Abstract
Saponins comprise a class of plant natural products that incorporate a lipophilic terpenoid core, to which is appended one or more carbohydrate residues. They are amphiphilic molecules and often exhibit toxic biological profiles, likely as a result of their roles as vital components in protective coatings to defend against phytopathogen infection and insect predation. The most notable of adjuvant-active saponins investigated for vaccine development come from the Chilean Soapbark Tree, Quillaja saponaria (i.e., QS). More than 30 years ago, semi-purified extracts (i.e., Quil A) from the cortex of Quillaja saponaria were found to be highly effective as adjuvants in veterinary vaccines. However, due to significant and variable toxicity effects, Quil A was not deemed appropriate for human vaccines. More refined purification methods have led to multiple fractions which are derived from the original plant extract. As such, QS-21 to date appears to be one of the more scientifically interesting and robust adjuvants in use in vaccinology. The role of QS-21 as an adjuvant for use in a variety of cancer vaccine trials and its comparison to other adjuvants is discussed in this review.
Keywords: QS-21, adjuvant, vaccines, prostate cancer
INTRODUCTION
Attempts toward augmenting the immune response have been limited by the body’s inability to always recognize a molecule such as a tumor antigen as foreign. While bacterial, natural and synthetic molecules are not always optimal in inducing humoral and/or cellular components of the immune system, generation of specific immune responses against the immunogen appears to be limited by a host of factors. These include not only evaluating the conformation of the molecule, but how it is presented to the immune response and how it works with an adjuvant. To circumvent this, vaccine constructs have used carriers such as keyhole limpet hemocyanin (KLH) and/or adjuvants such as BCG, or combinations with monophosporyl lipid A or the saponins to enhance the vaccines’ immunogenicity. QS-21 to date appears to be one of the more scientifically interesting and robust adjuvants in use in vaccinology. We detail our views in its role in vaccine development.
DEVELOPING AND ENHANCING CANCER VACCINES
No one approach has significantly enhanced the generation of humoral or cellular responses which could correlate with a biologic change in the behavior of the cancer leading to an anti-tumor effect. Indeed, while an immunologic “signal” against the immunogen of choice, i.e., tumor antigen: protein, peptide, naked DNA where (CPG motifs can act as inherent adjuvants) can be obtained in vitro, there is often discordance in its biologic impact on the tumor. In other words, generating an antibody or T cell response does not necessarily correspond to tumor shrinkage. Unlike the success of vaccines against bacterial antigens, tumor vaccines are heavily reliant on several factors. Among these are the size and conformation of the antigen as well as the adjuvant. It should be kept in mind that the development of vaccines to combat infectious diseases has heavily relied on constructs employing subunit antigens. While the use of vaccines derived from defined molecular antigens offers advantages in terms of safety and precision in immune response targeting, they are typically less immunogenic than those employing lifeless or attenuated microorganisms. Often, subunit vaccines require an adjuvant as a critical component to potentiate immune response. The benefits of vaccine adjuvants lie in their ability to, (1) enable the use of otherwise impotent antigens; (2) extend the benefits of vaccines to poor responders; and (3) effect dose-sparing of rare and expensive antigens in short supply.
As an example, many different strategies have been undertaken toward developing an “ideal” immune-mediated therapy for treating cancer. Many of these approaches have been used in prostate cancer research with human prostate-derived cell lines such as LnCAP or PC-3 which have been genetically transduced with cytokine genes for interleukin-2 or GM-CSF [1, 2] in order to enlist ancillary immune cell recruitment. Alternative approaches included the transfection of prostate cancer cell lines infected with viral vectors such as fowlpox [3, 4], vaccinia [5], adenoviruses [6] or plasmids [7] which enhance antibody and potentially T cell responses. Others have demonstrated that synthetic mimes of known altered carbohydrate “self” antigens overexpressed on the cancer cell surface can elicit specific immune responses when coupled to carriers such as KLH and given with QS-21 but were unable to induce T cell immunity [8–11]. A common theme with all of these approaches is that while vigorous antibody titers are induced, there have been little or no antitumor responses in patients with high tumor volume nor have criteria been established which allow better definition as to what should be considered to be a “response” to the cancer. Can we develop specific biologic/immunologic parameters which can reflect that the immunologic target has been recognized and hit? Another limitation to these approaches is that there is no easy way to potentiate and quantitate T cell immunity, which most immunologists feel is critical to enhancing and assessing antitumor responsiveness, respectively. Finally, it remains unclear which antigen(s) is/are the “right target(s)” and which patient population would benefit from these approaches [12]. It has been thought that the heavier the tumor burden, the greater the likelihood that the immune system will be suppressed and that patients with minimal disease would be benefited. To counter this is the observation that there is no definitive way to quantify a clinical response to vaccine therapy in patients who have biochemically relapsed following definitive primary therapy such as surgery or radiation. Another point of interest is that any decline in a biomarker, such as Prostate Specific Antigen (PSA) may not reflect a change in the biology of the tumor, and in vitro evidence of anti-tumor immunity may not be indicative of what is taking place in vivo.
Multiple vaccine approaches have suggested that while immunologic tolerance could be broken, individual vaccines could not sufficiently affect an antitumor response as evidenced by continued disease progression even in the setting of robust antibody responses against the immunogen used for immunization. Therefore, the combination of a vaccine with a biologic modulator, cytokine or checkpoint inhibitor might be a reasonable option. Keep in mind that an ideal adjuvant should induce immunity via different means. For example, an ideal “biomaterial” adjuvant will perform three functions. First, is that it should deliver the antigen in a selective manner to antigen presenting cells. This has been accomplished through release of chemokines or cytokines, use of anti-dendritic cell antibodies, and even through particle size selection. Secondly, biomaterials themselves have been shown to activate innate immunity, but specific innate-activating ligands have also been included in adjuvant formulations. Finally, it should release the antigen appropriately into the dendritic cell.
SAPONINS AS ADJUVANTS
It has long been recognized that certain saponin molecules derived from varied plant sources possess remarkable adjuvant activity. Saponins comprise a class of plant natural products that incorporate a lipophilic terpenoid core, to which is appended one or more carbohydrate residues. They are amphiphilic molecules and often exhibit toxic biological profiles, likely a result of their roles as vital components in protective coatings to defend against phytopathogen infection and insect predation. The most notable of adjuvant-active saponins investigated for vaccine development come from the Chilean Soapbark Tree, Quillaja saponaria (i.e., QS). More than 30 years ago, semi-purified extracts (i.e., Quil A) from the cortex of Quillaja saponaria were found to be highly effective as adjuvants in veterinary vaccines [13–15]. However, due to significant and variable toxicity effects, Quil A was not deemed appropriate for human vaccines. This is not surprising, given the extensive degree of heterogeneity within Quil A (Fig. 1), composed of 20–30 distinct fractions as revealed by RP-HPLC. Efforts directed at separation and evaluation of several constituents of Quil A [13, 14] eventually led to the identification of specific molecular components that impart the immuno-stimulatory properties of the QS-extracts, highlighting the QS-21 fraction as containing potent adjuvant activity with relatively low toxicity [14–16]. Based on these initial findings, QS-21 has since advanced to several clinical trials as a potent adjuvant in several experimental vaccines, including those against malaria [17], HIV [18], and cancer [19].
Fig. 1.
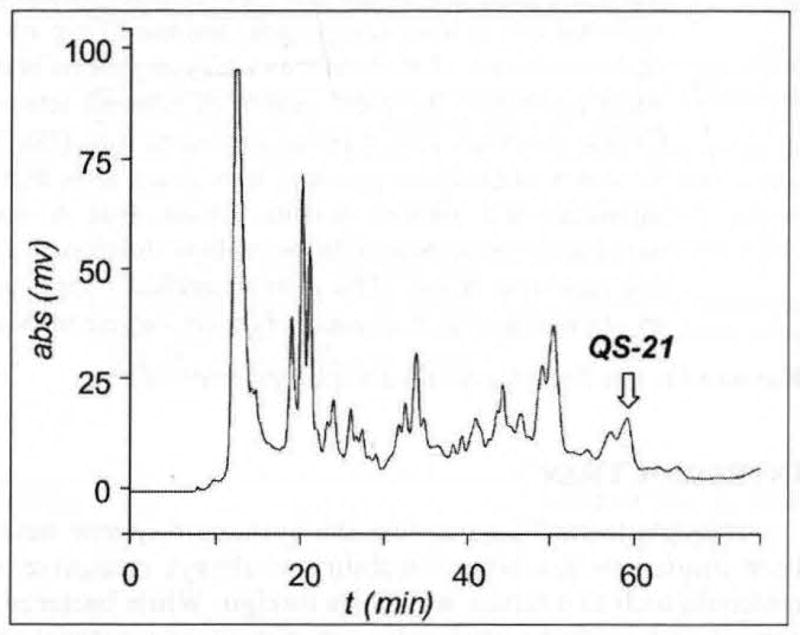
RP-HPLC trace of Quit A.
QS-21 – THE MOLECULE
QS-21 is not a single compound, but rather a mixture of isomeric molecules (Fig. 2) [20, 21]. These constituents are complex saponins incorporating Quillaic Acid as its central triterpene core. Attached to the C3 position of the triterpene is a sterically congested trisaccharide, composed of a branched β-D-GlcA residue incorporating β-D-Gal and β-D-Xyl along its periphery. Linked to the C28 carboxyl group of Quillaic Acid is a linear tetrasaccharide fragment, consisting of either a β-D-Api-(1,3)-β-D-Xyl-(1,4)-α-L-Rha-(1,2)-β-D-Fuc moiety for the QS-21-Api isomer (1), or β-D-Xyl-(1,3)-β-D-Xyl-(1,4)-α-L-Rha-(1,2)-β-D-Fuc moiety for the QS-21-Xyl isomer (2). The remaining component present in both isomeric forms of QS-21 is a pseudo-dimeric branched acyl chain attached to the C4-oxygen of the β-D-Fuc residue. Interestingly, this acyl chain component rapidly and reversibly migrates from the C4-position of β-D-Fuc to the C3-position via trans-esterification [22], adding yet another degree of isomeric heterogeneity in the QS-21 fraction. It is this complex mixture of saponins derived from isolation from the QS tree bark that has fueled vaccine clinical trials to date.
Fig. 2.
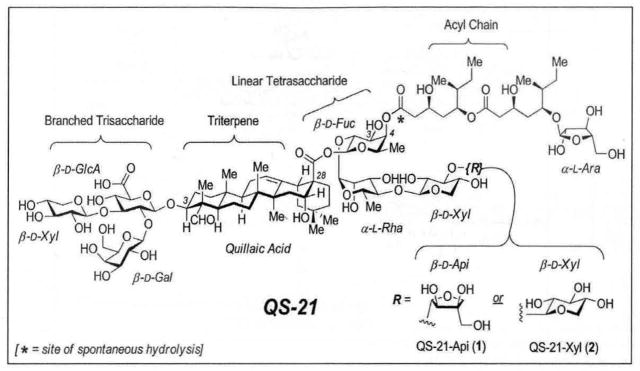
Principal isomeric constituents of QS-21.
Although QS-21 is currently viewed as one of the most potent vaccine adjuvants, there are liabilities associated with its use. First, the procurement of adequate quantities of QS-21 of consistent purity from natural sources is a challenging process. QS-21 constitutes an exceedingly minor component of the bark extracts, whose composition varies considerably even from tree to tree within the same local environment [23]. Moreover, recent metabolomic analysis of the Quil A revealed no less than 100 distinct saponin compounds [24], considerably more than that implied in the RP-HPLC traces consisting of only 20–30 fractions (i.e., Fig. 1). Current isolation protocols for QS-21 rely on protracted sequences of multiple water and MeOH extractions from the QS-bark in combination with several dialysis operations to acquire semi-purified extracts, which are then further purified by multiple chromatographic separations via silica and RP-HPLC [21]. While the end-product is a single fraction by RP-HPLC analysis, its molecular composition remains heterogeneous, and thus presents significant hurdles for regulatory approval in human vaccine development. Second, QS-21 adjuvant is not devoid of toxic side effects. Typical doses of QS-21 in cancer patients do not exceed 100–200μg, above which significant local erythema and systemic flu-like symptoms arise. Finally, the isomeric constituents are susceptible to hydrolytic degradation upon storage/formulation in aqueous solution at neutral or elevated pH. For example, the ester functionalities within the acyl chain undergo spontaneous hydrolysis at pH 7.4 over the course of several days at ambient temperature [22], wherein the terpene-glycoside by-product is found to be a poor adjuvant, and the acyl chain fragments demonstrated undesirable hemolytic effects. Notably, this lack of stability not only compromises its effectiveness in vivo, but also presents a daunting impasse in advancing QS-21 as a vaccine adjuvant in third world settings, the epidemic strongholds of malaria and HIV.
DEVELOPMENT OF QS-21
The therapeutic development of QS-21 to mitigate the undesired toxicity effects and hydrolytic lability has primarily involved the introduction of additives in the form of QS-based ISCOMS [25, 26]. For example, addition of MPL-oil/water emulsions [27], or cholesterol-phospholipid combinations [28], to partially purified QS-extracts have yielded promising results, albeit at the expense of increasing heterogeneity in molecular composition. Alternate strategies have relied on chemical modification of naturally derived QS-21 in the hopes of modulating efficacy [29], although the limited chemistry that can be performed in this context has provided only a very narrow window for structural modification with which to establish limited structure-activity-relationship profiles.
Another recent approach to address the heterogeneity, toxicity, and stability challenges of QS-21 is through structural modification of the adjuvant at the molecular level via de novo chemical synthesis of the carbohydrate portions of QS-21. This strategy has the advantages of accessing defined homogenous molecules, enabling chemical modification of the natural product with exquisite chemical control to generate analogues with increased potency and stability with attenuated toxicity. Advances on this front are based on the recent completion of the first synthesis of QS-21, both in its QS-21-Api (1)[30] and QS-21-Xyl (2) [31] isomeric forms. These highly modular syntheses involve the convergent assembly of the four principle substructure quadrants of the molecules (Fig. 3), including the tri- and tetrasaccharides 3 and 4, the acyl chain 5, and the triterpene core 6. Synthesis of the oligosaccharide portions 3 and 4 of QS-21 was made possible through the development of several novel carbohydrate coupling reactions, including a combination of sulfoxide-mediated dehydrative2 [32] and oxidative [33] glycosylation reactions. Enantioselective synthesis of the fatty acyl chain 5 employed the key steps of diastereo-selective asymmetric crotylation [34] and diastereo-selective aldol reaction [35]. By contrast, de novo synthesis of the selectively protected Quillaic acid ester 6 was not necessary. The bulk of the saponin constituents within the QS-extract contain the Quillaic Acid core, which could be obtained in gram quantities from acid hydrolysis of Quil A [36]. Access to these four structural quadrants of QS-21 in protected form allowed for their subsequent late-stage coupling, global deprotection, and RP-HPLC purification to provide synthetic QS-21 (i.e, SQS-21) in its pure isomeric forms. Importantly, the pure synthetic SQS-21 isomers, both separately and as a mixture, were found to exhibit comparable adjuvant activities to that reported for QS-21 with the GD3-KLH antigen conjugate [31, 37] and have since entered clinical evaluation with a GD3-KLH/GD2-KLH bivalent melanoma vaccine.
Fig. 3.
Preparation of synthetic QS-21 (SQS-21).
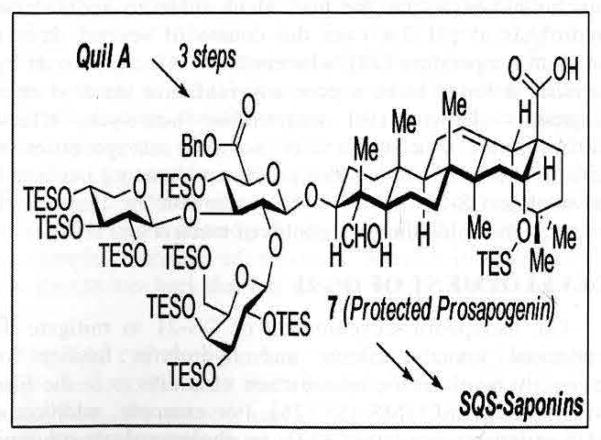
The most recent advances in the chemical synthesis of SQS-21 have led to the development a semisynthetic strategy in which the entire trisaccharide-triterpene half of the molecule can be isolated in gram quantities from chemical degradation of the crude tree bark extracts [38]. This advanced intermediate was selectively protected to furnish the protected prosapogenin 7 [39], which could be advanced to SQS-21 in a synthetic sequence that is streamlined to nearly half of its original length. This technology now enables rapid access to significant quantities of SQS-21 in pure form for further clinical evaluation. Moreover, these advances will not only allow for the rapid generation of systematically designed structural analogues to modulate potency, toxicity and stability, but also uniquely enable systematic generation of structurally defined analogues for investigations into the adjuvants unknown mechanism of immunostimulatory activity [40].
CLINICAL EXPERIENCE WITH QS-21 AND ITS DEVELOPMENT IN VACCINOLOGY
There has been longstanding experience with QS21 as an adjuvant in clinical trials with vaccines, the largest in melanoma and prostate cancer. Unlike other adjuvants which can induce cytokine activation via a Th1 or Th2 response, mice immunized with quillaja saponins as adjuvants developed a Th1 type response and produced antigen-specific cytotoxic T cells [29, 41]. Several papers by Livingston’s group [19, 29, 41, 42] have extensively studied multiple adjuvants including the saponins as well as the bacterial adjuvants in an effort to determine the most potent and the most effective in inducing an immune response. To date, the saponin fraction QS-21 was deemed the most potent single adjuvant but several other adjuvants also had potent adjuvant activity. While combinations of the optimal adjuvants induced an improved immune response compared to QS-21 alone, nevertheless, a new semi-synthetic saponin adjuvant GPI-0100 [8], (Fig. 5) containing the dodecylamide derivative of hydrolyzed naturally-occurring saponins was tested as well. Twelve different adjuvant combinations and GPI-0100 were compared for their ability to augment antibody responses against the GD3 and MUC1 antigens which are over-expressed on a variety of cancer cells. In addition, T-cell responses against GD3, MUC1 and keyhole limpet hemocyanin (KLH), the latter a carrier molecule used for conjugate carbohydrate vaccines were also assessed. These studies suggested that this newer formulation, GPI-0100, and five adjuvant combinations were superior to QS-21 alone for induction of IgM and IgG antibodies as determined by ELISA and FACS analyses against tumor cell lines, against MUC1 and/or GD3: QS-21 plus bacterial nucleotide CpG, QS-21 plus monophosphoryl lipid A (MPL), QS-21 plus non-ionic block copolymer CRL-1005, QS-21 plus Titermax and Titermax plus CpG. No T-cell immunity against GD3 or MUC1 was induced. The antibody responses against GD3 and MUC1 were, however, strongly correlated with IFN-γ release and delayed type hypersensitivity (DTH) responses against KLH. These results demonstrated that combinations of immunological adjuvants were able to augment antibody responses to these conjugates beyond that attainable with QS-21 alone.
Fig. 5.

Structure of GPI-0100.
Our own experience [8–11] with ten conjugate monovalent vaccine trials in patients with either castration resistant metastatic prostate cancer or in patients with biochemically relapsed prostate cancer following primary treatment such as surgery, radiation or both used well-studied ganglioside, glycolipid or glycoproteins including GM-2, Globo H, Tn(cluster), Thomsen-Friedenreich, MUC1-32mer, glycosylated MUC-1-106mer, MUC-2, respectively. All were conjugated to KLH and given with QS-21 inducing high titer IgM and IgG antibodies with specificity for the immunogen used in the vaccination. QS-21 caused mild redness, tenderness and itching at the vaccination site with rare episodes of systemic complaints such as fever or chills. These side effects were transient. However, in an attempt to determine whether a combination of antigens within a vaccine could induce higher antibody titers through mechanisms such as antibody-dependent cell-mediated lysis (ADCC) or complement lysis, a bivalent vaccine was tested in patients with micrometastatic disease as manifested by a rising biomarker, Prostate Specific Antigen (PSA) following surgery or radiation. In this study, GPI-0100, a semi-synthetic saponin was developed with modifications designed to augment stability and diminish the toxicity seen with QS-21. This study [34] was the first to use UF-GPI-0100 and a more purified form, GPI-0100-P in man. These batches were tested with doses ranging between 100 and 5000μg in group of five treated patients with rising PSAs. GPI-0100 was mixed with a bivalent vaccine containing the glycolipid Globo H and the glycosylated MUC-2 conjugated to KLH. There was slightly less overall local reactivity and no systemic side effects. While safe overall, antibody titers against Globo H and MUC-2 escalated with increasing dose levels. At the 5000μg dose level, toxicity remained minimal with only occasional grade II local toxicity and occasional sporadic grade I elevations in liver enzymes. Compared with a subsequent trial with the same bivalent vaccine plus QS-21 at the maximal tolerated dose of 100μg, the 5000μg dose of GP-01200 produced comparable antibody titers.
CONCLUSIONS
The development of vaccines for patients with cancers has been limited by inherent immunogenicity of the antigen, in addition to how it needs to be presented to the immune system. Unfortunately, the antigens under study are altered “self” antigens and are not truly cancer-specific. What is clearly needed is a means to enhance immunogenicity and at the same time determine more biologically meaningful readout or signals suggesting that we have not only hit the immunologic target but have impacted on the disease as well. This will allow for a “go” or “no go” approach in vaccine development. Secondly, the vaccine must be delivered in an optimal manner so that it is not destroyed by the reticulo-endothelial system prior to inducing some immunologic effect. The third and probably the most important, is that the immunologic adjuvant is critical to the induction of immune responses as many cancer antigens (altered “self” molecules) by themselves are unable to elicit an immunologic response. While our history of vaccinology has been fulfilled using QS-21 and its “iterations”, nevertheless, the clearest test of whether the vaccine truly works is to determine whether the immune readouts are associated with an impact on the biologic behavior of the tumor. This area remains active and will continue to be of interest to chemists, immunologists and oncologists irrespective of the disease.
Fig. 4.
Semisynthesis of SQS-saponins.
References
- 1.Simons JW, Jaffee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Simons JW, Mikhak B, Change J-F, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete Granulocyte-Macrophage Colony-Stimulating Factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–68. [PubMed] [Google Scholar]
- 3.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1–5. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elzey BD, Siemens DR, Ratliff TL, Lubroff DM. Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int J Cancer. 2001;94:842–49. doi: 10.1002/ijc.1556. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LE, Frye TP, Arnot AR, et al. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) Vaccine. 2006;24:293–03. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 8.Slovin SF, Ragupathi G, Adluri S, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–15. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovin SF, Ragupathi G, Musselli C, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–98. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 10.Slovin SF, Ragupathi G, Musselli C, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–02. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slovin SF, Ragupathi G, Fernandez C, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–22. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 12.Slovin SF. Emerging role of immunotherapy in the management of prostate cancer. Oncology. 2007;21:326–33. [PubMed] [Google Scholar]
- 13.Dalsgaard K. Thin layer chromatographic finger printing of commercially available saponins. Dansk Tidsskrift for Farmaci. 1970;44:327–3. [PubMed] [Google Scholar]
- 14.Dalsgaard K. Saponin Adjuvants .3. Isolation of a substance from Quillaja Saponaria Molina with adjuvant activity in Foot-and-Mouth-Disease vaccines. Archiv Fur Die Gesamte Virusforschung. 1974;44:243–54. [PubMed] [Google Scholar]
- 15.Kersten GFA, Teerlink T, Derks HJ. Incorporation of the major outer-membrane protein of Neisseria-Gonorrhoeae in saponin-lipid complexes (Iscoms) - chemical-analysis, some structural features, and comparison of their immunogenicity with 3 other antigen delivery systems. Infect Immun. 1988;56:432–48. doi: 10.1128/iai.56.2.432-438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja-Saponaria Molina Cortex. J Immuno. 1991;146:431–37. [PubMed] [Google Scholar]
- 17.Kashala O, Amador R, Valero, et al. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine. 2002;20:2263–77. doi: 10.1016/s0264-410x(02)00115-9. [DOI] [PubMed] [Google Scholar]
- 18.Evans TG, McElrath MJ, Mathews T, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immmunization in humans. Vaccine. 2001;19:2080–91. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 19.Livingston PO, Ragupathi G. Cancer vaccines targeting carbohydrate antigens. Human Vaccines. 2006;2:137–43. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen NE, Fairbrother WJ, Kensil CR, Lim A, Wheeler DA, Powell MF. Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by H-1 and natural abundance C-13 NMR spectroscopy. Carbohydr Res. 1996;280:1–14. doi: 10.1016/0008-6215(95)00278-2. [DOI] [PubMed] [Google Scholar]
- 21.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carr Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 22.Cleland JL, Kensil CR, Lim A, et al. Isomerization and formulation stability of the vaccine adjuvant QS-21. J Pharm Sci. 1996;85:22–28. doi: 10.1021/js9503136. [DOI] [PubMed] [Google Scholar]
- 23.Kamstrup S, San Martin R, Doberti A, Grande H, Dalsgaard K. Preparation and characterisation of quillaja saponin with less heterogeneity than Quil-A. Vaccine. 2000;18:2244–49. doi: 10.1016/s0264-410x(99)00560-5. [DOI] [PubMed] [Google Scholar]
- 24.Kite GC, Howes MJR, Simmonds MJ. Metabolomic analysis of saponins in crude extracts of Quillaja saponaria by liquid chromatography/mass spectrometry for product authentication. Rapid Commun Mass Spectrom. 2004;18:2859–70. doi: 10.1002/rcm.1698. [DOI] [PubMed] [Google Scholar]
- 25.Barr IG, Mitchell GF. ISCOMs (immunostimulating complexes): The first decade. Immunol Cell Biol. 1996;74:8–25. doi: 10.1038/icb.1996.2. [DOI] [PubMed] [Google Scholar]
- 26.Sanders MT, Brown LE, Deliyannis G, Pearse MJ. ISCOM (TM)-based vaccines: The second decade. Immunol Cell Biol. 2005;83:119–28. doi: 10.1111/j.1440-1711.2005.01319.x. [DOI] [PubMed] [Google Scholar]
- 27.Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 28.Drane D, Gittleson C, Boyle J, Moraskovsky E. ISCOMATRIX (TM) adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines. 2007;6:761–72. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- 29.Marciani DJ, Press JB, Reynold RC, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–51. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Wang P, Navarro-villalobos M, Rohde BD, Derryberry J, Gin DY. Synthetic studies of complex immunostimulants from Quillaja saponaria: Synthesis of the potent clinical immunoadjuvant QS-21 A(api) J Am Chem Soc. 2006;128:11906–915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng K, Adams MM, Damani P, Livungston PO, Ragupathi G, Gin DY. Synthesis of QS-21-xylose: Establishment of the immune-potentiating activity of synthetic QS-21 adjuvant with a melanoma vaccine. Angew Chem Int Ed. 2008;47:6395–8. doi: 10.1002/anie.200801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia BA, Gin DY. Dehydrative glycosylation with activated diphenyl sulfonium reagents. Scope, mode of C(1)-hemiacetal activation, and detection of reactive glycosyl intermediates. J Am Chem Soc. 2000;122:4269–79. [Google Scholar]
- 33.Di Bussolo V, Kim YJ, Gin DY. Direct oxidative glycosylations with glycal donors. J Am Chem Soc. 1998;120:13515–516. [Google Scholar]
- 34.Brown HC, Bhat KS. Chiral synthesis aia Organoboranes .7. Diastereoselective and Enantioselective Synthesis of erythro-beta-methylomoallyl and threo-beta-,ethylhomoallyl alchols via enantiomeric (Z)-Crotylborane and (E)-Crotylborane. J Am Chem Soc. 1986;108:5919–23. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 35.Braun M, Waldmuller D. Simple 3-step synthesis of (R)-4-amino-3-hydroxybutanoic and (S)-4-amino-3-hydroxybutanoic acid (Gabob) by stereoselective aldol addition. Synthesis-Stuttgart. 1989:856–85. [Google Scholar]
- 36.Elliott DF, Kon GAR, Sapogenins VI. Quillaic acid. J Chem Soc. 1939:1130–35. [Google Scholar]
- 37.Helling F, Shang A, Calves M, et al. G(D3) Vaccines for melanoma-superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–03. [PubMed] [Google Scholar]
- 38.Higuchi R, Tokimitsu Y, Fujioka T, Komori T, Kawaski T, Oakenful DG. Structure of Desacylsaponins Obtained from the Bark of Quillaja-Saponaria. Phytochemistry. 1987;26:229–35. [Google Scholar]
- 39.Deng K, Adams MM, Gin DY. Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants. J Am Chem Soc. 2008;130:5860–61. doi: 10.1021/ja801008m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Disc Today. 2003;8:934–43. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 41.Kim SK, Ragupathi G, Capello S, et al. Effect of immunological adjuvant combinations on the antibody and T cell-response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19:530–37. doi: 10.1016/s0264-410x(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 42.Liu G, Anderson D, Scaltreto H, Barbon J, Kensil CR. QS-21 structure/function studies: effect of acetylation on adjuvant activity. Vaccine. 2002;20:2808–15. doi: 10.1016/s0264-410x(02)00209-8. [DOI] [PubMed] [Google Scholar]