Abstract
We applied cardiac resynchronization therapy (CRT) for desynchronized heart failure patients. We evaluated clinical outcomes including morbidity, mortality, and echocardiographic parameters in 47 patients with implanted CRT in Korea from October 2005 to May 2013. The combined outcomes of hospitalization from heart failure, heart transplantation and death were the primary end point. Median follow-up period was 17.5 months. The primary outcomes listed above occurred in 10 (21.3%) patients. Two patients (4.3%) died after CRT and 8 (17%) patients were hospitalized for recurrent heart failure. Among patients hospitalized for heart failure, 2 (4.3%) patients underwent heart transplantation. The overall free rate of heart failure requiring hospitalization was 90.1% (95% CI, 0.81-0.99) over one year and 69.4% (95% CI, 0.47-0.91) over 3 yr. We observed improvement of the New York Heart Association classification (3.1±0.5 to 1.7±0.4), decreases in QRS duration (169.1 to 146.9 ms), decreases in left ventricular (LV) end-diastolic (255.0 to 220.1 mL) and end-systolic (194.4 to 159.4 mL) volume and increases in LV ejection fraction (22.5% to 31.1%) at 6 months after CRT. CRT improved symptoms and echocardiographic parameters in a relatively short period, resulting in low mortality and a decrease in hospitalization due to heart failure.
Keywords: Cardiac Resynchronization Therapy, Echocardiography, Heart Failure
INTRODUCTION
Cardiac resynchronization therapy (CRT) is indicated for the treatment of New York Heart Association (NYHA) functional class III or IV heart failure, with a wide QRS duration (QRS duration ≥120 ms) and an ejection fraction ≤35% (1). CRT has been shown to improve symptoms, exercise capacity, and left ventricular (LV) function; in addition, it reduced mortality and hospitalization rates for heart failure in several large multicenter clinical trials (2, 3, 4, 5). CRT devices have recently become a more common treatment for desynchronized heart failure patients in Korea. However, follow-up data regarding the effectiveness of CRT are sparse.
We analyzed the effectiveness of CRT by comparing clinical and echocardiographic parameters. We also evaluated mortality and morbidities such as hospitalization from heart failure and heart transplantation in patients with an implanted CRT device in Korea.
MATERIALS AND METHODS
Study population
We enrolled 47 patients who underwent CRT implantation at Samsung Medical Center, Gangneung Asan Hospital, and Hanmaeum General Hospital between October 2005 and May 2013. The criteria for CRT include New York Heart Association (NYHA) function class III/IV symptoms despite optimal medical therapy due to either ischemic or nonischemic cardiomyopathy with a left ventricular (LV) ejection fraction ≤35% and a QRS duration ≥120 ms on electrocardiography.
The primary end point was a composite of death from any cause, hospitalization from heart failure, or need of heart transplantation. Hospitalization with heart failure was defined by symptoms such as dyspnea, chest discomfort, and increased edema resulting in the need for admission for treatment with intravenous diuretics or inotropics.
Study design
Patients meeting the criteria for enrollment were evaluated for NYHA class, QRS duration on 12-lead electrocardiogram (ECG), and two-dimensional Doppler echocardiography measures (LV ejection fraction, LV end-diastolic diameter, LV end-systolic diameter, LV end-diastolic volume, LV end-systolic volume) at baseline. After this initial evaluation, patients underwent implantation of CRT with a right atrial lead, right ventricular lead, and a left ventricular lead, which was inserted into the lateral or posterolateral cardiac vein by a transvenous approach. NYHA functional class, QRS duration, and echocardiographic parameters were assessed at the 6-month follow-up visit. Combined outcomes of death, hospitalization from heart failure, and heart transplantation were assessed during the follow-up period.
CRT device implantation
The CRT device was implanted under local anesthesia with a transvenous approach via the left subclavian vein. The right ventricular lead was positioned in the RV apex or septum, and the right atrial lead was conventionally located in the right atrial appendage. The left ventricular lead was placed preferentially in a posterolateral or lateral vein after the coronary sinus venogram using an 8-Fr guiding catheter. The great cardiac vein or the middle cardiac vein were used only when other sites were not suitable or accessible. Only one patient required a left ventricular lead implanted via a thoracoscopic epicardial route; in this patient, the transvenous approach failed as there was no appropriate coronary venous branch.
Electrocardiograms
Standard 12-lead ECGs were obtained at baseline and at 6 months after CRT. Intraventricular conduction disturbances were defined according to criteria approved by the World Health Organization (6). Left bundle branch block (LBBB) was defined as QRS duration ≥120 ms, QS or rS in lead V1, broad (frequently notched or slurred) R waves in leads I, aVL, V5 or V6, and absent q waves in lead V5 and V6. Right bundle branch block (RBBB) was defined as QRS duration ≥120 ms, rsr', rsR', Rsr', or Qr in leads V1 or V2, and, occasionally, a wide and notched R wave and wide S waves in leads I, V5 and V6. Intraventricular conduction delay (IVCD) was defined as QRS ≥110 ms without typical features of LBBB or RBBB.
Echocardiography
Baseline and subsequent echocardiograms were obtained at 6 months after CRT implantation. The LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV ejection fraction (LVEF), left atrial (LA) width, and LA volume index (LAVI) were assessed according to the guidelines of the American Society of Echocardiography (7). LV and LA volume were estimated by Simpson's equation in 2- and 4-chamber views.
Statistical analysis
Data were expressed as the median with interquartile range or mean±standard deviation. Comparisons between groups were performed with the Student's t-test for continuous variables and the chi-square test for categorical data. For comparison of parametric variables between baseline and 6 months after CRT, the paired sample t-test was used. Cumulative clinical event-free rate curves for heart failure, heart transplantation, and mortality were determined according to the Kaplan-Meier method. Cox proportional hazards models were used to assess clinical factors and primary outcomes. Values of P<0.05 were considered significant. Statistical analysis was performed with SPSS 18.0 (SPSS Interactive Graphics, Version 18.0, SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the institutional review board (IRB) of Samsung medical center (IRB No. 2014-03-104). In addition, the local IRB at each participating hospital approved this study and waived the requirement for informed consent.
RESULTS
Clinical characteristics of study subjects
Forty-seven patients (27 men and 20 women) with a mean age of 62.3 yr were enrolled in this study. The median duration of follow-up was 17.5 months (interquartile range, 6-26.5 months). The baseline characteristics of the study population are described in Table 1. The cause of heart failure was ischemic in 6 (12.8%) patients and nonischemic in 41 (87.2%) patients. The mean NYHA class was 3.1±0.5, despite the use of optimal treatment, including angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers in 36 (76.6%) patients, beta-blockers in 34 (72.3%) patients, and diuretics in 33 (70.2%) patients. Implantation of a CRT device alone was performed in 7 (14.9%) patients, while a CRT-D device was implanted in 39 (85.1%) patients. Right ventricle lead placement was apical in 24 (51.1%) patients and septal in 22 (46.8%) patients. There were 32 (68.1%) patients with left bundle branch block, 4 (6.4%) patients with right bundle branch block, and 12 (25.5%) patients with nonspecific intraventricular conduction delay. A history of atrial fibrillation (AF) was observed in 10 patients (21.3%). Four patients underwent a CRT upgrade from a permanent pacemaker (PPM), initially implanted because of complete atrioventricular (AV) block. Although these patients had an atrial flutter fibrillation with slow ventricular rhythm in device interrogation after PPM implantation, the CRT upgrade was performed due to a high percentage of ventricular pacing, persistent severe HF symptoms and depressed EF. Two patients were in sinus rhythm after direct current cardioversion or catheter ablation. Three patients had a paroxysmal AF. Only 1 patient was implanted with CRT after AV junction ablation for biventricular pacing because of persistent AF.
Table 1.
Baseline clinical characteristics of the study population (n = 47)
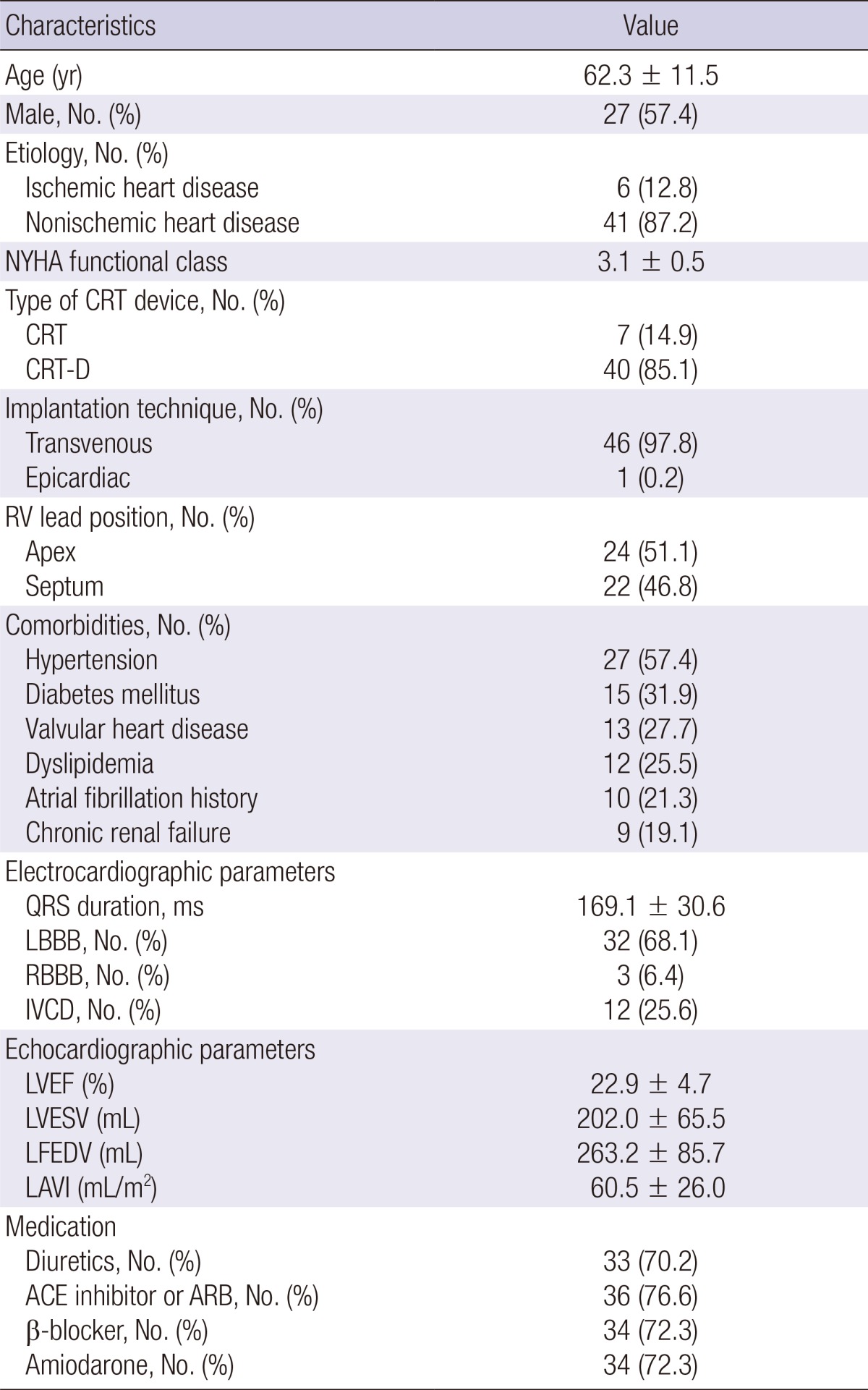
Data are presented as percentage or mean±SD. ACE, angiotensin- converting enzyme; ARB, angiotensin-receptor blocker; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillators; IVCD, interventricular conduction delay; LAVI, left atrial volume index; LBBB, left bundle branch block; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; RBBB, right bundle branch block.
Clinical, electrocardiographic, and echocardiographic parameters 6 months after CRT
Clinical, electrocardiographic, and echocardiographic changes during the first 6 months after CRT are shown in Table 2 and Fig. 1. NYHA functional class significantly improved from 3.1±0.5 to 1.7±0.4 (P<0.001). The duration of the QRS interval decreased from 169.1±30.6 ms to 146.9±22.8 ms (P<0.001). LVEDD decreased from 73.2±9.4 mm to 67.0±14.1 mm (P<0.001), LVESD decreased from 62.9±9.9 mm to 55.0±15.9 mm (P<0.001), LVESV decreased from 194.4±62.8 mL to 159.4±99.7 mL (P<0.001), LVEDV decreased from 255.0±83.9 mL to 220.1±111.4 mL (P=0.005), and LVEF improved from 22.5%±4.6% to 31.1%±12.6% (P=0.007). LAVI decreased from 62.4±28.7 mL/m2 to 52.3±23.6 mL/m2 (P=0.008), LA width decreased from 46.1±8.6 mm to 43.3±8.7 mm (P=0.002), and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels decreased from 3,697±5,054 pg/mL to 1,341±1,258 pg/mL (P=0.08). The median decrease in the LVESV observed during the first six months after CRT was 21.9% (interquartile range 7.5%-47.7%), the median decrease in the LVEDV was 10.4% (interquartile range 1.9%-38.4%), the median increase in the EF was 24.5% (interquartile range 2.7%-57.7%), and the median decrease in LAVI was 15% (interquartile range 4.9%-34.3%).
Table 2.
Comparison of changes in clinical, electrocardiographic and echocardiographic parameters before and after CRT

CRT, Cardiac resynchronization therapy; LA, left atrium; LAVI, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, LV end-systolic diameter; LVESV, left ventricular end-systolic volume; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Fig. 1.
Comparison of changes in echocardiographic, electrocardiographic, and NT-pro BNP parameters before and after CRT.
In the patients with primary outcomes, baseline NYHA functional class was higher (3.5±0.5 vs. 3.1±0.5, P=0.03), baseline QRS duration was prolonged (174.3±36.3 vs. 167.8±29.4 ms, P=0.12), and baseline LV cavity dilation was more severe (LVEDD, 78.2±11.5 vs. 71.4±7.9 mm, P=0.03; LVESD, 67.9±12.6 vs. 60.9±8.0 mm, P=0.03). Blunted improvement of echocardiographic parameters after CRT were observed in patients who reached primary outcomes; we observed a smaller decrease in LVEDD (73.4±16.4 vs. 65.0±12.9 mL, P=0.1), LVESD (60.5±18.7 vs. 53.2±14.8 mL, P=0.21). LVEDV (7.3%±29.8% vs. 15.9% ±29.0%, P=0.49), LVESV (10.7%±39.9% vs. 22.1%±34.0%, P=0.45), and EF (19.7%±61.5% vs. 49.4%±62.1%, P=0.19) (Table 3) also changed less in patients with primary outcomes.
Table 3.
Clinical and echocardiographic parameters in patients without and with primary end points

ACE-I, ACE inhibitors; ARB, angiotensin-receptor blockers; LAVI, left atrial volume index; LBBB, left bundle branch block; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, LV end-systolic diameter; LVESV, left ventricular end-systolic volume; RBBB, right bundle branch block; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Primary end point
The Kaplan-Meier curves of free rate of hospitalization from heart failure, any death, and heart transplantation are shown in Fig. 2. Of the 47 patients, 2 (4.3%) patients died; one patient died suddenly 2 months after CRT, while the other patient died from heart failure after orthopedic surgery. Eight (17.0%) patients were hospitalized for worsening heart failure; 2 (4.3%) of these patients underwent heart transplantation during the follow-up period. The overall free rate of heart failure requiring hospitalization was 90.1% (95% CI, 0.81-0.99) in one year, 69.4% (95% CI, 0.47-0.91) in 3 yr, and 46.2% (95% CI, 0.06-0.85) in 5 yr (Fig. 1). The heart transplantation free rate of patients was 97% (95% CI, 0.91-1.02) in 3 yr and 64% (95% CI, 0.12-1.17) in 6 yr (Fig. 2).
Fig. 2.
Kaplan-Meier curve of the free rate of hospitalization from heart failure, heart transplantation and death, along with composite primary outcomes.
The primary end point was not influenced by the use of beta-blockers, the cause of heart failure (ischemic or nonischemic), the configuration of the QRS complex (left or non-left bundle branch block), the base-line duration of the QRS interval (QRS duration <150 ms or ≥150 ms), or the baseline echocardiographic parameters (LVEDD ≥75 mm or <75 mm, LVESD ≥62 mm or <62 mm) (Table 4).
Table 4.
Univariate analysis of associations between clinical factors and primary outcomes

CI, confidence interval; LBBB, left bundle branch block; LVEDD, left ventricular end-diastolic diameter; LVESD, LV end-systolic diameter; LVESV, left ventricular end-systolic volume.
DISCUSSION
We found that CRT substantially improved symptoms, reduced QRS duration and improved echocardiographic parameters within the relatively short period of six months in Korean desynchronized heart failure patients. The clinical, electrocardiographic, and echocardiographic parameters in this study are similar to those of previous clinical trials (8, 9). The data are consistent with a CRT reduction in the degree of ventricular dyssynchrony (as evidenced by a shortened QRS interval), and this effect was accompanied by both an increase in the left ventricular ejection fraction and a decrease in the left ventricular end-diastolic and end-systolic dimension (5, 10). As a result, Korean patients with CRT experienced significant clinical improvements.
Ventricular remodeling consisting of LV dilation, cavity distortion, and deterioration in pump function was a sign of poor prognosis in moderate to severe heart failure (11). Reversed remodeling induced by CRT resulted in improved NYHA functional class, exercise capacity, and quality of life (4, 12). In our study, patients reaching primary outcomes had more baseline LV cavity dilatation and less reverse LV remodeling after CRT compared to those without primary outcomes; therefore, early implantation of a CRT device should be considered in these patients before progressive LV dilation.
To our knowledge, this was the first study that investigated mortality and morbidity after CRT in Korea. The free rate of hospitalization from heart failure was as good as 90% after 1 yr, and 69% after 3 yr; however, it decreased to 46% in 5 yr after CRT. Clinical outcomes after CRT confer greater benefits in a relatively short period (3 yr); however, that benefit was decreased over longer periods (5 yr). There were only two cases of death (4.3%) during the follow up period; this low mortality shows relatively good results in Korean patients who underwent CRT during a mid-term follow up period. Previous studies showed that the mortality rate was 10%/yr during 3 yr of follow up after CRT (9). Idiopathic dilated cardiomyopathy has a better response to treatment and a better prognosis than heart failure from ischemic heart disease in a number of studies (13, 14). This difference by etiology was explained by the rate of cardiac death due to progressive cardiac failure in ischemic heart disease (9). First and subsequent heart failure episodes after CRT were associated with 7- and nearly 19-fold respective increases in the risk of subsequent all-cause mortality in the MADIT-CRT study (15). In our study, the majority of the study population had idiopathic dilated cardiomyopathy. Heart transplantation was performed at the appropriate time in patients with progressive heart failure. This selection bias and appropriate treatment such as heart transplantation might underestimate the mortality and hospitalization from heart failure. However, our data showed that the benefit of CRT therapy for the prevention of heart failure was pronounced for 3 yr after CRT. CRT appears to be the optimal treatment in certain patients; our study showed a prominent decrease in mortality along with a decrease in hospitalization from heart failure.
This study has several limitations. First, comparisons to this analysis are limited by retrospective design, as confounders that were not evaluated might have influenced outcomes. Second, although each center recruited patients consecutively and follow-up loss is rarely observed, the inclusion of two tertiary referral centers may have led to selection bias because more patients with advanced heart failure were included. Third, because the main etiology of CRT indication was non-ischemic heart disease, it is possible that the prognosis for primary outcomes such as heart failure or death was fairly good. Lastly, our study population was small.
In conclusion, CRT improves clinical symptoms and electrocardiographic/echocardiographic parameters over a relatively short period, resulting in a decrease in mortality and hospitalization due to heart failure.
Footnotes
The authors have no conflicts to disclose.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 6.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:976–981. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Abreu CD, Xavier RM, Nascimento JS, Ribeiro AL. Long-term outcome after Cardiac Resynchronization Therapy: a nationwide database. Int J Cardiol. 2012;155:492–493. doi: 10.1016/j.ijcard.2011.12.083. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini M, Lunati M, Santini M, Tritto M, Curnis A, Bocchiardo M, Vincenti A, Pistis G, Valsecchi S, Denaro A, et al. Long-term survival in patients treated with cardiac resynchronization therapy: a 3-year follow-up study from the InSync/InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2006;29:S2–S10. doi: 10.1111/j.1540-8159.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 10.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ, Bourgoun M, Foster E, Zareba W, Goldenberg I, et al. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 12.Gras D, Leclercq C, Tang AS, Bucknall C, Luttikhuis HO, Kirstein-Pedersen A. Cardiac resynchronization therapy in advanced heart failure the multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311–320. doi: 10.1016/s1388-9842(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 13.Likoff MJ, Chandler SL, Kay HR. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:634–638. doi: 10.1016/0002-9149(87)91183-0. [DOI] [PubMed] [Google Scholar]
- 14.Adams KF, Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Hall WJ, Beck CA, Moss AJ, Barsheshet A, McNitt S, Polonsky S, Brown MW, Zareba W. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2011;58:729–737. doi: 10.1016/j.jacc.2011.04.024. [DOI] [PubMed] [Google Scholar]




