Abstract
We aimed to identify a vasoreactive subset of patients with idiopathic pulmonary arterial hypertension (IPAH) in Korea and to show their clinical characteristics and prognosis. Data on patients who were diagnosed with IPAH at Asan Medical Center between January 1994 and March 2013 were retrospectively collected. Acute vasodilator testing was performed with inhaled nitric oxide during diagnostic right heart catheterization. A positive acute response was defined as a reduction in mean pulmonary arterial pressure (PAP) ≥10 mmHg to an absolute level of mean PAP <40 mmHg without a decrease in cardiac output. Among a total of 60 IPAH patients included for analysis, 9 (15%) showed a positive acute response to acute vasodilator testing. Acute responders showed significantly lower peak velocity of a tricuspid regurgitation jet on echocardiography (4.1±0.3 m/s vs. 4.6±0.6 m/s; P=0.01) and significantly lower mean PAP hemodynamically (47±10 mmHg vs. 63±17 mmHg; P=0.003) than non-responders at baseline. The survival rate of acute responders was 88% at 1, 3, 5, and 10 yr, respectively, which was significantly higher than that of non-responders (85%, 71%, 55%, and 40%, respectively; P=0.029). In conclusion, Korean IPAH patients with vasoreactivity showed better baseline hemodynamic features and survival than those without vasoreactivity.
Graphical Abstract
Keywords: Hypertension, Pulmonary; Vasodilator Agents; Calcium Channel Blockers; Survival Rate
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare disease characterized by a progressive increase in pulmonary vascular resistance (PVR) that ultimately results in right heart failure and death (1). Idiopathic pulmonary arterial hypertension (IPAH) is a sporadic category of the disease without a family history of PAH and identifiable risk factors (2). The natural history of IPAH is well established based on the National Institutes of Health (NIH) registry, which showed a median survival of 2.8 yr and 1-, 3-, and 5-yr survival rates of 68%, 48%, and 34%, respectively (3). Before the recent advent of targeted therapies such as prostanoids, endothelin receptor antagonists, and phosphodiesterase inhibitors, conventional therapies such as calcium channel blockers (CCBs), warfarin, diuretics, and digoxin were used. In 1992, to evaluate the long-term efficacy of CCBs in patients with primary pulmonary hypertension, Rich et al. (4) assessed the acute responses of pulmonary arterial pressure (PAP) and PVR to high doses of CCBs at the time of diagnosis, and followed up these patients for 5 yr with treatment with high-dose CCBs. The patients who responded acutely to high doses of CCBs showed a significantly better outcome (a 5-yr survival rate of 94%) than patients who did not respond. Following the study of Rich et al. (4), many studies were conducted on agents used in acute vasodilator testing and on the definition of a positive acute response. In another key study reported in 2005, Sitbon et al. (5) reported the baseline characteristics and survival of a long-term CCB responder group among IPAH patients. The long-term CCB responder group had a significantly better survival (a 5-yr survival rate of 97%) than a long-term CCB failure group. Based on these studies, current guidelines recommend that acute vasodilator testing be performed during diagnostic workups for IPAH to identify the subset of patients showing long-term beneficial effects with CCBs and a better prognosis (1, 6).
Although several prospective cohort studies demonstrating some proportion of acute vasodilator responders among PAH patients have been reported in Western countries (7, 8, 9), no study to date has examined the characteristics or survival of acute vasodilator responders among IPAH patients in Korea. The aim of this retrospective study was to demonstrate a vasoreactive subset of Korean IPAH patients, to characterize their clinical and hemodynamic features, and to compare their survival with that of IPAH patients without vasoreactivity.
MATERIALS AND METHODS
Study patients
Patients who were diagnosed with PAH at Asan Medical Center, a tertiary referral hospital in Seoul, Korea, between January 1994 and March 2013 were screened for this study. Through a retrospective review of the electronic medical records, PAH was assessed with the results of right heart catheterization (RHC) or echocardiography. The hemodynamic definition of PAH on RHC included mean PAP >25 mmHg at rest, mean pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, and PVR >3 Wood units (1). If RHC was not performed, PAH was diagnosed by the echocardiographic definition of systolic PAP ≥40 mmHg measured by Doppler echocardiography (10). Patients' specific diagnoses were reclassified according to the most updated classification of pulmonary hypertension (11). The IPAH patients whose results of both RHC and acute vasodilator testing were available were included in the analysis. The patients with other types of PAH, such as PAH associated with connective tissue diseases, portal hypertension, or congenital heart diseases, were excluded. The included patients were grouped by the results of acute vasodilator testing into acute responders or non-responders.
Right heart catheterization (RHC) and acute vasodilator testing
Diagnostic RHC for PAH was performed in the medical intensive care unit. A 7.5 Fr Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA, USA) was advanced into the pulmonary artery through the right internal jugular vein or the right subclavian vein. Proper positioning of the catheter was confirmed by chest radiography. Baseline hemodynamic values, including mean right atrial pressure (RAP), mean PAP, mean PCWP, and mixed venous oxygen saturation (SvO2), were measured. Cardiac output was measured in triplicate by the thermodilution technique using Vigilance Monitor (Edwards Lifesciences). The PVR was calculated by dividing (mean PAP - mean PCWP) by the cardiac output.
Acute vasodilator testing was performed after baseline hemodynamic data were recorded. The specific protocol for the test followed previously described methods with minor modifications (12). Briefly, patients inhaled a mixture of air-nitric oxide (NO) of 10, 20, and 40 parts per million (ppm) and hemodynamic variables were measured after every 10 min. In the case of a positive response to inhaled NO, a fast-release preparation of oral nifedipine 10 mg was challenged after recovery to baseline hemodynamic values. Consecutive doses of nifedipine 10 mg were administered at 20-min intervals up to a total dose of 60 mg with recording of hemodynamic measurements. The testing was terminated when 1) the patient developed symptoms including nausea, flushing, headache, dyspnea, or chest discomfort; 2) systolic blood pressure decreased to <90 mmHg; or 3) the maximal vasodilator dose was reached. A positive acute response was defined as a reduction in mean PAP ≥10 mmHg to an absolute level of mean PAP <40 mmHg without a decrease in cardiac output (1, 6).
Data collection
Baseline data were collected from the patients' medical charts. These data included age at diagnosis, sex, duration of symptoms until diagnosis, symptoms at diagnosis, signs of right heart failure such as pitting edema of lower extremities or ascites, World Health Organization (WHO) functional class, smoking history, and results of the 6-min walk test.
Echocardiographic studies were performed using the Philips echocardiography system (Koninklijke Philips N.V., Eindhoven, the Netherlands). Echocardiographic measurements, such as the ejection fraction of the left ventricle, the peak velocity of a tricuspid regurgitation jet, or the presence of an inferior vena cava plethora or a pericardial effusion, were checked. Hemodynamic data at baseline and during acute vasodilator testing were also obtained.
The date of diagnosis was defined as when the patient underwent the first diagnostic RHC, and the time from the date of diagnosis to the last follow-up date was calculated. Survival status and date of death were determined based on medical charts and the expiration date of the Korean national health insurance service without considering the cause of death. Patients were only counted as deceased if they died during the follow-up period. All other cases, including cases lost to follow-up, were censored. The medication that was prescribed for IPAH was checked.
Statistical analyses
All values were expressed as the mean±standard deviation or the median (interquartile range) for continuous variables and as the number or percentage for categorical variables. Baseline clinical characteristics and echocardiographic and hemodynamic measurements were compared between acute responders and non-responders using a Mann-Whitney U test for continuous variables and a Fisher's exact test for all categorical variables except the WHO functional class. Differences in the proportions of WHO functional classes between the 2 groups were analyzed with a linear-by-linear association test. The Wilcoxon signed-rank test was performed for hemodynamic values measured at baseline and during acute vasodilator testing. The survival rate was estimated using the Kaplan-Meier method and comparison of survival between groups was done using a log-rank test. A P value <0.05 was considered to be statistically significant. Analysis was performed with the use of PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study protocol was approved by the institutional review board of Asan Medical Center (IRB No. 2013-0853). Informed consent was waived by the board.
RESULTS
Baseline characteristics
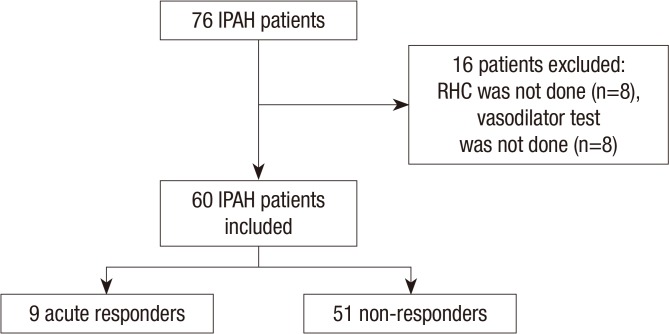
Of the 159 patients screened, 76 were diagnosed with IPAH. Sixteen IPAH patients were excluded from the study because they did not undergo RHC and were diagnosed by echocardiography only (n=8) or they underwent RHC only without acute vasodilator testing (n=8). Nine patients (15%) showed a positive acute response on the acute vasodilator test (Fig. 1). The baseline characteristics of the 60 IPAH patients are summarized in Table 1. The mean age of acute responders was 38±16 yr (range, 15-67 yr) and the female-to-male ratio was 8:1. It took a median of 36 months (interquartile range, 4-84 months) to diagnose IPAH from the onset of symptoms in acute responders. Most patients complained of dyspnea upon exertion. No significant differences in clinical features were noted between acute responders and non-responders at baseline. Echocardiographically, the peak velocity of a tricuspid regurgitation jet was significantly lower in acute responders than in non-responders (4.1±0.3 m/s vs. 4.6±0.6 m/s; P=0.01). On hemodynamics, acute responders showed significantly lower mean PAP than non-responders (47±10 mmHg vs. 63±17 mmHg; P=0.003). Mean PAP decreased significantly by 21±7 mmHg (range, 11-31 mmHg) to 26±9 mmHg (range, 12-39 mmHg) during acute vasodilator testing (Table 2). PVR also decreased significantly without a reduction in cardiac output. In all acute responders, both mean PAP and PVR decreased by >20%.
Fig. 1.
Flow diagram of patient selection for analysis. IPAH, idiopathic pulmonary arterial hypertension; RHC, right heart catheterization.
Table 1.
Baseline clinical, echocardiographic, and hemodynamic data of the idiopathic pulmonary arterial hypertension patients
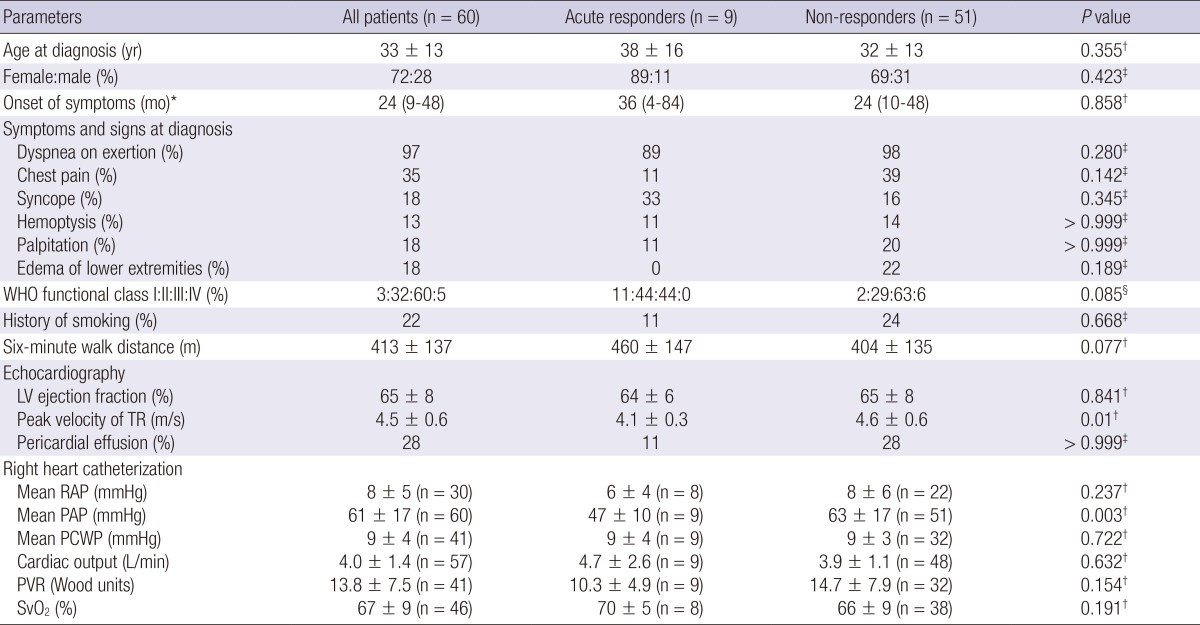
Data are presented as the mean±SD, *the median (interquartile range), or the percentage. Comparison between acute responders and non-responders using a †Mann-Whitney U test, ‡Fisher's exact test, or §linear-by-linear association test. WHO, World Health Organization; LV, left ventricle; TR, tricuspid regurgitation jet; RAP, right atrial pressure; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; SvO2, mixed venous oxygen saturation.
Table 2.
Hemodynamic values reached during acute vasodilator testing in acute responders
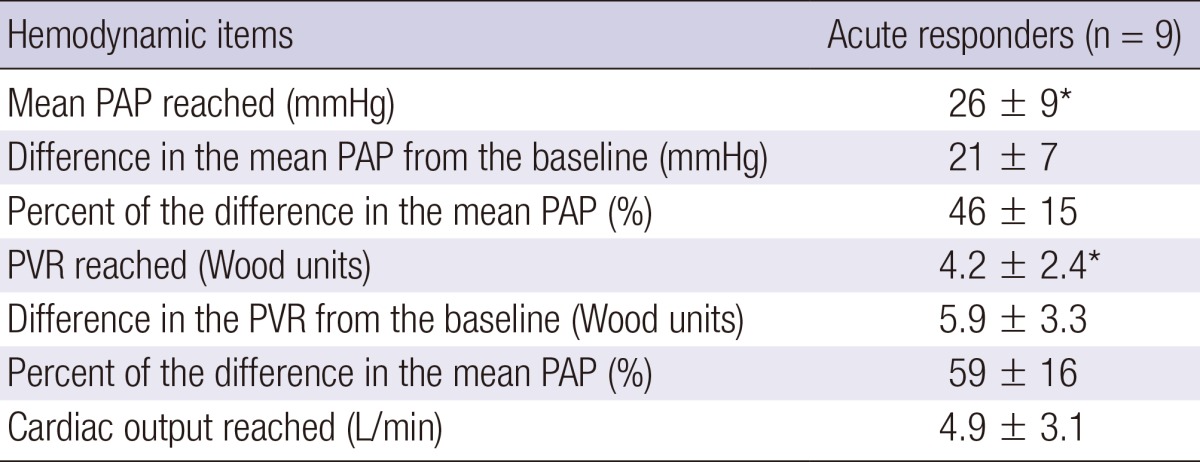
Data are presented as the mean±SD. *P=0.008, comparison with baseline hemodynamic values using a Wilcoxon signed-rank test. PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance.
Survival
Eight patients among the acute responders were initially treated with a fast-release preparation of nifedipine or a controlled release formulation of nifedipine at a daily dose of 59±26 mg (range, 30-90 mg) (Table 3). One patient in the acute responder group was not treated with CCBs but with bosentan because the patient did not show a positive acute response to oral nifedipine during acute vasodilator testing. Because the targeted agents-prostanoids, endothelin receptor antagonists, and phosphodiesterase inhibitors-except beraprost were not approved in Korea until 2004, most patients in the non-responder group diagnosed before September 2003 were initially treated with beraprost. Many non-responders diagnosed after September 2003 received treatment with targeted agents that included sildenafil, iloprost, bosentan, or ambrisentan.
Table 3.
Initial treatment of the idiopathic pulmonary arterial hypertension patients after diagnosis
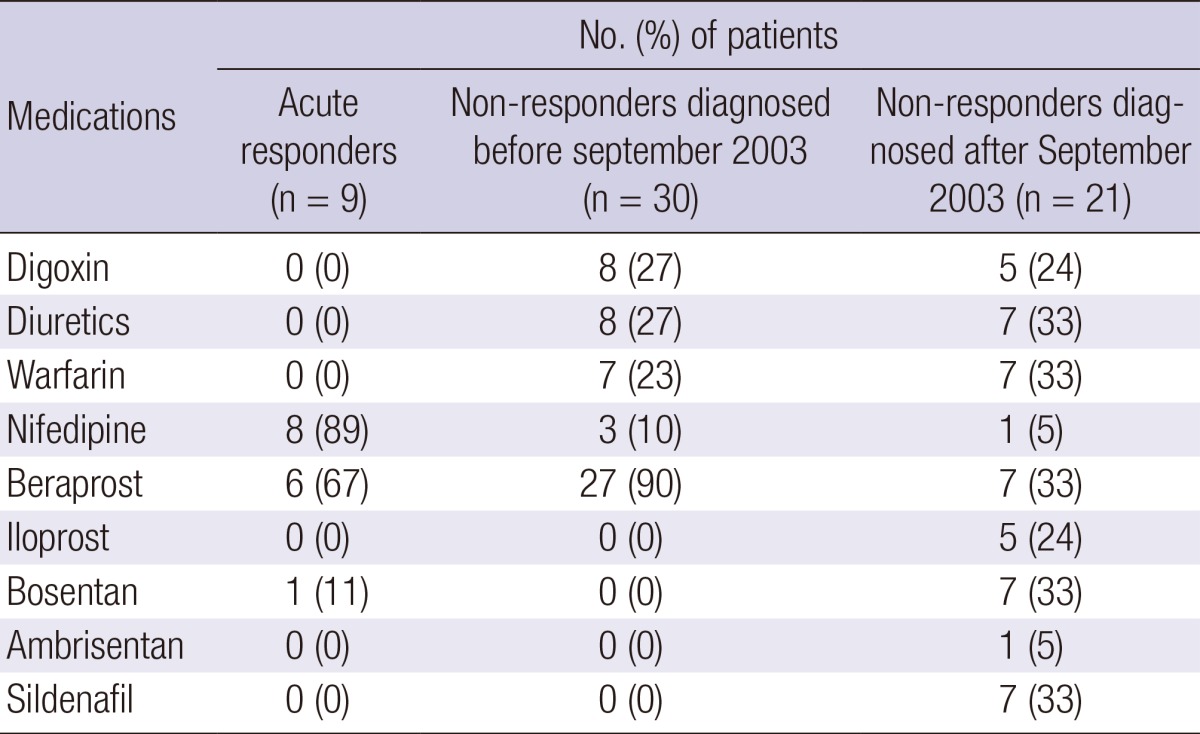
Data are presented as the number (%).
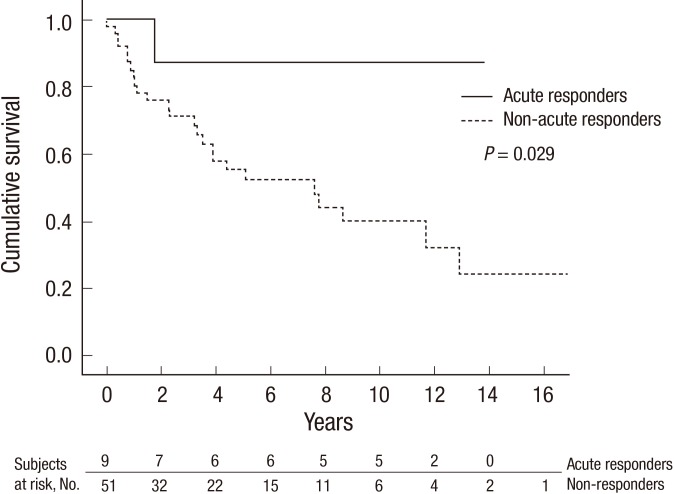
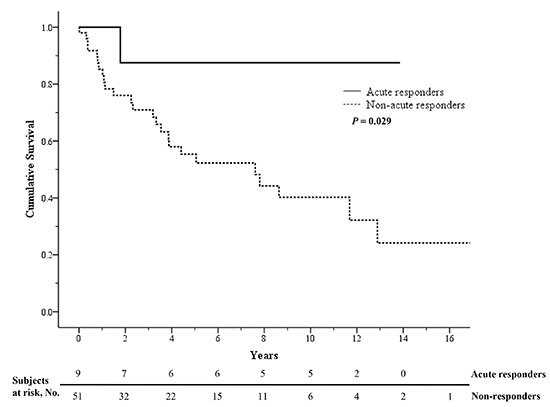
All included patients were followed up for a mean of 62±56 months. During the follow-up period, patients visited the hospital every 2 months on average. The mean duration of follow-up in acute responders was 96±63 months (range, 12-166 months). A total of 26 patients in this study died. In the acute responder group, only the patient who was not treated with CCBs died (at 21 months after diagnosis). Acute responders showed a significantly higher survival rate than non-responders (P=0.029 by the log-rank test). Kaplan-Meier survival estimates were 88% for acute responders at 1, 3, 5, and 10 yr, and 85%, 71%, 55%, and 40% for non-responders, respectively (Fig. 2).
Fig. 2.
Kaplan-Meier survival estimates in idiopathic pulmonary arterial hypertension patients depending on vasoreactivity. When the survival rate of acute responders (solid line) was compared to that of non-responders (dashed line), the difference was significant (5-yr survival rates, 88% versus 55%; P = 0.029 by the log-rank test).
DISCUSSION
Our present study shows that a vasoreactive subset exists among Korean IPAH patients and that the prognosis of this group is as favorable as that of Western IPAH patients with vasoreactivity. To the best of our knowledge, this is the second report about the baseline characteristics and prognosis of acute responders among IPAH patients in Asia. Although one prospective cohort study on Chinese idiopathic and familial PAH patients (13) and another retrospective study on Japanese PAH patients (14) were reported in 2007 and 2012, respectively, data about acute responders were not described. The first study on Asian IPAH patients with vasoreactivity was reported in 2009 (15). In that study, aerosolized iloprost was evaluated as an acute vasodilator agent in Chinese IPAH patients and acute responders were followed up for 12 months. Although clinical and hemodynamic improvement in acute responders was assessed after treatment with diltiazem for 12 months, long-term survival was not shown because of the limited duration of follow-up.
The proportion of acute responders among IPAH patients varies depending on the definition of a positive acute response used or the vasodilatory agents used in each study: 55% in the NIH registry (7), 26.6% reported by Rich et al. (4), 12.6% reported by Sitbon et al. (5), 10.3% in the French registry (8), 13.5% reported by Jing et al. (15), and 15% in our present study. The baseline clinical and hemodynamic characteristics of acute responders in our study were similar to those of acute responders reported by two previous studies (5, 15). The acute responders were in a better WHO functional class, had higher exercise tolerance on the 6-min walk test, and showed lower RAP, mean PAP, and PVR, and higher cardiac output and SvO2 than non-responders, although none of these differences except for mean PAP were statistically significant in our study. Based on these findings that acute responders had a less severe disease at diagnosis, we hypothesize that the proportion of acute responders among IPAH patients is higher when IPAH is diagnosed early on its disease course than when diagnosed later. The diagnostic gap between onset of symptoms and diagnosis was more than 2 yr in the NIH registry and this has not been closed in more recent registries (3, 8, 16, 17). Nearly 75% of PAH patients were diagnosed in WHO functional class III or IV in the French registry and the REVEAL registry (8, 16). If this diagnostic delay is reduced, IPAH patients may be more frequently diagnosed as being vasoreactive. Our hypothesis needs to be verified in the future.
The survival of acute responders in our present study was comparable to that of acute responders in previous studies (4, 5). All acute responders, except 1 who did not receive CCB therapy, survived for a mean of 96 months after initiation of CCB therapy. The 8 acute responders were treated with nifedipine at a daily dose of 59±26 mg (range, 30-90 mg). This dose of nifedipine was much lower than that used in the previous studies (172±41 mg (4), and 102±27 mg or 144±68 mg [5]). The treatment was changed to sildenafil after 30 months of nifedipine therapy in one acute responder and to ambrisentan after 154 months in another acute responder. The other acute responders maintained the initial dose of nifedipine. A distinctive feature of our present study was that long-term beneficial effects of CCBs in acute responders were observed with a relatively low dose of nifedipine.
Since the report of Rich et al. (4) in 1992, many studies dealing with the acute vasodilator test had defined a positive acute response as a decrease in both mean PAP and PVR by ≥20%. After Sitbon et al. (5) showed that a new criterion-a decrease in mean PAP by ≥10 mmHg to <40 mmHg with unchanged or increased cardiac output-identified patients who benefited from long-term CCB therapy better than the previous criterion, current guidelines recommend the new criterion as a positive acute response (1, 6). Given that the 6 patients who met the previous criterion but did not achieve the new one showed an excellent long-term outcome in the study reported by Sitbon et al. (5), the new criterion is more specific but less sensitive than the previous one (1). In our present study, only 1 patient met the previous criterion but not the current one. This patient was not treated with CCBs and the long-term response to CCBs was unknown.
In our present study, 4 non-responders received treatment with nifedipine. Although 1 of the 4 patients did not satisfy the positive criterion on the acute vasodilator test, a considerable reduction in both mean PAP (from 76 mmHg to 53 mmHg) and PVR (6.9 Wood units to 5.9 Wood units) was seen. The patient had been treated with a controlled release formulation of nifedipine 30 mg/day, had improved clinically and hemodynamically, and had been under regular follow-up for 119 months without changing medication. Two other patients initially receiving nifedipine therapy did not show clinical improvement and 1 of them died 4 months after initiation of nifedipine therapy. The last patient was treated with nifedipine due to concurrent systemic hypertension.
Sixteen IPAH patients (21% of screened IPAH patients) were excluded from our current analyses because their acute vasodilator test results were unavailable. RHC was not performed in 8 of the 16 excluded patients due to the following reasons: decompensated right heart failure (n=2), hemodynamic instability and/or respiratory distress (n=2), sudden cardiac death during workups (n=2), patient refusal (n=1), and an unknown cause (n=1). Of these patients, 6 patients might have had an advanced disease and a selection bias might have occurred because they were not included in the analysis. Only RHC without acute vasodilator testing was done in the 8 other patients and there was no plausible cause. Although the nature of a single-center study prevents the results from being generalized to the Korean population, the proportion of excluded cases implies that RHC and acute vasodilator testing are not always performed in PAH patients in Korea and, as a result, some acute responders cannot be identified.
A study on the adherence of clinicians to the PAH diagnosis guidelines was recently reported (18). Across the United States, 791 PAH patients who were examined by 60 pulmonologists, cardiologists, and rheumatologists between August 2005 and July 2007 participated in that study. RHC was not done in 77 of these patients (9.7%). In addition, acute vasodilator testing was performed in 32 of 91 patients who received CCB therapy for PAH and only 6 patients were proven to be vasoreactive. There has been no report about how often RHC and acute vasodilator testing are conducted in Korea when diagnosing PAH. Because echocardiography is a non-invasive method of evaluating hemodynamics and availability of echocardiography is high, RHC and subsequent acute vasodilator testing, which are invasive methods, are sometimes not performed when diagnosing PAH in Korea. As our study demonstrates, the prognosis for a vasoreactive subset of Korean IPAH patients is excellent. Therefore, acute vasodilator testing should be included in diagnostic workups for PAH to identify those patients who can benefit from CCB monotherapy and to prevent complications that can develop when PAH patients without vasoreactivity are treated with CCBs.
Our study has several limitations to note. First, due to its retrospective nature, a selection bias might occur and some of the baseline data were missing, particularly the hemodynamic data. Second, the study patients we evaluated might not be representative of all Korean IPAH patients because they were enrolled from a single tertiary referral hospital. Third, beraprost was prescribed together with nifedipine in 6 of the 8 acute responders. Although long-term beneficial effects of beraprost on the hemodynamic and clinical outcomes in PAH patients are not proven (19, 20) and beraprost is only weakly recommended by the current guidelines (21), concurrent beraprost therapy might have affected the long-term survival of acute responders in our study. Finally, our study did not show direct hemodynamic improvement in acute responders because RHC was not repeated during the follow-up period.
In conclusion, vasoreactive patients exist among Korean IPAH patients in a similar proportion to among Western IPAH patients. Korean IPAH patients with vasoreactivity can benefit from long-term treatment with CCBs and their prognosis is excellent. Therefore, to identify and treat such a subgroup adequately, acute vasodilator testing should be performed at the time of diagnosis of RHC for IPAH in Korea.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 4.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 7.Weir EK, Rubin LJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Elliott CG, Fishman AP, Goldring RM, Groves BM, et al. The acute administration of vasodilators in primary pulmonary hypertension. Experience from the National Institutes of Health Registry on Primary Pulmonary Hypertension. Am Rev Respir Dis. 1989;140:1623–1630. doi: 10.1164/ajrccm/140.6.1623. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 9.Tueller C, Stricker H, Soccal P, Tamm M, Aubert JD, Maggiorini M, Zwahlen M, Nicod L Swiss Society for Pulmonary Hypertension. Epidemiology of pulmonary hypertension: new data from the Swiss registry. Swiss Med Wkly. 2008;138:379–384. doi: 10.4414/smw.2008.11915. [DOI] [PubMed] [Google Scholar]
- 10.Murata I, Takenaka K, Yoshinoya S, Kikuchi K, Kiuchi T, Tanigawa T, Ito K. Clinical evaluation of pulmonary hypertension in systemic sclerosis and related disorders. A Doppler echocardiographic study of 135 Japanese patients. Chest. 1997;111:36–43. doi: 10.1378/chest.111.1.36. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Sitbon O, Humbert M, Jagot JL, Taravella O, Fartoukh M, Parent F, Herve P, Simonneau G. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium-channel blockers in primary pulmonary hypertension. Eur Respir J. 1998;12:265–270. doi: 10.1183/09031936.98.12020265. [DOI] [PubMed] [Google Scholar]
- 13.Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132:373–379. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 14.Sakao S, Tanabe N, Kasahara Y, Tatsumi K. Survival of Japanese patients with pulmonary arterial hypertension after the introduction of endothelin receptor antagonists and/or phosphodiesterase type-5 inhibitors. Intern Med. 2012;51:2721–2726. doi: 10.2169/internalmedicine.51.8162. [DOI] [PubMed] [Google Scholar]
- 15.Jing ZC, Jiang X, Han ZY, Xu XQ, Wang Y, Wu Y, Lv H, Ma CR, Yang YJ, Pu JL. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J. 2009;33:1354–1360. doi: 10.1183/09031936.00169608. [DOI] [PubMed] [Google Scholar]
- 16.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 17.Joo H, Park J, Lee SD, Oh YM. Comorbidities of chronic obstructive pulmonary disease in Koreans: a population-based study. J Korean Med Sci. 2012;27:901–906. doi: 10.3346/jkms.2012.27.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, Channick RN, Rubin LJ Pulmonary Arterial Hypertension-Quality Enhancement Research Initiative. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest. 2013;143:324–332. doi: 10.1378/chest.11-3060. [DOI] [PubMed] [Google Scholar]
- 19.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins IM, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119–2125. doi: 10.1016/s0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Song JH, Park DA, Lee JS, Lee SD, Oh YM. Systematic review and meta-analysis of pulmonary hypertension specific therapy for exercise capacity in chronic obstructive pulmonary disease. J Korean Med Sci. 2013;28:1200–1206. doi: 10.3346/jkms.2013.28.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galiè N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S78–S84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]