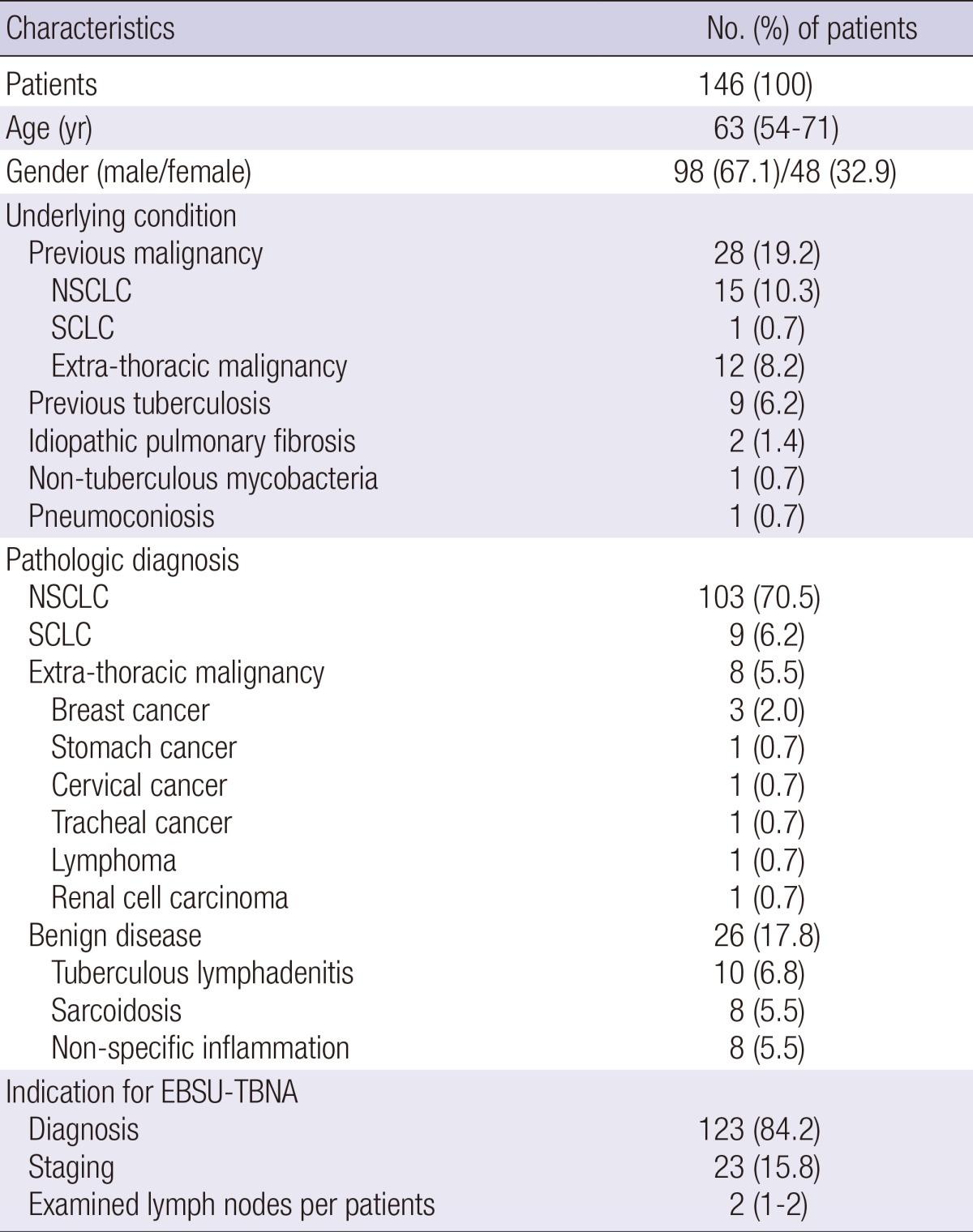
Data are shown as No. (%) or median (interquartile range). NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; EBUS-EBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
Fig. 1 shows the results for lymph nodes sampled by EBUS-TBNA. Of the total of 283 nodes, 25 that had no PET/CT scan and one that had an inadequate sample by EBUS-TBNA were excluded. In total, 116 nodes were confirmed as malignant and 15 nodes were identified to have obvious benign etiologies by EBUS-TBNA. Of the 126 nodes that were non-diagnostic by EBUS-TBNA, 41 subsequently underwent surgical resection; 37 of these 41 nodes were found to be benign and 4 were malignant. However, 80 nodes that had non-diagnostic EBUS-TBNA results that were not confirmed by surgery and five nodes that had neo-adjuvant chemotherapy before surgery were also excluded. Consequentially, 120 malignant nodes and 52 benign nodes were included in the analysis.
The characteristics of the 172 lymph nodes included in the analysis are shown in Table 2. The median short-axis diameter on chest CT was 10 (IQR, 8-14) mm and 11 (6.4%) nodes showed calcifications. High uptake (SUVmax>2.5) on integrated PET/CT was identified in 143 (83.1%) nodes. Of the 120 malignant lymph nodes, non-small-cell lung cancer was the most common (n=104, 60.5%) and of the 52 benign lymph nodes, tuberculosis was the most common benign etiology (n=11, 6.4%).
Table 2.
Characteristics of lymph nodes included in the analysis
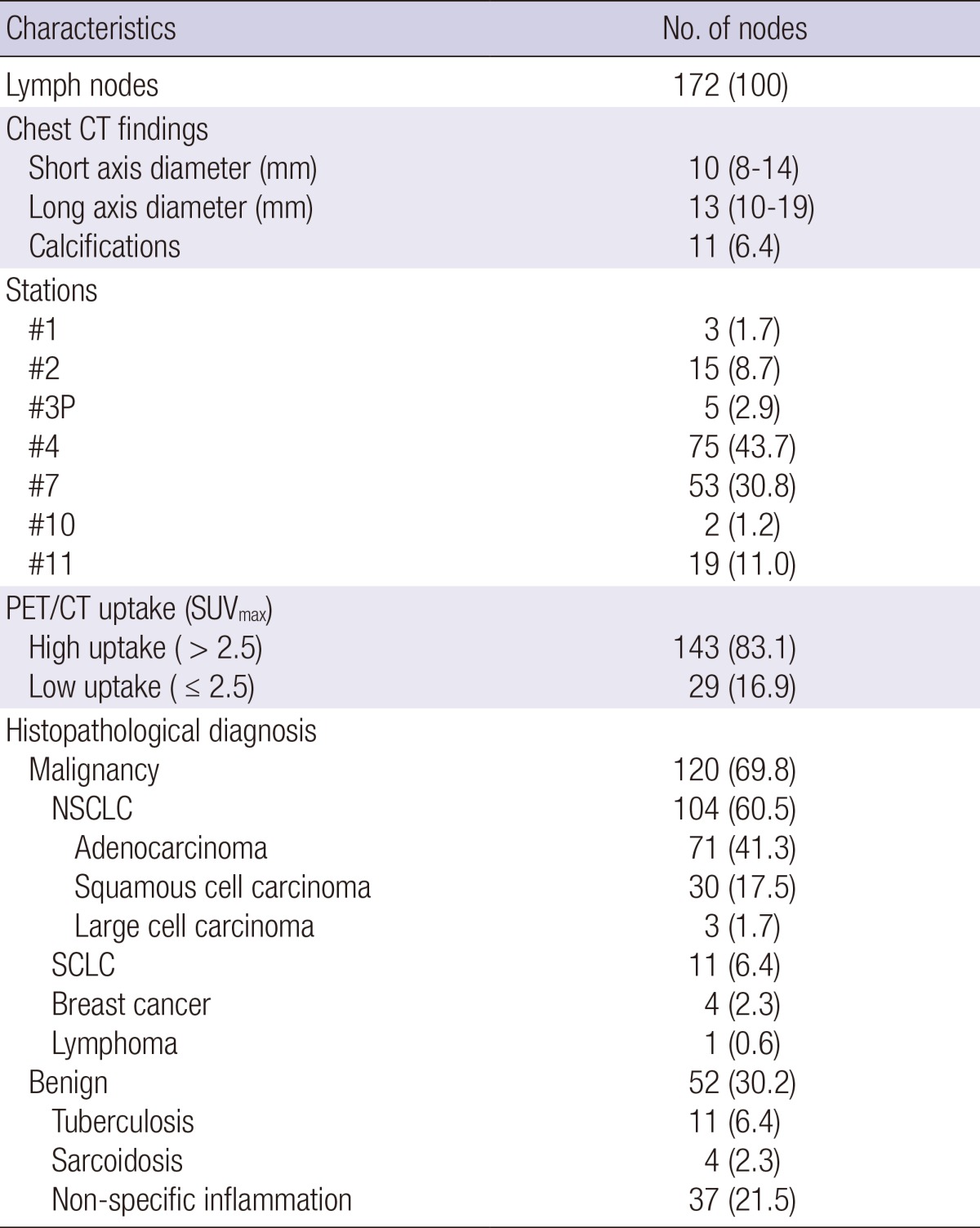
Data are shown as No. (%) or median (interquartile range). #1, low cervical, supraclavicular, and sternal notch; #2, paratracheal; #3P, retrotracheal; #4, lower paratracheal; #5, subaortic; #7, subcarinal; #8, paraesophageal; #10, hilar; #11, interlobar; PET, positron emission tomography; CT, computed tomography; SUVmax, maximum standardized uptake; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
EBUS findings of lymph nodes
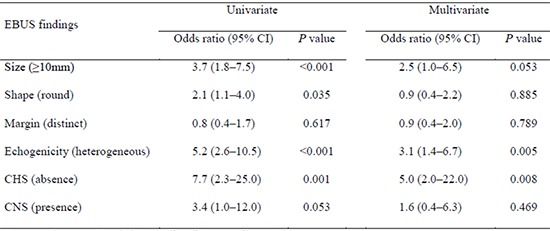
Sonographic findings of lymph nodes are shown in Table 3. Of the 120 malignant nodes, 69 (57.5%) were 10 mm or more in short-axis diameter, 65 (54.2%) had round shapes, 76 (63.3%) had distinct margins, 92 (76.7%) had heterogeneous echogenicity, 116 (96.7%) had absence of CHS, and 21 (17.5%) had presence of CNS. Of the 52 benign nodes, 14 (26.9%) were 10 mm or more in short-axis diameter, 19 (36.5%) had round shapes, 35 (67.3%) had distinct margins, 20 (38.5%) had heterogeneous echogenicity, 41 (78.8%) had absence of CHS, and 49 (94.2%) had presence of CNS. The size (P<0.001), shape (P=0.034), echogenicity (P=0.001), and CHS (P=0.001) differed significantly between malignant and benign lymph nodes. In the univariate analysis, the odds ratio (OR) for nodal metastasis was statistically significant for size (≥10 mm, OR 3.7; P<0.001), shape (round, OR 2.1; P=0.035), echogenicity (heterogeneous, OR 5.2; P<0.001), and CHS (absence, OR 7.7; P=0.001). In the multivariate analysis, the OR for metastasis in echogenicity (heterogeneous, OR 3.1; P=0.005) and CHS (absence, OR 3.1; P=0.005) remained statistically significant (Table 4).
Table 3.
EBUS findings of lymph nodes included in the analysis
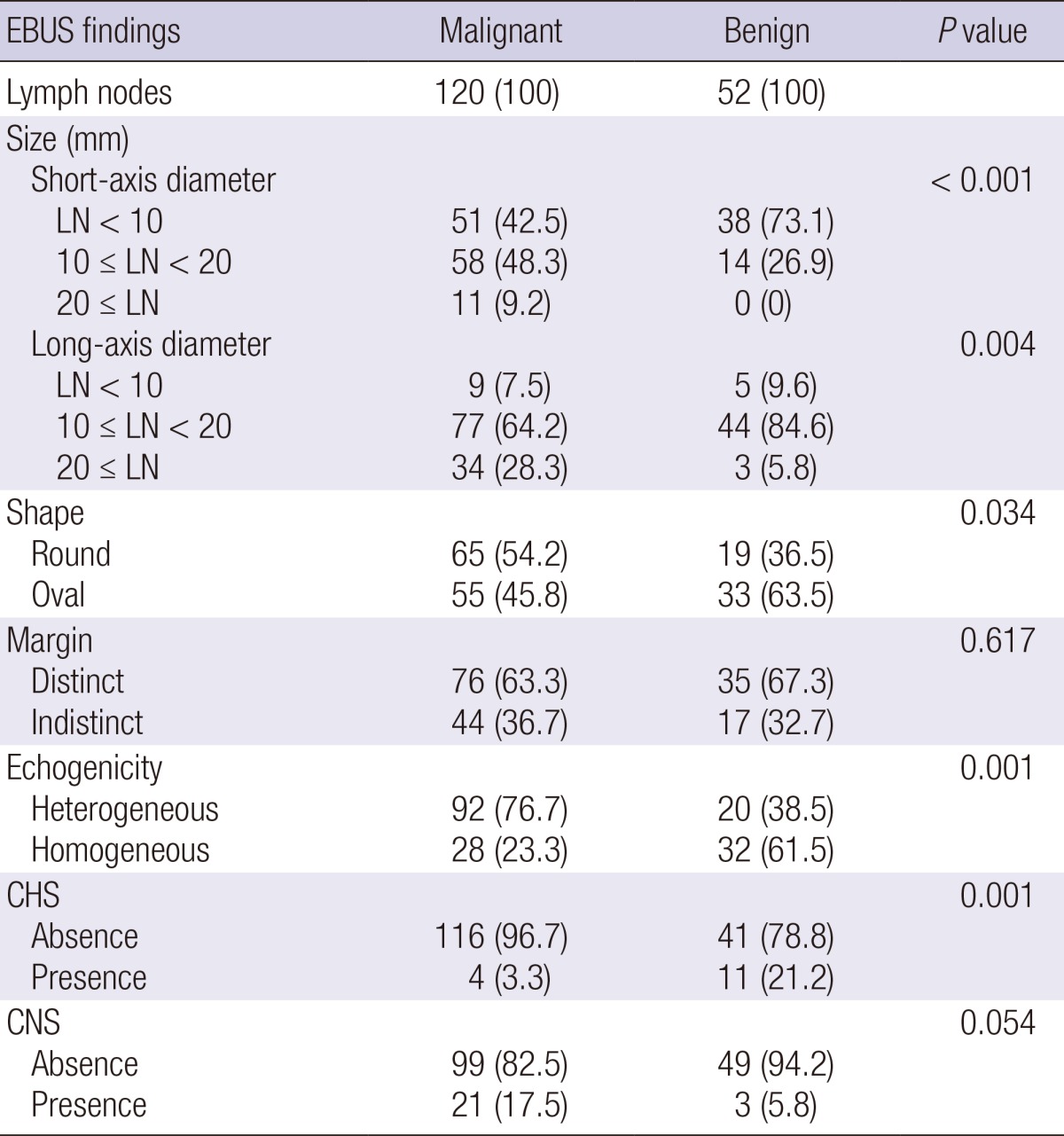
Data are shown as No. (%) or median (interquartile range). EBUS, endobronchial ultrasound; LN, lymph node; CHS, central hilar structure; CNS, Coagulation necrosis sign.
Table 4.
Logistic regression analysis of EBUS findings for prediction of nodal metastasis
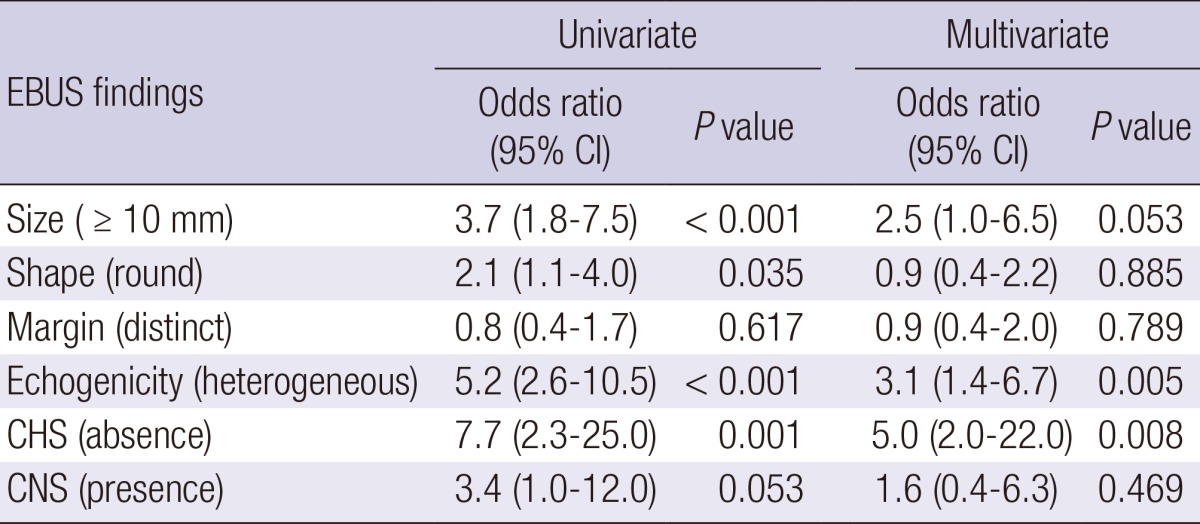
Data are shown as No. (%) or median (interquartile range). EBUS, endobronchial ultrasound; CHS, central hilar structure; CNS, Coagulation necrosis sign; CI, confidence interval.
Diagnostic yield according to modality
Table 5 presents comparisons of diagnostic yields according to diagnostic modality. The diagnostic sensitivity, specificity, PPV, NPV, and accuracy of chest CT were 68.3%, 61.5%, 80.4%, 45.7%, and 66.3%, respectively. The diagnostic sensitivity, specificity, PPV, NPV, and accuracy of integrated PET/CT were 93.3%, 40.4%, 78.3%, 72.4%, and 77.3%, respectively. The diagnostic sensitivity, specificity, NPV, and accuracy of EBUS-TBNA were 96.7%, 100.0%, 92.9%, and 97.7%, respectively.
Table 5.
Comparisons of diagnostic yields according to diagnostic modality
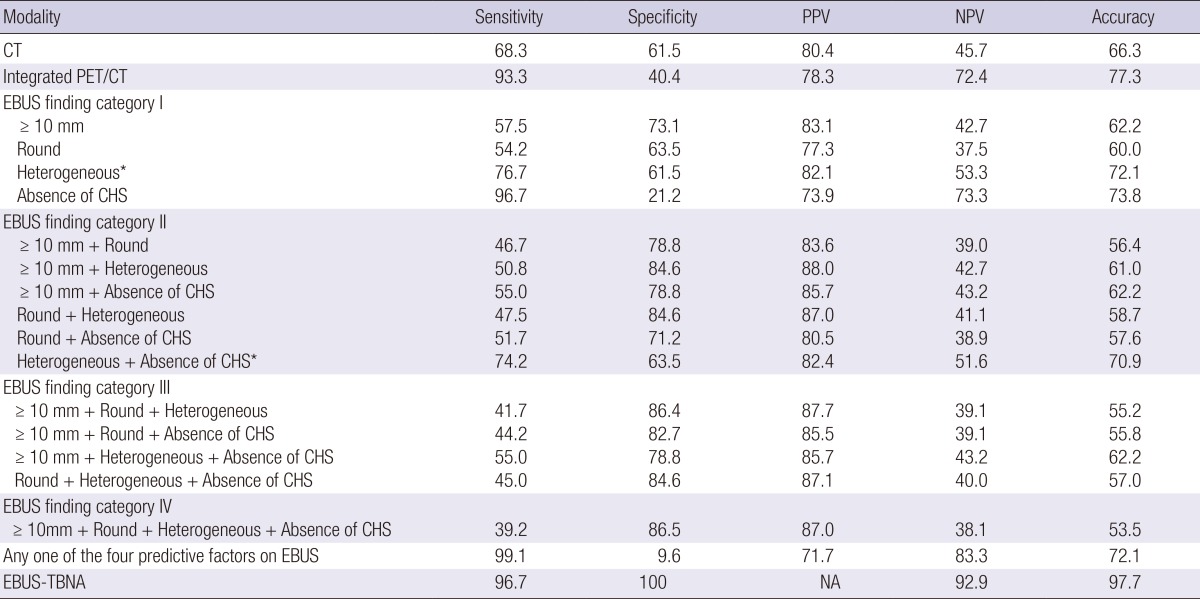
Data are shown as percentage. EBUS finding categories as prediction model for nodal metastasis were composed with combinations of the four predictive factors on EBUS; size (≥10 mm), shape (round), echogenicity (heterogeneous), and CHS (absence). Diagnostic sensitivity and specificity for nodal metastasis between CT, integrated PET/CT, and each EBUS categories using McNemar's test were significantly different (P<0.05) except *P values between CT and EBUS finding category (CT vs. EBUS category I [heterogeneous] and CT vs. EBUS category II [heterogeneous + absence of CHS]). PPV, positive predictive value; NPV, negative predictive value; PET,positron emission tomography; CT, computed tomography; CHS, central hilar structure; CNS, Coagulation necrosis sign; EBUS-EBNA, endobronchial ultrasound-guided transbronchial needle aspiration; NA, not applicable.
To evaluate the diagnostic yields of EBUS findings, EBUS finding categories as prediction models for nodal metastasis were composed with combinations of the four predictive factors that had P values of <0.05 in the univariate analyses: size, shape, echogenicity, and CHS (Tables 4, 5). EBUS finding category I was considered positive for malignancy when nodes had single predictive factors, category II was considered positive for malignancy when nodes had any two predictive factors, category III was considered positive for malignancy when nodes had any three predictive factors, and category IV was considered positive for malignancy when nodes had all four predictive factors. The diagnostic yields were then evaluated according to each EBUS finding category. Additionally, nodes that had any one of the four predictive factors were considered positive for malignancy and the diagnostic yields were evaluated. The diagnostic sensitivity and specificity between CT and integrated PET/CT, between CT and each EBUS finding category, and between integrated PET/CT and each EBUS finding category were significantly different (McNemar's test, P<0.05) except the *P values between CT and two EBUS finding categories (CT vs. heterogeneous and CT vs. heterogeneous + absence of CHS).
As shown in Table 5, a single sonographic finding during EBUS did not have sufficient diagnostic yield for predicting metastasis, compared with CT and integrated PET/CT. As the EBUS finding categories increased, the specificity and PPV tended to increase; however, sensitivity tended to decrease and NPV did not increase. In EBUS finding category IV, when the lymph node had all of the predictive factors, the diagnostic sensitivity, specificity, PPV, NPV, and accuracy for metastasis were 39.2%, 86.5%, 87.0%, 38.1%, and 53.5%, respectively. In contrast, when the lymph node had any one of the predictive factors, the diagnostic sensitivity, NPV, and accuracy for metastasis increased to 99.1%, 83.3%, and 72.1%, respectively, which were higher sensitivity and NPV yields than chest CT and integrated PET/CT. However, specificity and PPV decreased to 9.6% and 71.7%, respectively.
DISCUSSION
In the current study, nodal size ≥10 mm, round shape, heterogeneous echogenicity, and absence of CHS on EBUS were identified as predictive factors for nodal metastasis in univariate analyses, and heterogeneous echogenicity and absence of CHS remained significant in a multivariate analysis. These results are similar to a previous report of the utility of sonographic findings in predicting nodal metastasis. Fujiwara et al. (9) retrospectively evaluated the sonographic findings during EBUS-TBNA in 487 patients with malignancies and found that round shape, distinct margins, heterogeneous echogenicity, and presence of CNS were independent predictive factors for metastasis. However, in our data, presence of CNS and distinct margin on EBUS were not statistically significant, while nodal size was a significant factor. These differing results could be explained in part by the previous study including only patients with malignancies, whereas our study included patients with benign diseases. Schmid-Bindert et al. (8) also reported the utility of ultrasound criteria for predicting nodal metastasis for 281 nodes in 145 patients with mediastinal lymphadenopathy by EBUS-TBNA, which included 126 malignant and 155 non-malignant nodes. They showed that the nodal size ≥10 mm, round shape, heterogeneous echogenicity, and absence of CHS could be predictive factors for metastasis and heterogeneous echogenicity and absence of CHS had the highest OR values, consistent with our data, and indicated that ultrasound criteria could potentially increase the diagnostic accuracy of EBUS-TBNA.
However, a single sonographic finding during EBUS did not seem to have sufficient diagnostic yield for predictions. As shown in Table 3, considerable proportions of the 52 benign nodes showed the predictive factors on EBUS, which would be considered positive signs for malignant nodes. Additionally, as shown in Table 5, the diagnostic yields for predicting metastasis with a single predictive factor on EBUS were not higher than with imaging modalities, such as CT and integrated PET/CT, which are performed routinely. When we evaluated the diagnostic yield of EBUS finding categories, as the number of combination EBUS findings increased, the specificity and PPV tended to increase; however, sensitivity tended to decrease and NPV did not increase. These data suggest that EBUS finding should be used to determine which nodes should be targeted but not used to exclude malignancies. Therefore, our data may suggest that when any of the predictive factors is observed during the EBUS procedure, then subsequent TBNA might be considered, which may provide higher diagnostic yield than chest CT and integrated PET/CT, especially in terms of sensitivity and NPV.
From the current study, whether using EBUS finding categories can help in providing additional diagnostic yield to EBUS-TBNA remains unclear. When nodes that had any one of the four predictive factors on EBUS were considered positive for malignancy, the sensitivity was slightly higher than EBUS-TBNA but, the specificity, NPV, and accuracy of EBUS findings were not superior to EBUS-TBNA (Table 5). Although some previous studies comparing EUS morphology to EUS-fine needle aspiration (FNA) have indicated that EUS FNA is superior to imaging by EUS alone (13, 23), there are no accurate data on the additional diagnostic yield of using EBUS findings to EBUS-TBNA.
The current study had some limitations. First, relatively large numbers of nodes were excluded from the analysis because not all patients underwent surgical sampling for non-diagnostic lymph nodes from EBUS-TBNA due to the observational study design. Patients who were diagnosed with advanced malignancies by EBUS-TBNA alone did not necessarily need further surgical sampling regardless of accompanying suspected nodes with non-diagnostic EBUS-TBNA results in the same patients, considering the risk and ethical issues of surgical sampling in advanced cancer patients, and the known high PPV of EBUS-TBNA (2). Second, because there might have been some true malignant lymph nodes smaller than 10 mm on chest CT that had low PET/CT uptake and were not sampled by EBUS-TBNA, there is a possibility of selection bias in identifying the target lymph node. However, in the present study, most of the sampled lymph nodes (n=143, 83.1%) had high PET/CT uptake (Table 2) and more than half of the lymph nodes (n=89, 57.0%) were smaller than 10 mm (Table 3). Thus, the possible influence of selection bias on the results of our study does not appear to be significant. Third, the sonographic findings of targeted lymph nodes during EBUS-TBNA were evaluated by agreement by two bronchoscopists. Although the interpretations of sonographic findings were performed under blinded conditions without ROSE, the interpretation of sonographic finding was performed in real time during EBUS-TBNA for a relatively short period of time and the interpretation of sonographic findings could be partly subjective according to physicians, these could have influenced our results.
In conclusion, nodal size ≥10 mm, round shape, heterogeneous echogenicity, and absence of CHS on EBUS could be predictive factors for nodal metastasis. However, no single EBUS finding had sufficient diagnostic yield, compared with chest CT and integrated PET/CT. When any one of the predictive factors is observed on EBUS, subsequent TBNA might be considered, which may provide higher diagnostic yield than chest CT or integrated PET/CT.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Jhun BW, Park HY, Jeon K, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Han J, Um SW. Nodal stations and diagnostic performances of endobronchial ultrasound-guided transbronchial needle aspiration in patients with non-small cell lung cancer. J Korean Med Sci. 2012;27:46–51. doi: 10.3346/jkms.2012.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varela-Lema L, Fernandez-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33:1156–1164. doi: 10.1183/09031936.00097908. [DOI] [PubMed] [Google Scholar]
- 3.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–1396. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax. 2009;64:757–762. doi: 10.1136/thx.2008.109868. [DOI] [PubMed] [Google Scholar]
- 5.Joo H, Kim HR, Oh YM, Kim YH, Shim TS, Kim D-K, Park SI, Kim WS, Kim DS, Choi CM. The efficacy of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer in a university hospital. Tuberc Respir Dis. 2011;71:180–187. [Google Scholar]
- 6.Kang HJ, Hwangbo B. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration. Tuberc Respir Dis (Seoul) 2013;75:135–139. doi: 10.4046/trd.2013.75.4.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol. 2009;4:947–950. doi: 10.1097/JTO.0b013e3181add88d. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Bindert G, Jiang H, Kähler G, Saur J, Henzler T, Wang H, Ren S, Zhou C, Pilz LR. Predicting malignancy in mediastinal lymph nodes by endobronchial ultrasound: a new ultrasound scoring system. Respirology. 2012;17:1190–1198. doi: 10.1111/j.1440-1843.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M, Shibuya K, Hiroshima K, Nakatani Y, Yoshino I. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest. 2010;138:641–647. doi: 10.1378/chest.09-2006. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T, Anayama T, Shingyoji M, Kimura H, Yoshino I, Yasufuku K. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol. 2012;7:1009–1014. doi: 10.1097/JTO.0b013e31824cbafa. [DOI] [PubMed] [Google Scholar]
- 11.Memoli JS, El-Bayoumi E, Pastis NJ, Tanner NT, Gomez M, Huggins JT, Onicescu G, Garrett-Mayer E, Armeson K, Taylor KK, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest. 2011;140:1550–1556. doi: 10.1378/chest.11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts SA, Mahon BS, Evans R. Coagulation necrosis in malignant mediastinal nodes on endoscopic ultrasound: a new endosonographic sign. Clin Radiol. 2005;60:587–591. doi: 10.1016/j.crad.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Schmulewitz N, Wildi SM, Varadarajulu S, Roberts S, Hawes RH, Hoffman BJ, Durkalski V, Silvestri GA, Block MI, Reed C, et al. Accuracy of EUS criteria and primary tumor site for identification of mediastinal lymph node metastasis from non-small-cell lung cancer. Gastrointest Endosc. 2004;59:205–212. doi: 10.1016/s0016-5107(03)02692-0. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse A, Leveen MB, Etemad B, Matsuda K, Patel RS, et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54:714–719. doi: 10.1067/mge.2001.119873. [DOI] [PubMed] [Google Scholar]
- 15.Yang WT, Metreweli C. Colour Doppler flow in normal axillary lymph nodes. Br J Radiol. 1998;71:381–383. doi: 10.1259/bjr.71.844.9659130. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005;184:1691–1699. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 17.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–479. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 18.Catalano MF, Sivak MV, Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–446. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Inoue K, Yamamoto R, Kinoshita H. Patterns of internal echoes in lymph nodes in the diagnosis of lung cancer metastasis. World J Surg. 1992;16:986–993. doi: 10.1007/BF02067013. discussion 93-4. [DOI] [PubMed] [Google Scholar]
- 20.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, Choi JY, Kwon OJ, Shim YM, Kim S. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 21.Hong R, Halama J, Bova D, Sethi A, Emami B. Correlation of PET standard uptake value and CT window-level thresholds for target delineation in CT-based radiation treatment planning. Int J Radiat Oncol Biol Phys. 2007;67:720–726. doi: 10.1016/j.ijrobp.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, Nam BH, Zo JI, Hwangbo B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134:368–374. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 23.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628–633. doi: 10.1111/j.1572-0241.2004.04064.x. [DOI] [PubMed] [Google Scholar]